Abstract
Group living is ubiquitous in the animal kingdom and confers a number of benefits and costs. In nature, animal habitats are complex, diverse, and constantly changing. As one of the most important ecological factors, temperature can act directly on the physiology and behavior of ectotherms, and its effect might be related to the context. Here, we used crucian carp (Carassius auratus) as an animal model to investigate how the individual and collective behaviors of the fish respond to two different temperatures (15 °C vs. 25 °C) across three contexts (e.g., open water, food, and food + shelter). Compared to those at 25 °C, the fish at 15 °C had lower individual swimming speed, synchronization of speed, group speed, and longer time spent in the shelter with a lower foraging speed, but such effects of temperature were not found in terms of collective behavior (e.g., interindividual distance, nearest neighbor distance, distance to group center, or group polarization). The individual swimming speeds of the fish increased with increasing environmental complexity at both temperatures. The fish shoals had a higher foraging speed and better group coordination and cohesion in the food context than in the food + shelter context. In the food + shelter context, fish spent time on moving in and out the shelter under a pattern of high swimming speed. Consequently, groups are less efficient at foraging in food + shelter contexts than in food contexts at only 25 °C. Our results suggest that the effects of temperature on the individual and collective behavior of fish are dependent on context.
Significance statement
Establishing how collective behavior emerges is crucial to our understanding of animal societies. The collective behavior and structure of animal groups may change considerably depending on the context, which can alter collective behavior through adaptive changes in individuals’ behavior. Among the various environmental factors, temperature is an ‘ecological master factor’ that influences individuals’ physiology and behavior. Shoals of crucian carp exhibit distinct patterns of response to temperature between individual- and group-level behaviors across contexts. In the food + shelter context, fish at 15 °C spend more time hiding within the shelter, resulting in a lower foraging speed and a longer latency to forage with a smaller group size than those of fish at 25 °C. Our study provides new insights into the consequences of ambient temperature on the collective behavior of group-living animals in nature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Group living is widespread in animal taxa, and collective behavior is an emergent phenomenon arising from the local interactions of the members of animal groups. (Krause and Ruxton 2002; Delcourt and Poncin 2012; Herbert-Read et al. 2016; Schaerf et al. 2017). Living in groups can confer a number of ecological benefits, such as enhanced antipredator strategies and vigilance (Treherne and Foster 1981; Krause 1994; Fels et al. 1995), improved foraging speed (Creel and Creel 1995; Herbert-Read et al. 2017), increased reproductive success (Westneat et al. 2000; Bekkevold et al. 2002; Pilastro et al. 2003), reduced heat loss (Andrews and Belknap 1986), and decreased energetic cost of locomotion (Killen et al. 2012; Marras et al. 2015). Such benefits, however, could be partly offset by some costs of group living, including greater visibility to predators (Cresswell 1993; Seebacher and Krause 2017), increased competition for resources (Krause and Ruxton 2002; Pitcher et al. 1982; Webster and Hart 2006a), and increased risk of ectoparasite infection (Brown and Brown 1986; Van Vuren 1996; Han et al. 2015). This potential trade-off between the benefits and costs of group living is a dynamic process that is influenced by a number of ecological factors, such as individual physiology and cognition (Herbert-Read et al. 2011; Von Rueden et al. 2015; Seebacher and Krause 2017), aquatic temperature (Cooper et al. 2018), ecological context (Jolles et al. 2017; Rodriguez-Pinto et al. 2020; Yang et al. 2021; Li et al. 2022), and seasonal alternation (Chen et al. 2019).
Fish that rely on ambient heat regulate their body temperature to maintain their physiological functions at an optimum level. Changes in temperature can directly influence physiological processes related to energy metabolism (Zeng et al. 2010; Sandblom et al. 2014; Lefevre 2016), appetite (Chen et al. 2019), digestive capacity and growth (Legler et al. 2010; Zeng et al. 2018), and swimming performance (Claireaux et al. 2006; Zeng et al. 2009). Several researchers have indicated that temperature can increase hunger levels by increasing the metabolic rate of predators, which in turn affects their predation motivation (Allan et al. 2015; Domenici et al. 2019). Subjected to climate and seasonal changes, surface water in temperate zones can fluctuate widely by up to 20 °C diurnally (Temple and Johnston 1997; Zeng et al. 2009). Moreover, the behavior of fish exhibits rhythms in response to seasonal temperature changes in the water body (Häfker and Tessmar-Raible 2020). As one of the most crucial impacts in the aquatic environment, temperature changes have direct effects on the physiological and biochemical processes of fish. For example, the physiological functions (e.g., swimming ability) of fish increase with increasing temperature within the appropriate temperature range (Lee et al. 2003; Zeng et al. 2009; Peng et al. 2014; Pang et al. 2016). However, at a lower temperature, mitochondrial function is reduced due to a decrease in the metabolic capacity for muscle production (Randall and Brauner 1991; Guderley 2004; Day and Butler 2005), thereby reducing all aspects of fish organism function. Temperature also affects the water environment in which the fish live. When the temperature of a water body decreases, the viscosity of the water body also increases (Temple and Johnston 1997; Lee et al. 2003; Zeng et al. 2009). These changes in temperature are likely to cause modifications to individual functions and hence collective behavior (e.g., structure and function), which in turn will have a significant impact on the group's foraging performance. Therefore, the first goal of our study was to examine the effect of temperature on the individual and collective behavior of group-living fish.
In nature, animal habitats are complex and diverse and are usually compounded by various informational cues that span multiple sensory modalities (Heit et al. 2002; Dall et al. 2005). Habitats that fluctuate significantly in terms of food and shelter (Pitcher and Parrish 1993; Killen et al. 2016) can result in animals responding more flexibly to different contexts (Sih et al. 2011; Richter et al. 2012). Differences in context may lead to corresponding behavioral responses in animals (Herbert-Read et al. 2016; Seebacher and Krause 2017; Jolles et al. 2020). For example, hiding under a shelter or refuge can reduce the risk of predation and routine energy consumption by fish (Maximino et al. 2010; Matsuzaki et al. 2012). Moreover, the group structure and function of group-living animals change greatly according to the context in which they live. For instance, animal groups potentially behave more cohesively when under attack and are more dispersed when foraging (Hoare et al. 2004; Schaerf et al. 2017). While animal groups are more cohesive in situations where food sources are scarce or scattered, they are more dispersed in situations where food is plentiful (Lihoreau et al. 2017; Jolles et al. 2018). However, natural changes in water temperature may affect the foraging efficiency of fish groups in using resources across contexts. Previous studies have focused more on the effects of single contexts on fish collective behavior (Smith et al. 2009; Herbert-Read et al. 2016), while few studies have examined how animal groups function across contexts at given temperatures (Jolles et al. 2018; Hansen et al. 2020; Yang et al. 2021; Li et al. 2022). Thus, the second goal of our study was to test whether individual and collective behavior and their functions across contexts are temperature-dependent.
Crucian carp (Carassius auratus) is a freshwater fish that is widely distributed in rivers, lakes, and reservoirs in Eurasia. It is also a common freshwater economic fish in China and that displays schooling foraging habits. The common seasonal water temperature fluctuations in summer and winter in the upper region of the Yangtze River, China (Pang et al. 2014; Fu et al. 2018), were considered the acclimation temperatures, where 15 °C is the typical water temperature in winter and 25 °C is the typical water temperature in summer (Long et al. 2007). Here, we used crucian carp as an animal model to examine how individual and collective behaviors respond to different water temperatures and whether fish groups exhibit repeatable behavioral differences across contexts.
Materials and methods
Fish
Three hundred two-month-old crucian carp were obtained from a local fish farm in Yongchuan district, Chongqing, immediately transported to our laboratory at Chongqing Normal University, China, and kept in four cyclic temperature-controlled tanks (2 m length × 1 m width × 0.5 m height, water depth of 0.4 m), each containing 75 fish at 20 °C. The water temperature of the two tanks was heated at a rate of 2 °C per day to 25 °C using an aquarium thermoregulator at a starting temperature of 20.2 ± 0.1 °C, while the other two tanks were cooled to 15 °C at the same rate using a chiller (CA-1000A, RESUN, Guangzhou, China). After the target temperatures were reached, all the fish were acclimatized to the given temperature (15 and 25 °C) for at least four weeks. We placed plastic water plants at the bottom of each tank to increase the environmental enrichment. The fish were fed to satiation with mixed bloodworms to ensure that the fish could feed on the mixed bloodworms well during the collective behavioral trials. Before each feeding, both the air and water pumps were turned off to calm the water surface, and the fish were allowed to feed for one hour. After that, a siphon was used to remove residual food and feces within the tank. The dissolved oxygen level was kept above 7.0 mg/L with a 10 L: 14 D photoperiod.
Experimental overview
There were two acclimation temperatures (15 °C and 25 °C) for our study, with 150 fishes at each acclimation temperature and a total of 300 fishes. After acclimatization, 120 fish of similar size were selected at each temperature (Mean ± SE, 15 °C, body size 7.84 ± 0.22 cm; 25 °C, body size 7.73 ± 0.23 cm) and randomly formed into groups of six fish, with 20 groups at each temperature. Given the spatial constraints present in the holding compartment, each group consists of six fish, which conforms with the findings of previous studies in which the group size was controlled between 4 and 8 individuals (Jolles et al. 2017, 2018; Yang et al. 2021; Li et al. 2022; Cao et al. 2023). Each group of each temperature was tested for their collective behavior across three contexts for a total 10 of days. Each group of each temperature underwent a total of five test trials: one in the open water context (10 min), two in the food context (10 min), and two in the food + shelter context (10 min).
Collective behavior
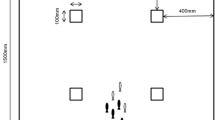
A white circular acrylic tank (80 cm in diameter × 20 cm wall height, Fig. 1) was used to test the collective behavior of the fish. The testing temperature was controlled to be the same as that used during the acclimatization period. To maintain the water temperature at the target value (15 °C and 25 °C), a thermal insulation cover was placed around the outside of the tank wall, which allowed the change in water temperature to be less than 0.2 °C before and after filming. Given the body height of the fish at their current developmental stage and to minimize the possibility of vertical overlap between different individuals swimming in a shoal, we kept the water in the tank at a depth of 6.0 cm. The bottom and inner walls of the tank were pasted with nontoxic white sticker to increase the difference in color between the fish and the tank. The tank was positioned inside a shelf (1.5 m length × 1.5 m width × 2.0 m height) illuminated from the top (test environment light at ~ 240 lx). A ring of green opaque cloth was draped around the shelf to minimize the potential influence of the external environment on the collective behavior of the fish. A high-resolution Sony camera (HDR-PJ820E, 25 frames per second, 1080p) was placed vertically above the tank to film the collective behavior of the fish. (1) In the open water context, no food or shelter was placed in the tank. (2) For the food context, three identical food patches (10 cm diameter × 1 cm height) with 9 grids each inside were placed in an equilateral triangular space at the bottom edge of the tank. Five frozen bloodworms were randomly placed in the nine grids of the food patches when the fish were acclimating within a plastic cylinder at the center of the tank. (3) In the food + shelter context, a shelter composed of five green plastic plants (e.g., these plants were also present in their acclimation waters) was placed in the center of the tank, creating a 20 × 20 cm concealed area. At the same time, five bloodworms were randomly placed in the 9 cells of the food patches when the fish were acclimating within the plastic cylinder.
Schematic of the tank in which the groups of fish were tested across three different contexts: (i) open water context, an environment without food or plant shelter; (ii) food context, an environment with three patches of food; and (iii) food + shelter context, an environment with food patches as well as plant shelter
We exposed each group of six fish at each temperature to a total of 5 tests (5 days in total), one in the open water context (day 1), two in the food context (days 2–3) and two in the food + shelter context (days 4–5). Similar to two previous studies (Jolles et al. 2017, 2018), we tested the groups in random order at each temperature but employed a fixed context order to avoid confounding the behavior of the fish in earlier contexts with experience acquired with the foraging patches and shelter. Before each trial, the fish were transferred from their holding compartments to a cylinder (12 cm in diameter) at the center of the tank without air exposure and allowed to acclimate for 5 min. Afterward, the fish were released by remotely raising the cylinder. At this moment, the collective behavior was filmed for the given recording time. After the trials, the fish were transferred back to their holding compartments, and five bloodworms were fed to each experimental fish to ensure that they maintained an appetite until the next trial. To avoid the influence of potential pheromones (e.g., feces, uneaten foods, and chemical alarm cues) from the previous fish group on the next group's behavioral expression, all the water in the tank was replaced with new aged tap water at the same water temperature. All trials were conducted between 8:30 am and 5:30 pm each day to minimize the potential effect of diurnal rhythms on the collective behavior of the fish. The room was maintained at a consistent and low sound level to reduce the impact of external sound sources on the groups' behavior.
Data collection and calculation
To minimize observer bias, blinded methods were use when all behavioral data were recorded and analyzed. After obtaining a video of the collective behavior, the video was converted to the AVI format using video converter software. The tracking software idTracker (version 1.10, https://www.idtracker.es/home) was used to analyze all the videos. The two-dimensional coordinate data (i.e., pixel values on the x- and y-axes) of six fish in the group per time (1/24 s in our study) in the visual range (i.e., the open area beyond the shelter) were obtained. We obtained the pixel-to-actual distance ratio by transforming the coordinate data with the actual size of the tank (i.e., 11.62 pixels/cm in our study), which was used to obtain a set of actual movement data (measured in cm) of the fish groups in the active field of the test. Then, we calculated the following parameters via the axial data: (1) individual swimming speed, (2) synchronization of speed, (3) interindividual distance (IID), (4) nearest neighbor speed (NND), (5) group polarization (P), (6) distance to group center, (7) group speed, and (8) group percentage time moving (PTM). The calculation of each parameter is as follows:
-
(1)
Individual swimming speed (cm/s): In our study, the individual swimming speeds of juvenile crucian carp were taken from the median speed of six fish in a group.
$$V\left(t\right)=\sqrt{{(x\left(t\right)-x\left(t-1\right))}^{2}+{(y\left(t\right)-y\left(t-1\right))}^{2}} /\Delta t$$(1)where V(t) is the individual swimming speed (cm/s); x, y(t) and x, y(t-1) denote the horizontal or vertical coordinate values of individual fish at moments t and t-1, respectively; and ∆t is the time interval between the two coordinate points (set at 1/24 s in our study).
-
(2)
Synchronization of speed (Sv): Sv is an assessment of the synchronization of individual swimming speeds that range between 0 and 1. The higher the value is, the greater the synchronization of individual swimming speeds.
$${S}_{v}=1-\left|\left({v}_{i}-{v}_{j}\right)/({v}_{i}+{v}_{j})\right|$$(2)where vi and vj are the individual swimming speeds of fish i and j in the shoal at an instantaneous time instant, respectively.
-
(3)
Interindividual distance (IID, cm): IID refers to the average interindividual distance between all individuals in a shoal to assess group cohesion. The lower the IID is, the more cohesive the group.
$${\text{IID}}\left(t\right)=\frac{1}{n}\sum\nolimits_{i\ne j}^{n}\sqrt{({x}_{i}\left(t\right)-{x}_{j}\left(t\right){)}^{2}+({y}_{i}\left(t\right)-{y}_{j}\left(t\right){)}^{2}}$$(3)where xi and yi are the horizontal and vertical coordinates, respectively, of fish i and j in the shoal at instant t.
-
(4)
Nearest neighbor distance (NND, cm): This measurement quantifies group cohesion and is the minimum distance (cm) of each fish among a matrix of distances between all individuals in a group.
$${\text{NND}}\left(t\right)={min}_{i\ne j}\sqrt{({x}_{i}\left(t\right)-{x}_{j}\left(t\right){)}^{2}+({y}_{i}\left(t\right)-{y}_{j}\left(t\right){)}^{2}}$$(4)where xi and yi are the values of the horizontal and vertical axes, respectively, of fish i and j in the fish group, and fish j indexes all neighbors of fish i at time t.
-
(5)
Group polarization (P, no unit): This measurement quantifies the degree of alignment of a group of fish when swimming and can be calculated as the magnitude of the mean movement vector of all individuals (Miller and Gerlai 2012; Gimeno et al. 2016). The group polarization ranges from 0 to 1. The polarization value is 1 when all the individuals moving in a group are perfectly aligned and 0 when all the individuals’ movement vectors completely cancel each other.
$$P\left(t\right)=\frac{1}{n}\left|{\sum }_{i=1}^{n}{v}_{i}(t)\right|$$(5)where vi (t) is the movement vector per unit time of an individual fish i, and the direction of movement is from the point of time t-1 to the location point of time t. n indicates the number of members of the group (e.g., n = 6 in our study).
-
(6)
Group center (Gx, Gy)
$${G}_{x, y}\left(t\right)=({x,y}_{1}\left(t\right)+{x,y}_{2}\left(t\right)+{x,y}_{3}\left(t\right)+{x,y}_{4}\left(t\right)+{x,y}_{5}\left(t\right)+{x,y}_{6}\left(t\right))/6$$(6)where G(t) represents the mean of the horizontal or vertical axes of the six fish in a group at time t.
-
(7)
Distance to the group center (cm)
$$D\left(t\right)=\sqrt{(x\left(t\right)-{G}_{X}\left(t\right){)}^{2}+(y\left(t\right)-{G}_{y}\left(t\right){)}^{2}}$$(7)where x(t), y(t) and Gx, Gy(t) are the values of the horizontal or vertical coordinates of the individual fish and the centroid of the group at time t, respectively.
-
(8)
Group speed (GV, cm/s)
$${G}_{v}\left(t\right)=\sqrt{({G}_{x}\left(t\right)-{G}_{x}\left(t-1\right){)}^{2}+({G}_{y}\left(t\right)-{G}_{y}(t-1){)}^{2}}/\Delta t$$(8)where Gx (t) and Gy (t) are the values of the horizontal and vertical coordinates of the group center at time t, and Gx (t-1) and Gy (t-1) are the values of the horizontal and vertical coordinates of the group center at time t-1, and ∆t is the interval between the two coordinates of the group center (e.g., 1/24 s in our study).
-
(9)
Group percentage time spent moving (PTM, %)
$${\text{PTM}}={T}_{{\text{moving}}}/{T}_{{\text{total}}}\times 100$$(9)where Tmoving is the total time that the group spent performing swimming and Ttotal is the total duration of the video capture (e.g., 600 s in our study). A fish group was considered to be moving when its instantaneous group speed was greater than 1.75 cm/s (Tang et al. 2017).
(10) Foraging speed (ind/min)
$$F=i/t$$(10)where i represents the number of bloodworms ingested by the shoal and t (min) is the total time taken to consume all 15 bloodworms.
Additionally, the following parameters were manually analyzed to evaluate the group dynamics in the context of food and shelter. (1) Number of times the fish group left the shelter (n): This refers to the number of times a group of juvenile crucian carp (consisting of more than four individuals) entered and exited the shelter, with each entry and exit counted as one occurrence. (2) Duration of stay in shelter (s): This refers to the total time that a group of fish, consisting of more than four individuals, spends inside shelters. (3) Group size (individuals): This represents the number of individuals in a group when they emerge from shelters.
Data analysis and statistics
Because 12 videos could not be analyzed by the idTracker software, these fish groups were excluded from the following statistical analysis of individual and group behavior. The obtained data were first analyzed with Excel (v.2021) to obtain the individual and collective behavioral parameters, as mentioned above. The statistical software package IBM SPSS Statistics (v.22.0) was subsequently used to perform the statistical analysis of these parameters. The software Origin (v.2021) was used to plot all the figures. All the data are expressed as the means ± SEs, and the significance level for all the tests was set as P < 0.05.
The data were first tested for normality and homogeneity of variance using the Kolmogorov‒Smirnov test. A linear mixed model (LMM) was used to examine the effects of temperature and context on the behavioral parameters. This model used temperature (15 °C and 25 °C) and context (open water, food and food + shelter) as fixed effects, behavioral parameters as the dependent variables, and group ID as a random effect. If differences were reported by this LMM, differences in individual and group behavioral parameters between the two temperatures within the same context were compared using independent sample t tests, and differences among contexts within the same temperature were compared using one-way ANOVA followed by Duncan’s test. If the data distribution did not conform to the normality analysis, a nonparametric test was used, with the Kruskal‒Wallis test used for between-group analysis and the Mann‒Whitney U test used for within-group analysis. The Mann‒Whitney U test was also used to test the differences in the number of times the fish group left the shelter, duration of stay in shelter, and group size between two temperatures.
We used Pearson’s correlation to examine the potential correlations between the three key components of collective motion. Finally, the repeatability of the approach for individual and collective behaviors at two temperatures across the three contexts was assessed using the intraclass correlation coefficient (ICC), which serves as a reliable measure of measurement or rating consistency. The ICC is computed as a ratio, where ICC = between-cluster variance/total variance (both within and between clusters), thus yielding a value ranging from 0 to 1 (Liljequist et al. 2019).
Results
We found that both temperature and context impacted the individual behavior of the fish (Fig. 2 and Table 1). Across the two temperatures, the fish acclimated at 25 °C moved faster, with individual swimming speeds higher at 25 °C than at 15 °C, irrespective of context (Fig. 2a). Similarly, individuals acclimated at 25 °C had a greater synchronization of speed than did those acclimated at 15 °C in both the open water context and the food context (Fig. 2b). In contrast, at the two temperatures, the fish had the lowest individual swimming speeds in the open water context compared with those in the food context and food + shelter context (Fig. 2a). At 15 °C, the fish swam faster and exhibited greater synchronization of speed in the food + shelter context than in the other two contexts (Fig. 2). However, the fish exhibited a lower synchronization of speed in the food + shelter context than in the other two contexts at 25 °C (Fig. 2b).
Effects of temperature and context on individual swimming speed and synchronization of speed in juvenile crucian carp. The boxes show the median, interquartile range, and whiskers (1.5 × the interquartile range). Boxes topped by the same lowercase letter (a, b, and c) do not differ significantly among the three contexts within a given temperature, while the pound sign (#) indicates a significant difference in parameters between the two temperatures within a given context
Apart from group polarization, temperature and context impacted the group speed and the group PTM of the fish (Fig. 3 and Table 1). The group speed and the group PTM were lower in the 15 °C treatment than in the 25 °C treatment (Fig. 3a, c). In different contexts, both the group speed and the group PTM increased from the open water context to the food + shelter context. The group speed and the group PTM were greater in the food + shelter context than in both the open water and food contexts. In addition, group polarization was modulated only by context and not by temperature (Fig. 3b and Table 1). The fish groups in the food context were arranged more compactly than were those in the other two contexts. No temperature effect on group cohesion was found in the three contexts (Fig. 4 and Table 1). However, among the three contexts, fish in the food context had the closest group structure, with the lowest values for IID, NND, and distance to the group center, compared to those in both the open and food + shelter contexts (Fig. 4 and Table 1).
Effects of temperature and context on collective behavior in juvenile crucian carp. The boxes show the median, interquartile range, and whiskers (1.5 × the interquartile range). Boxes topped by the same lowercase letter (a, b, and c) do not differ significantly among the three contexts within a given temperature, while the pound sign (#) indicates a significant difference in parameters between the two temperatures within a given context
Effects of temperature and context on group cohesion in juvenile crucian carp. The boxes show the median, interquartile range, and whiskers (1.5 × the interquartile range). Boxes topped by the same lowercase letter (a, b, and c) do not differ significantly among the three contexts within a given temperature, while the pound sign (#) indicates a significant difference in parameters between the two temperatures within a given context
Both the foraging speed and foraging latency were regulated by temperature and context (Fig. 5). In both the food and the food + shelter contexts, the foraging speed of the shoals was lower in the 15 °C treatment than in the 25 °C treatment (Fig. 5a). Furthermore, the fish had greater foraging speeds in the food context than in the food + shelter context at 25 °C, but such a contextual effect was not found at 15 °C (Fig. 5a). On average, in the food context, the fish first fed at 42 s and 48 s after the start of the experiment at 15 °C and 25 °C, respectively (Fig. 5b). In contrast, in the food + shelter context, the fish started to feed at 182 s and 102 s after the start of the experiment at 15 °C and 25 °C, respectively (Fig. 5b).
Group foraging performance of the two temperature groups across contexts. The boxes show the median, interquartile range, and whiskers (1.5 × the interquartile range). Boxes topped by the same lowercase letter differ significantly in group efficiency between the two contexts at 25 °C, while the pound sign (#) indicates a significant difference in group efficiency between the two temperatures within the food context
With the presence of the shelter in the tank, the fish tended to split into subgroups, which were dependent on temperature. Between 15 °C and 25 °C, there were differences in the sizes of the groups that left the shelter at any time, the frequency of swimming from the shelter, and the duration of stay in the shelter (Table 2). At 15 °C, due to the longer time (396 ± 29 s) fish spent in the shelter, the fish shoal left the shelter less frequently, and the average fish group size was 2.5 ± 0.2 individuals per outing. In contrast, the fish shoal spent less time (186 ± 20 s) in the shelter at 25 °C than at 15 °C, and the average fish group size was 5.1 ± 0.1 individuals per outing (Table 2). The variables related to individual and group behavior, except for the nearest neighbor distance, were found to be consistent across contexts at 15 °C; however, only individual swimming speed exhibited repeatability across contexts at 25 °C (Table 3).
Discussion
Our study aimed to investigate the effect of temperature on the individual and collective behavior of juvenile crucian carp in different contexts. By analyzing movement data from the fish groups, we found that fish at a higher temperature (25 °C) had greater spontaneous activity than did those at a lower temperature (15 °C), as evidenced by increased time in movement and swimming speed and increased feeding efficiency. Under colder conditions, fish tended to stay in shelters, reducing their foraging activity. Furthermore, compared with the open water context and food + shelter context, the effect of temperature on group structure was only evident in the food context, in which fish exposed to higher temperatures were more dispersed, while those at lower temperatures were more densely arranged. In the different contexts, the locomotor performance of fish groups increased with increasing habitat complexity. Compared with those in the other two contexts, the fish in the food context were the most aligned and arranged themselves more closely together. In contrast, shoals exposed to food + shelter were the most dispersed, and they usually took more time before starting their first group foraging. The presence of shelter in the environment made the fish groups more likely to split into subgroups, with larger group sizes occurring at 25 °C than at 15 °C.
Previous studies have shown that the effect of temperature on individual fish behavior is modulated by the behavioral strategies of the fish (Fu et al. 2018). The spontaneous activity of damselfish (Pomacentrus moluccensis) increases with increasing temperature (Biro et al. 2010). However, the locomotor activity of common carp (Cyprinus carpio) decreases with increasing temperature (Fu et al. 2012), while the spontaneous activity of Chinese bream (Parabramis pekinensis) does not change with increasing temperature (Peng et al. 2016). Such behavioral strategy choices may be modulated by the different physiological states of fish and the environment in which the individual is situated. In our study, compared to the fish at 15 °C, the fish at 25 °C had greater individual swimming speeds (e.g., spontaneous activity) and foraging speeds. Moreover, the fish groups spent more time hiding in the shelter at 15 °C than at 20 °C, suggesting that this behavioral strategy of hiding in shelters in crucian carp groups may be modified by temperature. At lower temperatures, physiological activity and metabolic functions are reduced, resulting in lower spontaneous activity (Claireaux et al. 2000; Joaquim et al. 2004; Zeng et al. 2009; Fu et al. 2018). Under such thermal conditions, the fish may have chosen to compensate for this functional decline by reducing movements and instead staying in the shelter.
Generally, the faster the group moves, the greater the synchronization of speeds, and the greater the IID and coherence of the group (Jolles et al. 2017; Schaerf et al. 2017; Wang et al. 2019; Li et al. 2022). In the foraging context, fish usually exhibit a faster foraging speed and may form looser shoals to reduce competition for food between group members (Hoare et al. 2004; Schaerf et al. 2017). An empirical study showed that the IID and group polarization of stickleback (Gasterosteus aculeatus) increase with increasing group speed (Jolles et al. 2017). In zebrafish (Danio rerio), the faster the group swims, the looser the group becomes (Miller and Gerlai 2012). Compared to those in an open water context, qingbo showed a significant increase in group speed but a decrease in group cohesion and group coordination in the presence of both food and shelter (Yang et al. 2021). In our study, fish in the open water context swam the slowest, those in the food context swam at moderate speeds, and those in the food + shelter context had the highest swimming speeds. Interestingly, the fish in the food context were the most cohesive and closely aligned, having the greatest group polarization and the smallest IID. The groups in the food + shelter context had the greatest group swimming speeds but were not as well aligned as the groups in the food context were. Moreover, although the groups in the food + shelter contexts had the greatest movement speeds, their synchronization of speed was not positively related to individual swimming speeds. There was no significant difference in the synchronization of speed (both 0.65 ± 0.02) between the two temperatures for the fish in the food + shelter context. This may be attributed to the presence of shelters allowing for increased environmental complexity, which may reduce anxiety in fish (Maillet et al. 2015). Environmental contexts might temporarily reduce heterogeneity in behavioral expressions; for example, foraging motivation may change in response to food availability or predation risk (Krause and Ruxton 2002; Jolles et al. 2019). A complex habitat provides a variety of ecological niches for animals and facilitates the coexistence of multiple behavioral strategies within a community (Xu 2020). Furthermore, the physical structure of shelters may also reduce visual contact between individuals but also increase barriers to movement; shelters provide more physical structures while increasing the cost of animal movement (Amat et al. 2018), and animals may be less likely to move in complex habitats (Skalski and Gilliam 2002). Additionally, the presence of more complex environments, as compared to a single biological context, introduces multiple physical dimensions in fish, thereby diverting attention to some extent (Heit et al. 2002; Dall et al. 2005). The presence of shelters increased the potential for fragmentation of intergroup behavioral strategies and led to differences in the behavioral tendencies of members of different groups. In our study, in contexts without shelter grouping is the primary option for safety and it also increases competition for food among group members. In the food + shelter context, however, some individuals that were less hungry tended to stay in the shelter, but others tended to go out for some motivational activities, such as searching for food, resulting in a longer latency to consume food compared to the food context. In this way, although the fish in the food + shelter context exhibited the fastest movement speed, the synchronization of swimming speed did not show a corresponding change, and the group coordination was lower than that in the food context.
Animal foraging behavior is regulated by various factors. It has been shown that under high temperature conditions (especially in hot summers), the activation of the GH/IGF-I axis increases metabolic demand for growth in fish (Gabillard et al. 2003) and can also regulate the expression of appetite centers in the Atlantic cod (Gadus morhua) and Chinese perch (Siniperca chuatsi) (Kehoe and Randall 2008; Song et al. 2017). Additionally, uncertainty about distribution of food patches or predators in the environment may also allow foraging behavior to change. Previous studies have indicated that during foraging, an animal's energy intake increases, but the risk of exposure to predators also increases (Andrews and Belknap 1986). When not foraging, animals can scan the environment for predators or rest in a shelter, thus reducing the risk of predation but decreasing energy intake (Higginson et al. 2012). The presence of shelters, in addition, enhances environmental complexity, potentially impeding communication between group members and consequently reducing their foraging speed (Tang and Schwarzkopf 2013). Consistent with the findings of previous studies (Hansen et al. 2015; Allan et al. 2015), our study found that the foraging speed of fish at 15 °C was lower than that at 25 °C in all three contexts. Compared with those in the 15 °C treatment, the fish in the 25 °C treatment may have had greater metabolic demands for basic energy expenditure, growth, and appetite, and they were more strongly motivated to forage at larger group sizes under the 25 °C treatment. Fish often delay feeding when shelters exist. The presence of shelters may not only provide shelter from potential risks but also reduce the animals' visibility to the habitats (Lima and Zollner 1996; Tang and Schwarzkopf 2013). In our study, fish first entered the shelter after being released into the tank to scan their surroundings or to rest in the shelter to balance foraging benefits and costs. The food context was relatively simple in terms of environmental cues, and the fish could quickly observe and assess the potential risks of their surroundings and find food to then make group decisions when compared to the food + shelter context. It is noteworthy that the juvenile crucian carp used in the present study were obtained from a local fish farm, implying a probable lack of exposure to really natural predators, and suggesting that their antipredatory behavior likely stems from innate evolutionary responses.
In conclusion, changes in temperature and context lead to differences in individual behavior of group members, which can further lead to changes in collective behavior and group functioning. Even at the same temperature, collective behaviors are modulated according to the different contexts in which they occur and are temperature-dependent. Consistent differences in collective behavior can occur even when the same group is faced with different contexts. In the food context where only a single stimulus cue (e.g., food) is present, the fish groups show the tightest and neatest structure. However, with the addition of shelter to the tank, the environmental complexity increased, and the behavioral tendencies of the group members changed. It becomes more difficult for fish groups to assess the potential risk of their surroundings over time, and fish may need to spend more time to trade off the benefits of foraging against these risks. Such adaptive variation in fish groups may impact colony survival and reproduction. Currently, in the face of global climate change and frequent human activities, animals may face multiple survival stresses and challenges. Whether animal groups develop adaptive variations in response to rapid environmental changes in their habitats as well as the associated behavioral responses deserves attention. Future studies should focus on the collective behavioral patterns of animals and explore the links between collective behavior, functional performance, mechanisms, and ecological processes in the face of global climate change.
Data availability
Our manuscript has data included as electronic supplementary material.
References
Allan BJM, Domenici P, Munday PL, McCormick MI (2015) Feeling the heat: the effect of acute temperature changes on predator–prey interactions in coral reef fish. Conserv Physiol 3:cov011. https://doi.org/10.1093/conphys/cov011
Amat I, Desouhant E, Gomes E, Moreau J, Monceau K (2018) Insect personality: What can we learn from metamorphosis. Curr Opin Insect Sci 27:46–51. https://doi.org/10.1016/j.cois.2018.02.014
Andrews RV, Belknap RW (1986) Bioenergetics benefits of huddling by deer mice (Peromyscus maniculatus). Comp Biochem Physiol A 85:775–778. https://doi.org/10.1016/0300-9629(86)90294-X
Bekkevold D, Hansen MM, Loeschcke V (2002) Male reproductive competition in spawning aggregations of cod (Gadus morhua, L.). Mol Ecol 11:91–102. https://doi.org/10.1046/j.0962-1083.2001.01424.x
Biro PA, Beckmann C, Stamps JA (2010) Small within-day increases in temperature affects boldness and alters personality in coral reef fish. Proc R Soc Lond B 277:71–77. https://doi.org/10.1098/rspb.2009.1346
Brown CR, Brown MB (1986) Ectoparatism as a cost of coloniality in cliff swallows (Hirundo phyyhonota). Ecology 67:1206–1218. https://doi.org/10.2307/1938676
Butler DN (2005) The effects of acclimation to reversed seasonal temperatures on the swimming performance of adult brown trout Salmo trutta. J Exp Biol 208:2683–2692. https://doi.org/10.1242/jeb.01669
Cao B, Luo H, Zeng LQ (2023) Effects of external cues and group mate body size on the collective behavior of shoaling crucian carp. Behav Process 208:104873. https://doi.org/10.1016/j.beproc.2023.104873
Chen T, Wong MKH, Chan BCB, Wong AOL (2019) Mechanisms for temperature modulation of feeding in goldfish and implications on seasonal changes in feeding behavior and food intake. Front Endocrinol 10:133. https://doi.org/10.3389/fendo.2019.00133
Claireaux G, Webber DM, Lagardère JP, Keer SR (2000) Influence of water temperature and oxygenation on the aerobic metabolic scope of Atlantic cod (Gadus morhua). J Sea Res 44:257–265. https://doi.org/10.1016/S1385-1101(00)00053-8
Claireaux G, Couturier C, Groison AL (2006) Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). J Exp Biol 209:3420–3428. https://doi.org/10.1242/jeb.02346
Cooper B, Adriaenssens B, Killen SS (2018) Individual variation in the compromise between social group membership and exposure to preferred temperatures. Proc R Soc B 285:20180884. https://doi.org/10.1098/rspb.2018.0884
Creel S, Creel NM (1995) Communal hunting and pack size in African wild dogs, Lycaon pictus. Anim Behav 50:1325–1339. https://doi.org/10.1016/0003-3472(95)80048-4
Cresswell W (1993) Escape responses by redshanks, Tringa totanus, on attack by avian predators. Anim Behav 46:609–611. https://doi.org/10.1006/anbe.1993.1231
Dall SRX, Giraldeau L, Olsson O, McNamara JM, Stephens DW (2005) Information and its use by animals in evolutionary ecology. Trends Ecol Evol 20:187–193. https://doi.org/10.1016/j.tree.2005.01.010
Delcourt J, Poncin P (2012) Shoals and schools: back to the heuristic definitions and quantitative references. Rev Fish Biol Fisher 22:595–619. https://doi.org/10.1007/s11160-012-9260-z
Domenici P, Allan BJM, Lefrançois C, McCormick MI (2019) The effect of climate change on the escape kinematics and performance of fishes: implications for future predator–prey interactions. Conserv Physiol 7:coz078. https://doi.org/10.1093/conphys/coz078
Fels D, Rhisiart AA, Vollrath F (1995) The selfish crouton. Behaviour 132:49–55. https://doi.org/10.1163/156853995X00270
Fu C, Cao ZD, Fu SJ (2012) The influence of temperature and starvation on resting metabolic rate and spontaneous activity in juvenile Cyprinus Carpio. Chinese J Zool 47:85–90
Fu C, Peng JL, Fu SJ (2018) Effects of acclimation temperature on locomotion performance and behavior of pale chub. Chinese J Ecol 37:1889–1896
Gabillard JC, Weil C, Rescan PY, Navarro I, Gutierrez J, Le Bail PY (2003) Environmental temperature increases plasma GH levels independently of nutritional status in rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 133:17–26. https://doi.org/10.1016/S0016-6480(03)00156-4
Gimeno E, Quera V, Beltran FS, Dolado R (2016) Differences in shoaling behavior in two species of freshwater fish (Danio rerio and Hyphessobrycon herbertaxelrodi). J Comp Psychol 130:358–368. https://doi.org/10.1037/com0000041
Guderley H (2004) Locomotor performance and muscle metabolic capacities: impact of temperature and energetic status. Comp Biochem Physiol B 139:371–382. https://doi.org/10.1016/j.cbpc.2004.04.001
Han BA, Park AW, Jolles AE, Altizer S (2015) Infectious disease transmission and behavioural allometry in wild mammals. J Anim Ecol 84:637–646. https://doi.org/10.1111/1365-2656.12336
Hansen MJ, Schaerf TM, Ward AJ (2015) The effect of hunger on the exploratory behaviour of shoals of mosquitofish Gambusia holbrooki. Behaviour 152:1659–1677
Hansen MJ, Ligocki IY, Zillig KE, Stell AE, Todgham AE, Fangue NA (2020) Risk-taking and locomotion in foraging threespine sticklebacks (Gasterosteus aculeatus): the effect of nutritional stress is dependent on social context. Behav Ecol Sociobiol 74:12. https://doi.org/10.1007/s00265-019-2795-4
Heit B, Tavener SA, Raharjo E, Kubes P (2002) An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol 159:91–102. https://doi.org/10.1083/jcb.200202114
Herbert-Read JE, Perna A, Mann RP, Schaerf TM, Sumpter DJ, Ward AJ (2011) Inferring the rules of interaction of shoaling fish. P Natl Acad Sci USA 108:18726–18731. https://doi.org/10.1073/pnas.1109355108
Herbert-Read JE, Romanczuk P, Krause S et al (2016) Proto-cooperation: group hunting sailfish improve hunting success by alternating attacks on grouping prey. Proc R Soc B 283:20161671. https://doi.org/10.1098/rspb.2016.1671
Herbert-Read JE, Rosén E, Szorkovszky A, Ioannou CC, Rogell B, Perna A, Sumpter DJT (2017) How predation shapes the social interaction rules of shoaling fish. Proc R Soc B 284:20171126. https://doi.org/10.1098/rspb.2017.1126
Higginson AD, Fawcett TW, Trimmer PC, McNamara JM, Houston AI (2012) Generalized optimal risk allocation: foraging and antipredator behavior in a fluctuating environment. Am Nat 180:589–603. https://doi.org/10.1086/667885
Hoare DJ, Couzin ID, Godin J-GJ, Krause J (2004) Context-dependent group size choice in fish. Anim Behav 67:155–164. https://doi.org/10.1016/j.anbehav.2003.04.004
Häfker NS, Kristin Tessmar-Raible K (2020) Rhythms of behavior: are the times changin’? Curr Opin Neurobiol 60:55–65. https://doi.org/10.1016/j.conb.2019.10.005
Joaquim N, Wagner GN, Gamperl AK (2004) Cardiac function and critical swimming speed of the winter flounder (Pleuronectes americanus) at two temperatures. Comp Biochem Physiol A 138:277–285. https://doi.org/10.1016/j.cbpb.2004.03.016
Jolles JW, Boogert NJ, Sridhar VH, Couzin ID, Manica A (2017) Consistent individual differences drive collective behavior and group functioning of schooling fish. Curr Biol 27:2862-2868.e7. https://doi.org/10.1016/j.cub.2017.08.004
Jolles JW, Briggs H, Araya-Ajoy Y, Manica A, Boogert NJ (2019) Personality, plasticity and predictability in sticklebacks: bold fish are less plastic and more predictable than shy fish. Anim Behavi 154:193–202. https://doi.org/10.1016/j.anbehav.2019.06.022
Jolles JW, Laskowski KL, Boogert NJ, Manica A (2018) Repeatable group differences in the collective behaviour of stickleback shoals across ecological contexts. Proc R Soc B 285:20180884. https://doi.org/10.1098/rspb.2017.2629
Jolles JW, King AJ, Killen SS (2020) The role of individual heterogeneity in collective animal behaviour. Trends Ecol Evol 35:278–291. https://doi.org/10.1016/j.tree.2019.11.001
Kehoe AS, Randall H (2008) The effects of temperature on feeding and expression of two appetite-related factors, neuropeptide Y and cocaine- and amphetamine regulated transcript, in Atlantic cod, Gadus morhua. J World Aquacult Soc 39:790–796. https://doi.org/10.1111/j.1749-7345.2008.00215.x
Killen SS, Marras S, Steffensen JF, McKenzie DJ (2012) Aerobic capacity influences the spatial position of individuals within fish schools. Proc R Soc B 279:357–364. https://doi.org/10.1098/rspb.2011.1006
Killen SS, Fu C, WuQ WYX, Fu SJ (2016) The relationship between metabolic rate and sociability is altered by food deprivation. Funct Ecol 30:1358–1365. https://doi.org/10.1111/1365-2435.12634
Krause J (1994) The influence of food competition and predation risk on size-assortative shoaling in juvenile chub (Leuciscus cephalus). Ethology 96:105–116. https://doi.org/10.1111/j.1439-0310.1994.tb00886.x
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, Oxford, UK
Lee CG, Farrell AP, Lotto A, MacNutt MJ, Hinch SG, Healey MC (2003) The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J Exp Biol 206:3239–3251. https://doi.org/10.1242/jeb.00547
Lefevre S (2016) Are global warming and ocean acidification conspiring against marine ectotherms? A meta-analysis of the respiratory effects of elevated temperature, high CO2 and their interaction. Conserv Physiol 4:cow009. https://doi.org/10.1093/conphys/cow009
Legler ND, Johnson TB, Heath DD, Ludsin SA (2010) Water temperature and prey size effects on the rate of digestion of larval and early juvenile fish. Trans Am Fisher Soc 139:868–875. https://doi.org/10.1577/T09-212.1
Li WX, Ma HH, Yu CX, Liu S, Lei JM, Zeng LQ (2022) Effects of water temperature rising and ecological context on collective behaviour of gibel carp. Acta Ecol Sin 42:9359–9370
Lihoreau M, Charleston MA, Senior AM, Clissold FJ, Raubenheimer D, Simpson SJ, Buhl J (2017) Collective foraging in spatially complex nutritional environments. Phil Trans R Soc B 372:20160238. https://doi.org/10.1098/rstb.2016.0238
Liljequist D, Elfving B, Skavberg Roaldsen K (2019) Intraclass correlation–A discussion and demonstration of basic features. PLoS ONE 14:e0219854. https://doi.org/10.1371/journal.pone.0219854
Lima SL, Bednekoff PA (1999) Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am Nat 153:649–659. https://doi.org/10.1086/303202
Lima SL, Zollner PA (1996) Anti-predatory vigilance and limits to collective detection of predatory attack: spatial and visual separation between foragers. Behav Ecol Sociobiol 38:355–363. https://doi.org/10.1007/s002650050252
Long TY, Zheng M, Guo WH, Hou YQ (2007) Applying the ecological amplitude to nutrition restrictive factors of Jialing River in the city zone of Chongqing. J Chongqing Univer 30:81–86
Maillet Z, Halliday WD, Blouin-Demers G (2015) Exploratory and defensive behaviours change with sex and body size in eastern garter snakes (Thamnophis sirtalis). J Ethol 33:47–54. https://doi.org/10.1007/s10164-014-0416-2
Marras S, Killen S, Lindström J, McKenzie D, Steffensen J, Domenici P (2015) Fish swimming in schools save energy regardless of their spatial position. Behav Ecol Sociobiol 69:219–226. https://doi.org/10.1007/s00265-014-1834-4
Matsuzaki SIS, Sakamoto M, Kawabe K, Takamura N (2012) A laboratory study of the effects of shelter availability and invasive crayfish on the growth of native stream fish. Freshw Biol 57:874–882. https://doi.org/10.1111/j.1365-2427.2012.02743.x
Maximino C, de Brito TM, Colmanetti R, Pontes AAA, De Castro HM, De Lacerda RI, Morato S, Gouveia AJ (2010) Parametric analyses of anxiety in zebrafish scototaxis. Behav Brain Res 210:1–7. https://doi.org/10.1016/j.bbr.2010.01.031
Miller N, Gerlai R (2012) From schooling to shoaling: patterns of collective motion in zebrafish (Danio rerio). PLoS ONE 7:e48865. https://doi.org/10.1371/journal.pone.0048865
Pang X, Yuan XZ, Cao ZD, Fu SJ (2014) The effects of fasting on swimming performance in juvenile qingbo (Spinibarbus sinensis) at two temperatures. J Therm Biol 42:25–32. https://doi.org/10.1016/j.jtherbio.2014.02.014
Pang X, Fu SJ, Zhang YG (2016) Acclimation temperature alters the relationship between growth and swimming performance among juvenile common carp (Cyprinus carpio). Comp Biochem Physiol 199:111–119. https://doi.org/10.1016/j.cbpa.2016.06.011
Peng J, Cao ZD, Fu SJ (2014) The effects of constant and diel-fluctuating temperature acclimation on the thermal tolerance, swimming capacity, specific dynamic action and growth performance of juvenile Chinese bream. Comp Biochem Physiol A 176:32–40. https://doi.org/10.1016/j.cbpa.2014.07.005
Peng J, Zeng LQ, Cao ZD, Fu SJ (2016) The effects of constant and diel-fluctuating temperature acclimation on the spontaneous activity in juvenile Parabramis pekinensis. J Chongqing Norm Univer Nat Sci 33:27–30
Pilastro A, Benetton S, Bisazza A (2003) Female aggregation and male competition reduce costs of sexual harassment in the mosquitofish Gambusia holbrooki. Anim Behav 65:1161–1167. https://doi.org/10.1006/anbe.2003.2118
Pitcher TJ, Parrish JK (1993) Functions of shoaling behaviour in teleosts. In: Pitcher TJ (ed) Behaviour of teleost fishes. Chapman and Hall, London, UK, pp 363–439
Pitcher TJ, Magurran AE, Winfield IJ (1982) Fish in large shoals find food faster. Behav Ecol Sociobiol 10:149–151. https://doi.org/10.1007/BF00300175
Randall D, Brauner C (1991) Effects of environmental factors on exercise in fish. J Exp Biol 160:113–126. https://doi.org/10.1242/jeb.160.1.113
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. https://doi.org/10.1111/j.1469-185X.2007.00010.x
Richter S, Kipfer T, Wohlgemuth T, Calderón Guerrero C, Ghazoul J, Moser B (2012) Phenotypic plasticity facilitates resistance to climate change in a highly variable environment. Oecologia 169:269–279. https://doi.org/10.1007/s00442-011-2191-x
Rodriguez-Pinto II, Rieucau G, Handegard NO, Boswell KM (2020) Environmental context elicits behavioural modification of collective state in schooling fish. Anim Behav 165:107–116. https://doi.org/10.1016/j.anbehav.2020.05.002
Sandblom E, Gräns A, Axelsson M, Seth H (2014) Temperature acclimation rate of aerobic scope and feeding metabolism in fishes: implications in a thermally extreme future. Proc R Soc B 281:20141490. https://doi.org/10.1098/rspb.2014.1490
Schaerf TM, Dillingham PW, Ward AJW (2017) The effects of external cues on individual and collective behavior of shoaling fish. Sci Adv 3:e1603201. https://doi.org/10.1126/sciadv.1603201
Seebacher F, Krause J (2017) Physiological mechanisms underlying animal social behaviour. Phil Trans R Soc B 372:20160231. https://doi.org/10.1098/rstb.2016.0231
Sih A, Ferrari MCO, Harris DJ (2011) Evolution and behavioural responses to human-induced rapid environmental change. Evol Appl 4:367–387. https://doi.org/10.1111/j.1752-4571.2010.00166.x
Skalski GT, Gilliam JF (2002) Feeding under predation hazard: testing models of adaptive behavior with stream fish. Am Nat 160:158–172. https://doi.org/10.1086/341012
Smith K, Wiegmann D, Newman S, Miner J (2009) Individual differences in exploratory and antipredator behaviour in juvenile smallmouth bass (Micropterus dolomieu). Behaviour 146:283–294
Song Y, Zhao C, Liang XF, He S, Tian C, Cheng XY, Lv LL, Guo WJ, Tao YX (2017) Effects of fasting, temperature, and photoperiod on preproghrelin mRNA expression in Chinese perch. Fish Physiol Biochem 43:803–812. https://doi.org/10.1007/s10695-016-0335-y
Tang L, Schwarzkopf L (2013) Foraging behaviour of the Peaceful Dove (Geopelia striata) in relation to predation risk: group size and predator cues in a natural environment. Emu-Austral Ornithol 113:1–7. https://doi.org/10.1071/MU12023
Tang ZH, Wu H, Huang Q, Kuang L, Fu SJ (2017) The shoaling behavior of two cyprinid species in conspecific and heterospecific groups. Peer J 5:e3397. https://doi.org/10.7717/peerj.3397
Temple GK, Johnston IA (1997) The thermal dependence of fast-start performance in fish. J Therm Biol 22:391–401. https://doi.org/10.1016/S0306-4565(97)00058-2
Treherne JE, Foster WA (1981) Group transmission of predator avoidance in a marine insect: the Trafalgar effect. Anim Behav 29:911–917. https://doi.org/10.1016/S0003-3472(81)80028-0
Van Vuren D (1996) Ectoparasites, fitness, and social behaviour of yellow-bellied marmots. Ethology 102:686–694. https://doi.org/10.1111/j.1439-0310.1996.tb01159.x
Volkoff H, Hoskins LJ, Tuziak SM (2010) Influence of intrinsic signals and environmental cues on the endocrine control of feeding in fish: Potential application in aquaculture. Gen Comp Endocrinol 167:352–359. https://doi.org/10.1016/j.ygcen.2009.09.001
Von Rueden C, Gavrilets S, Glowacki L (2015) Solving the puzzle of collective action through inter-individual differences. Phil Trans R Soc B 370:20150002. https://doi.org/10.1098/rstb.2015.0002
Wang L, Tang JY, Qin YL, Zeng LQ, Peng JL, Fu SJ (2019) Effect of starvation on energy metabolism, fish behavior, and schooling behavior of Spinibarbus sinensis. Acta Ecol Sin 39:1095–1104
Webster MM, Hart PJ (2006a) Kleptoparasitic prey competition in shoaling fish: effects of familiarity and prey distribution. Behav Ecol 17:959–964. https://doi.org/10.1093/beheco/arl037
Webster MM, Hart PJ (2006b) Subhabitat selection by foraging threespine stickleback (Gasterosteus aculeatus): previous experience and social conformity. Behav Ecol Sociobiol 60:77–86. https://doi.org/10.1007/s00265-005-0143-3
Westneat DF, Walters A, McCarthy TM, Hatch MI, Hein WK (2000) Alternative mechanisms of nonindependent mate choice. Anim Behav 59:467–476. https://doi.org/10.1006/anbe.1999.1341
Xu WJ (2020) Environmental complexity during early life stage affects mosquitofish personality. MSc Thesis, Anhui University, China
Yang Y, Ling H, Fu SJ, Zeng LQ (2021) Effects of ecological context and metabolic phenotype on collective behaviour of qingbo Spinibarbus sinensis. Acta Ecol Sin 41:4447–4459
Zeng LQ, Cao ZD, Fu SJ, Peng JL, Wang YX (2009) Effect of temperature on swimming performance in juvenile southern catfish (Silurus meridionalis). Comp Biochem Physiol A 153:125–130. https://doi.org/10.1016/j.cbpa.2009.01.013
Zeng LQ, Zhang YG, Cao ZD, Fu SJ (2010) Effect of temperature on excess post-exercise oxygen consumption in juvenile southern catfish (Silurus meridionalis Chen) following exhaustive exercise. Fish Physiol Biochem 36:1243–1252. https://doi.org/10.1007/s10695-010-9404-9
Zeng LQ, Fu C, Fu SJ (2018) The effects of temperature and food availability on growth, flexibility in metabolic rates and their relationships in juvenile common carp. Comp Biochem Physiol A 217:26–34. https://doi.org/10.1016/j.cbpa.2017.12.011
Acknowledgements
We thank Chunhua Wang and Jinqiu Bi for their help with the experiment. We also thank two anonymous reviewers for their valuable comments on the previous draft of this manuscript. Our study was supported by the Project of the Natural Science Foundation of Chongqing (cstc2021jcyj-msxm0498) and grants from the Science and Technology Research Program of Chongqing Municipal Education Commission (KJQN201900540, KJQN202000539) to L-QZ.
Author information
Authors and Affiliations
Contributions
HL, BC, and L-QZ conceived the idea and designed the study. HL and BC carried out the experiments and analyzed the data. HL and L-QZ led the writing of the manuscript. All authors commented on previous versions of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
In our study, data were collected from juvenile crucian carp (C. auratus). All animal handling and experiments were conducted in strict accordance with both the ethical requirements and the recommendations for animal care of the Key Laboratory of Animal Biology of Chongqing, China (permit number: FU2021092302); the requirements for environmental and housing facilities for laboratory animals in China (GB/T14925-2001); the state measures for the quality control of experimental animals in China; and regulations on the control of experimental animals in China. Additionally, all the experiments also complied with the local animal welfare laws (i.e., the measures of Chongqing municipality for the administration of experimental animals) of Chongqing city, China.
Additional information
Communicated by J. G. Frommen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, H., Cao, B. & Zeng, LQ. The effect of temperature on the collective behavior of crucian carp (Carassius auratus) is related to context. Behav Ecol Sociobiol 78, 55 (2024). https://doi.org/10.1007/s00265-024-03473-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03473-4