Abstract
Background
Despite technical advances in minimally invasive gastrectomy for gastric cancer, an increased incidence of postoperative pancreatic fistula (POPF) has been reported. POPF can cause infectious and bleeding complications, which could lead to surgery-related death; therefore, reduction of the post-gastrectomy POPF risk is crucial. This study aimed to investigate the importance of pancreatic anatomy as a predictor of POPF in patients undergoing laparoscopic or robotic gastrectomy.
Methods
Data were collected from 331 consecutive patients who underwent laparoscopic or robotic gastrectomy for gastric cancer. The thickness of the pancreas anterior to the most ventral level of the splenic artery (TPS) was measured. The correlation between TPS and POPF incidence was investigated using univariate and multivariate analyses.
Results
The cutoff value of TPS was 11.8 mm, which predicted a high drain amylase concentration on postoperative day 1, and patients were categorized into thin (Tn group) and thick TPS groups (Tk group). There was no significant difference in the background characteristics between the two groups, except for sex (P = 0.009) and body mass index (P < 0.001). The incidences of POPF grade B or higher (2% vs. 16%, P < 0.001), all postoperative complications of grade II or higher (12% vs. 28%, P = 0.004), and postoperative intra-abdominal infections of grade II or higher (4% vs. 17%, P = 0.001) were significantly higher in the Tk group. Multivariable analysis identified that high TPS was the only independent risk factor for grade B or higher POPF and grade II or higher postoperative intra-abdominal infectious complications.
Conclusions
The TPS is a specific predictive factor for POPF and postoperative intra-abdominal infectious complications in patients undergoing laparoscopic or robotic gastrectomy. Careful pancreatic manipulation during suprapancreatic lymphadenectomy is necessary for patients with increased TPS (> 11.8 mm) to avoid postoperative complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Gastric cancer was the fifth most common cancer and the fourth leading cause of cancer-related mortality worldwide in 2020 [1]; it has a high incidence, especially in East Asian countries [2]. Minimally invasive surgery, such as laparoscopic (LG) or robotic gastrectomy (RG), is widely used as a curative surgical treatment for gastric cancer. Despite advances in surgical techniques, the incidence of postoperative pancreatic fistula (POPF), one of the most common postoperative complications, has increased significantly [3, 4]. POPF can cause sepsis, bleeding, intra-abdominal infection, and anastomotic leakage, which could lead to surgery-related death; therefore, reduction of the post-gastrectomy POPF risk is crucial. Several factors, including sex, age, body shape, blood reports, cancer progression, and pancreatic anatomy, have been reported as POPF predictors [5,6,7,8,9,10,11,12,13]. Additionally, POPF is known to occur due to direct physical or thermal pancreatic injury, including compression [14]. Unintentional damage to the pancreas or prolonged duration of pancreatic compression mainly occurs during suprapancreatic lymph node dissection (No.7, 8a, 9, and 11p). Although pancreatic thickness is reportedly a predictor of POPF risk [10, 12], a thick pancreas, which appears anterior to the most protuberant part of the splenic artery loop, has become an obstacle for suprapancreatic lymphadenectomy. We hypothesized that the thickness of the pancreas in front of the most ventral level of the splenic artery arch after branching from the celiac artery (TPS) is a reliable risk predictor for postoperative complications after gastrectomy. In measuring TPS, we can predict the patients who may develop POPF. Thus, for patients who show a greater TPS, it is possible to perform lymphadenectomy more carefully. Moreover, as TPS is simple and easily measured through thin-slice computed tomography (CT), it can be introduced relatively easily in clinical practice. Therefore, this study aimed to evaluate the importance of pancreatic anatomy as a predictor of POPF in patients undergoing LG or RG.
Materials and methods
Patients
This retrospective cohort study included data of 331 consecutive patients who underwent LG or RG for gastric cancer at the Department of Gastroenterological Surgery, Ishikawa Prefectural Central Hospital, Ishikawa, Japan, between April 2019 and December 2022. Twelve patients for whom the drain amylase concentration on postoperative day (POD) 1 was not recorded and 48 patients who underwent gastrectomy without suprapancreatic lymph node dissection were excluded. For the 282 patients included, TPS was measured using preoperative CT.
This study was performed in accordance with the World Medical Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects, and written informed consent for surgery and the use of clinical data were obtained from all patients included in this study. This study was approved by the ethics committee of Ishikawa Prefectural Central Hospital (approval no. 2042).
Surgical indication and procedures
According to the latest Japanese Gastric Cancer Treatment Guidelines, we performed LG or RG with D1 + or D2 lymph node dissection, according to the cancer stage [15]. Gastric cancer at any stage was considered an indication for LG or RG. The indications for LG and RG did not differ, and we selected either procedure according to patient preference. All LGs and RGs were performed or assisted by a qualified surgeon from the Japanese Society for Endoscopic Surgery, and all RGs were performed using the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). During suprapancreatic lymph node dissection, the pancreas was gently compressed with a soft sponge for the shortest required duration. After exposing the splenic artery, nerve fibers were pulled caudally to obtain a good surgical field of view. LG was performed with five ports: one for a scope, two for an operator, and two for a first assistant; however, in cases where the operator forceps unintentionally pressed the pancreas during the suprapancreatic lymph node dissection, we added an additional port to avoid accidental pancreatic compression. A 19 Fr Blake drain was placed in the suprapancreatic region.
Measurement of the pancreatic thickness
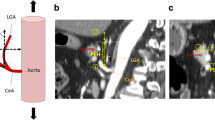
Preoperative contrast-enhanced abdominal CT scans of all patients were reviewed. We searched for a slice in which the arch of the splenic artery ran at the ventral level after branching from the celiac artery. The thickness of the axial CT slice was 0.5 or 1 mm. The thickness of the pancreas anterior to the most ventral point of the splenic artery was labeled as the TPS (Fig. 1), which was measured and recorded for all patients.
TPS measurement using CT images. TPS can be measured with axial CT images. The white arrow indicates the splenic artery and yellow, the pancreas anterior to the most ventral level of the splenic artery. a a case where TPS is 0 mm, b a case where TPS is 17.8 mm. TPS thickness of the pancreas anterior to the most ventral level of the splenic artery, CT computed tomography
Definition of outcomes
For all patients, the drain output and serum amylase levels were measured on PODs 1 and 3. We defined a drain amylase concentration > 1000 U/L on POD 1 as a high drain amylase concentration, which has been reported to be an indicator of pancreas-related intra-abdominal abscess [16, 17]. POPF was defined with the International Study Group on Pancreatic Fistula Definition [18]. However, suprapancreatic fluid collection with inflammatory findings detected by CT with high CRP (> 20 on POD 3), without clinical or radiological evidence of anastomotic leakage was also regarded as POPF, despite the low drain amylase concentration. The severity of postoperative complications was determined using the Clavien–Dindo (CD) classification [19]. Grade II or higher adverse events that occurred within 30 postoperative days were defined as significant postoperative complications, which included intra-abdominal infectious complications, such as intra-abdominal abscess, pancreatic fistula, and anastomotic leakage. The primary endpoint of this study was the incidence of POPF grade B or higher, and the secondary endpoints were the incidences of POPF and postoperative intra-abdominal infections.
Statistical analysis
All categorical variables are expressed as numbers (percentages) and continuous variables as medians (ranges). A scatterplot was used to analyze correlations, and Spearman’s rank correlation coefficient was used to assess the strength of the correlation. To determine the optimal cutoff values of TPS as a predictor of POPF, receiver operating characteristic (ROC) curves were calculated. To evaluate the differences in categorical and continuous variables, Fisher’s exact, Chi-squared, and Mann–Whitney U tests were used, as appropriate. If there were more than eight events per cofounder for multivariable analysis, logistic regression analysis was adopted, not propensity scores [20]. Statistical significance was set at P < 0.05. All statistical analyses were performed using the EZR statistical software (Easy R, Saitama Medical Center, Jichi Medical University, Japan) [21] based on R and R commander.
Results
This study included 282 patients. Spearman’s rank correlation coefficient was 0.35 (P < 0.001); therefore, there was a weak correlation between TPS and drain amylase concentration on POD 1 on the scatterplot (Fig. 2). The ROC curve indicated that the area under the curve was highest when the TPS was 11.8 mm (Fig. 3), which was considered as the optimal TPS cutoff value. We categorized the patients into thin (Tn group, n = 215) and thick (Tk group, n = 67) TPS groups. Patient characteristics and surgical details of the two groups are described in Table 1. There were significant differences in sex (P = 0.009) and body mass index (BMI) (P < 0.001) between the two groups. Surgical outcomes of the two groups are shown in Table 2. Amylase level in the drained fluid (D-Amy) on POD 1 (P < 0.001), the incidence of grade II or higher complications (P = 0.004), all POPF (P = 0.004), POPF grade B (P < 0.001), and grade II or higher intra-abdominal infectious complications (P = 0.001) were significantly higher in the Tk group. In the multivariable logistic regression analysis, TPS was identified as the only independent risk factor for all POPF (odds ratio [OR] 2.55, 95% confidence interval [CI] 1.42–4.98, P = 0.009) (Table 3), grade B or higher POPF (OR 7.52, 95% CI 2.46–23.0, P < 0.001) (Table 4), and intra-abdominal infection (OR 4.89, 95% CI 1.91–12.6, P < 0.001) (Table 5).
Correlation between TPS and postoperative drain amylase concentration. The scatterplot of the correlation between TPS and drain amylase concentration on postoperative day 1 is shown. The vertical axis of the plot is scaled logarithmically. There is a weak correlation between the two (r = 0.35, P < 0.001). TPS thickness of the pancreas anterior to the most ventral level of the splenic artery
Optimal TPS value. The receiver operation characteristic curve for predicting high drain amylase concentration over 1000 U/L on postoperative day 1 based on TPS is shown. The cutoff value of TPS is 11.8 mm, which predicts a high drain amylase concentration on postoperative day 1, because the area under the curve is highest. TPS thickness of the pancreas anterior to the most ventral level of the splenic artery
Discussion
The primary endpoint that TPS predicts POPF of grade B or higher was achieved with a high OR of 7.59. The secondary endpoints were also achieved, except for postoperative complications of CD grade II or higher. Although a high BMI, male sex, advanced cancer stage, and extended lymphadenectomy have been reported to be associated with POPF or postoperative complications, they were not regarded as independent predictors of postoperative complications [5,6,7,8,9].
The POPF occurrence rate after LG is 1.7–7.2% [5, 22,23,24,25], which is higher than that after an open gastrectomy (OG) [22, 26, 27]. Pancreatic juice leakage occurs not only after pancreatic parenchymal injury but also following pancreatic compression [14]. Regarding surgical procedures, Itamoto et al. reported that a longer time of pancreas compression during minimally invasive gastrectomy was associated with a higher incidence of postoperative complications [28]. However, pancreatic compression is required during suprapancreatic lymphadenectomy to identify the dissection line between the lymph nodes to be dissected and the pancreas or artery. Therefore, a thick or protruding pancreas that impedes lymphadenectomy is expected to be a risk factor for increasing the outflow of pancreatic juice. A novel procedure for pancreas-compressionless lymphadenectomy was reported by Tsujiura et al.; however, minimal pancreatic compression by an assistant’s forceps may be required according to the pancreatic anatomy to obtain a good surgical view or to avoid lateral thermal injuries by surgical instruments [29]. Previous studies have reported the impact of anatomical features of the pancreas on POPF: Kobayashi et al. reported that the process of the pancreatic head is a risk factor for POPF after LG for gastric cancer; Migita et al. reported that the length between the levels of the pancreatic body surface and root of the common hepatic artery is a predictor of POPF; Kumagai et al. reported that the length of the vertical line between the pancreas and aorta, and angle between a line drawn from the upper border of the pancreas to the root of the celiac artery and aorta are independent predictors of pancreatic fistula and/or postoperative complications and correlate with drain amylase concentration after LG for gastric cancer; and Kinoshita et al. reported that the maximum vertical length between the upper border of the pancreas and root of the left gastric artery on a preoperative sagittal CT is a specific and independent predictor of POPF in LG [10,11,12,13]. However, the splenic artery is known for its tortuosity after branching from the celiac trunk [30, 31]. We hypothesized that a thick pancreas, anterior to the most protuberant section of the splenic artery loop, could be a more precise predictor of the part of the pancreas that needs to be compressed during suprapancreatic lymphadenectomy and POPF. Our study concluded that a TPS ≥ 11.8 mm is an independent risk factor of POPF of grade B or higher, with a higher OR (7.53) than those of previously reported predictors.
Additionally, a previous study reported a significant positive correlation between BMI and pancreatic volume [32]; BMI and TPS positively correlated in our study. However, since BMI was not a significant indicator of POPF and intra-abdominal infectious complications in the univariable and multivariable analyses, TPS is not an indicator of pancreatic thickness or volume; instead, it indicates the part of the pancreas that can hinder suprapancreatic lymphadenectomy.
To ensure safe and effective suprapancreatic lymphadenectomy, we used the outermost layer-oriented medial approach [33,34,35]. In this technique, the thin loose connective tissue layer between the autonomic nerve sheaths of the arteries and adipose tissue, including lymph nodes, is dissected [33,34,35]. After exposing the autonomic nerve sheath, we pulled the nerve sheath caudally to identify the No. 11p lymph nodes instead of compressing the pancreatic body. We presumed that the use of this technique led to our finding that there was no significant difference in the POPF occurrence between D1 + and D2 lymph node dissections, although D2 lymphadenectomy is reportedly a risk factor for POPF [9].
POPF occurs more frequently after LG compared with OG [22, 27, 36, 37]. A Japanese nationwide prospective cohort study using the National Clinical Database reported a higher incidence of POPF after LG than after an open procedure. Kinoshita et al. reported that the anatomical location of the pancreas was one of the reasons for the high POPF incidence after LG, because limited forceps mobility and unintentionally strong compression may have led to pancreatic trauma [10]. In the present study, we examined LG and RG during the same period, at the same institution, and with the same surgical indication. Although RG is expected to decrease the incidence of POPF with the use of articulated forceps, which reduce unintentional pancreatic compression, and laparoscopic coagulating shears, which are frequently used in LG, to decrease lateral thermal injuries, the impact of the robotic approach on POPF reduction remains controversial [38,39,40]. Similarly, our study did not detect statistically significant differences between the impacts of the laparoscopic and robotic approaches on the incidences of POPF grade B or higher (P = 0.61), all POPF (P = 0.31), and intra-abdominal infectious complications (P = 0.49). This may be because pancreatic compression, conducted to provide a good surgical view, is mainly performed laparoscopically using the assistant’s forceps. Therefore, direct laparoscopic pancreatic compression should be avoided as much as possible to decrease the incidence of POPF.
As we reported, TPS is an independent predictor not only for POPF grade B or higher, but also for all POPF and intra-abdominal infectious complications. Measuring TPS is simple and does not require any specific device. Therefore, we believe that the TPS is a good predictor of postoperative complications after minimally invasive gastrectomy. For patients with increased TPS over 11.8 mm, careful pancreatic manipulation is required. When performing the pancreas-contactless technique, the operator themself should gently perform the minimal number of necessary pancreatic compressions instead of an assistant to pay more awareness to the procedure [41].
This study had some limitations. First, it was conducted retrospectively at a single institution. Pancreatic compression time should be investigated to verify its positive relationship with TPS; however, it was difficult to check this because several surgical videos were unavailable. Furthermore, because the incidence of grade B or higher POPF and grade II or higher intra-abdominal infectious complications were low, only a few explanatory variables were included in the multivariable analysis to maintain statistical quality, and a larger sample size will strengthen the conclusion. Additionally, different operating platforms were included in this study and there was weakness in the analysis performance. Prospective studies with a larger sample size and that investigate the relationships between TPS and pancreatic compression time are necessary to strengthen the power of this study.
In conclusion, TPS is a specific predictive factor for POPF and postoperative intra-abdominal infections in patients undergoing LG and RG. By measuring TPS, patients at a high risk of developing POPF and postoperative intra-abdominal infections can be identified preoperatively. Additionally, careful pancreatic manipulation during suprapancreatic lymphadenectomy is necessary for patients with increased TPS (> 11.8 mm) to avoid postoperative complications.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Morgan E, Arnold M, Camargo MC, Gini A, Kunzmann AT, Matsuda T, Meheus F, Verhoeven RHA, Vignat J, Laversanne M, Ferlay J, Soerjomataram I (2022) The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: a population-based modelling study. EClinicalMedicine 47:101404
Hiki N, Honda M, Etoh T, Yoshida K, Kodera Y, Kakeji Y, Kumamaru H, Miyata H, Yamashita Y, Inomata M, Konno H, Seto Y, Kitano S (2018) Higher incidence of pancreatic fistula in laparoscopic gastrectomy. Real-world evidence from a nationwide prospective cohort study. Gastric Cancer 21:162–170
Guerra F, Giuliani G, Iacobone M, Bianchi PP, Coratti A (2017) Pancreas-related complications following gastrectomy: systematic review and meta-analysis of open versus minimally invasive surgery. Surg Endosc 31:4346–4356
Jiang X, Hiki N, Nunobe S, Kumagai K, Nohara K, Sano T, Yamaguchi T (2012) Postoperative pancreatic fistula and the risk factors of laparoscopy-assisted distal gastrectomy for early gastric cancer. Ann Surg Oncol 19:115–121
Tsujinaka T, Sasako M, Yamamoto S, Sano T, Kurokawa Y, Nashimoto A, Kurita A, Katai H, Shimizu T, Furukawa H, Inoue S, Hiratsuka M, Kinoshita T, Arai K, Yamamura Y, Gastric Cancer Surgery Study Group of Japan Clinical Oncology Group (2007) Influence of overweight on surgical complications for gastric cancer: results from a randomized control trial comparing D2 and extended para-aortic D3 lymphadenectomy (JCOG9501). Ann Surg Oncol 14:355–361
Wu J, Tang Z, Zhao G, Zang L, Li Z, Zang W, Li Z, Qu J, Yan S, Zheng C, Ji G, Zhu L, Zhao Y, Zhang J, Huang H, Hao Y, Fan L, Xu H, Li Y, Yang L, Song W, Zhu J, Zhang W, Li M, Qin X, Liu F (2022) Incidence and risk factors for postoperative pancreatic fistula in 2089 patients treated by radical gastrectomy: a prospective multicenter cohort study in China. Int J Surg 98:106219
Ojima T, Iwahashi M, Nakamori M, Nakamura M, Naka T, Ishida K, Ueda K, Katsuda M, Iida T, Tsuji T, Yamaue H (2009) Influence of overweight on patients with gastric cancer after undergoing curative gastrectomy: an analysis of 689 consecutive cases managed by a single center. Arch Surg 144:351–358
Yu HW, Jung DH, Son SY, Lee CM, Lee JH, Ahn SH, Park DJ, Kim HH (2013) Risk factors of postoperative pancreatic fistula in curative gastric cancer surgery. J Gastric Cancer 13:179–184
Kinoshita J, Yamaguchi T, Saito H, Moriyama H, Shimada M, Terai S, Okamoto K, Nakanuma S, Makino I, Nakamura K, Tajima H, Ninomiya I, Fushida S (2020) Comparison of prognostic impact of anatomic location of the pancreas on postoperative pancreatic fistula in laparoscopic and open gastrectomy. BMC Gastroenterol 20:325
Kobayashi N, Shinohara H, Haruta S, Ohkura Y, Mizuno A, Ueno M, Udagawa H, Sakai Y (2016) Process of pancreas head as a risk factor for postoperative pancreatic fistula in laparoscopic gastric cancer surgery. World J Surg 40:2194–2201
Kumagai K, Hiki N, Nunobe S, Kamiya S, Tsujiura M, Ida S, Ohashi M, Yamaguchi T, Sano T (2018) Impact of anatomical position of the pancreas on postoperative complications and drain amylase concentrations after laparoscopic distal gastrectomy for gastric cancer. Surg Endosc 32:3846–3854
Migita K, Matsumoto S, Wakatsuki K, Ito M, Kunishige T, Nakade H, Nakatani M, Kitano M, Nakajima Y (2016) The anatomical location of the pancreas is associated with the incidence of pancreatic fistula after laparoscopic gastrectomy. Surg Endosc 30:5481–5489
Ida S, Hiki N, Ishizawa T, Kuriki Y, Kamiya M, Urano Y, Nakamura T, Tsuda Y, Kano Y, Kumagai K, Nunobe S, Ohashi M, Sano T (2018) Pancreatic compression during lymph node dissection in laparoscopic gastrectomy: possible cause of pancreatic leakage. J Gastric Cancer 18:134–141
Japanese Gastric Cancer Association (2021) Japanese gastric cancer treatment guidelines. Gastric Cancer 24:1–21
De Sol A, Cirocchi R, Di Patrizi MS, Boccolini A, Barillaro I, Cacurri A, Grassi V, Corsi A, Renzi C, Giuliani D, Coccetta M, Avenia N (2015) The measurement of amylase in drain fluid for the detection of pancreatic fistula after gastric cancer surgery: an interim analysis. World J Surg Oncol 13:65
Iwata N, Kodera Y, Eguchi T, Ohashi N, Nakayama G, Koike M, Fujiwara M, Nakao A (2010) Amylase concentration of the drainage fluid as a risk factor for intra-abdominal abscess following gastrectomy for gastric cancer. World J Surg 34:1534–1539
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M, International Study Group on Pancreatic Surgery (ISGPS) (2017) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 161:584–591
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Cepeda MS, Boston R, Farrar JT, Strom BL (2003) Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol 158:280–287
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Obama K, Okabe H, Hosogi H, Tanaka E, Itami A, Sakai Y (2011) Feasibility of laparoscopic gastrectomy with radical lymph node dissection for gastric cancer: from a viewpoint of pancreas-related complications. Surgery 149:15–21
Katai H, Sasako M, Fukuda H, Nakamura K, Hiki N, Saka M, Yamaue H, Yoshikawa T, Kojima K, JCOG Gastric Cancer Surgical Study Group (2010) Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer 13:238–244
Inaki N, Etoh T, Ohyama T, Uchiyama K, Katada N, Koeda K, Yoshida K, Takagane A, Kojima K, Sakuramoto S, Shiraishi N, Kitano S (2015) A multi-institutional, prospective, phase II feasibility study of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (JLSSG0901). World J Surg 39:2734–2741
Miyai H, Hara M, Hayakawa T, Takeyama H (2013) Establishment of a simple predictive scoring system for pancreatic fistula after laparoscopy-assisted gastrectomy. Dig Endosc 25:585–592
Washio M, Yamashita K, Niihara M, Hosoda K, Hiki N (2020) Postoperative pancreatic fistula after gastrectomy for gastric cancer. Ann Gastroenterol Surg 4:618–627
Fujita T, Ohta M, Ozaki Y, Takahashi Y, Miyazaki S, Harada T, Iino I, Kikuchi H, Hiramatsu Y, Kamiya K, Konno H (2015) Collateral thermal damage to the pancreas by ultrasonic instruments during lymph node dissection in laparoscopic gastrectomy. Asian J Endosc Surg 8:281–288
Itamoto K, Hikage M, Fujiya K, Kamiya S, Tanizawa Y, Bando E, Terashima M (2021) The impact of pancreas compression time during minimally invasive gastrectomy on the postoperative complications in gastric cancer. Ann Gastroenterol Surg 5:785–793
Tsujiura M, Hiki N, Ohashi M, Nunobe S, Kumagai K, Ida S, Okumura Y, Sano T, Yamaguchi T (2017) “Pancreas-compressionless gastrectomy”: a novel laparoscopic approach for suprapancreatic lymph node dissection. Ann Surg Oncol 24:3331–3337
Brinkman DJ, Troquay S, de Jonge WJ, Irwin ED, Vervoordeldonk MJ, Luyer MDP, Nederend J (2021) Morphometric analysis of the splenic artery using contrast-enhanced computed tomography (CT). Surg Radiol Anat 43:377–384
Moraes DMV, Gutierres A, Colleoni Neto R, Lindemann IL, Rottenfusser R, Carlotto JRM (2022) Anatomy of the splenic artery: what does the surgeon need to know? Rev Col Bras Cir 49:e20223294
Kou K, Saisho Y, Jinzaki M, Itoh H (2014) Relationship between body mass index and pancreas volume in Japanese people. JOP 15:626–627
Kanaya S, Haruta S, Kawamura Y, Yoshimura F, Inaba K, Hiramatsu Y, Ishida Y, Taniguchi K, Isogaki J, Uyama I (2011) Video: laparoscopy distinctive technique for suprapancreatic lymph node dissection: medial approach for laparoscopic gastric cancer surgery. Surg Endosc 25:3928–3929
Suda K, Nakauchi M, Inaba K, Ishida Y, Uyama I (2016) Minimally invasive surgery for upper gastrointestinal cancer: our experience and review of the literature. World J Gastroenterol 22:4626–4637
Uyama I, Kanaya S, Ishida Y, Inaba K, Suda K, Satoh S (2012) Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg 36:331–337
Haverkamp L, Weijs TJ, van der Sluis PC, van der Tweel I, Ruurda JP, van Hillegersberg R (2013) Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis. Surg Endosc 27:1509–1520
Jeong O, Ryu SY, Zhao XF, Jung MR, Kim KY, Park YK (2012) Short-term surgical outcomes and operative risks of laparoscopic total gastrectomy (LTG) for gastric carcinoma: experience at a large-volume center. Surg Endosc 26:3418–3425
Ojima T, Nakamura M, Hayata K, Kitadani J, Katsuda M, Takeuchi A, Tominaga S, Nakai T, Nakamori M, Ohi M, Kusunoki M, Yamaue H (2021) Short-term outcomes of robotic gastrectomy vs laparoscopic gastrectomy for patients with gastric cancer: a randomized clinical trial. JAMA Surg 156:954–963
Kim YW, Reim D, Park JY, Eom BW, Kook MC, Ryu KW, Yoon HM (2016) Role of robot-assisted distal gastrectomy compared to laparoscopy-assisted distal gastrectomy in suprapancreatic nodal dissection for gastric cancer. Surg Endosc 30:1547–1552
Suda K, Nakauchi M, Inaba K, Ishida Y, Uyama I (2016) Robotic surgery for upper gastrointestinal cancer: current status and future perspectives. Dig Endosc 28:701–713
Ushiku H, Sakuraya M, Washio M, Hosoda K, Niihara M, Harada H, Miura H, Sato T, Nishizawa N, Tajima H, Kaizu T, Kato H, Sengoku N, Tanaka K, Naitoh T, Kumamoto Y, Sangai T, Yamashita K, Hiki N (2022) Pancreas-contactless gastrectomy for gastric cancer prevents postoperative inflammation. Surg Endosc 36:5644–5651
Acknowledgements
We would like to thank Editage for the English language editing.
Funding
This research received no specific grant from any funding agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Kengo Hayashi, Noriyuki Inaki, Yusuke Sakimura, Takahisa Yamaguchi, Yoshinao Obatake, Shiro Terai, Hirotaka Kitamura, Shinichi Kadoya, and Hiroyuki Bando have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hayashi, K., Inaki, N., Sakimura, Y. et al. Pancreatic thickness as a predictor of postoperative pancreatic fistula after laparoscopic or robotic gastrectomy. Surg Endosc 37, 5358–5367 (2023). https://doi.org/10.1007/s00464-023-10021-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10021-0