Abstract
Two classes of explanations for covariation between activity level and metabolic rate among conspecifics have been proposed. First, individual-level variation in activity exhibited during the measurement of metabolic rate should covary with routine metabolic rate because movement increases respiration (a methodological relationship). Second, energetic-based hypotheses posit relationships between individual activity measured under more natural conditions and baseline measures of metabolic rate, among other behavioral, physiological, and life-history traits (functional relationships). Here, we examined these potential relationships between individual behavior and metabolic rate in the mud crab (Panopeus herbstii). Specifically, we tested for (1) an effect of crab activity in metabolic chambers (activitychamber) on routine metabolic rate (RMR), and (2) an effect of crab activity in mesocosms that mimicked field conditions (activitymesocosm) on standard metabolic rate (SMR). To test for context dependence, we assessed both activity-metabolic rate relationships in the absence and presence of predation threat from toadfish (Opsanus tau) in the form of waterborne chemical cues. Individual variation in activitymesocosm and RMR was repeatable over time. In support of a methodological relationship, individual differences in RMR were partially explained by crab activitychamber. After accounting for this methodological relationship, individual SMR was inversely related to activitymesocosm, supporting an allocation model that predicts behavior and baseline metabolic rate compete for finite energy reserves. We found no evidence of context dependence in either activity-metabolic rate relationship. Thus, our study emphasizes the importance of considering methodological artifacts in elucidating functional relationships between individual behavior and energetics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Individual-level phenotypic variation in behavioral and energetic traits is ubiquitous in animal populations (Careau and Garland 2012). For example, behavioral traits such as activity level, boldness, and aggressiveness can vary substantially between conspecifics, and this variation is often consistent over time (referred to as behavioral types; Sih et al. 2004; Bell et al. 2009). Furthermore, the behavioral types of individuals are sometimes consistent across disparate ecological contexts, and variation in multiple behavioral traits (e.g., boldness and aggression) correlated at the individual level (collectively referred to as behavioral syndromes; Sih et al. 2004; Bell and Sih 2007). Such consistent individual differences (CIDs) in behavior are taxonomically widespread (Gosling 2001), and the causes and consequences of CIDs in behavior have become a burgeoning area of research (Sih et al. 2004, 2012).
Similarly, measures of baseline metabolic rate that represent the “idling” cost of metabolism, including basal, standard, and resting metabolic rate, can vary threefold among conspecifics of the same size, age, and sex (Speakman et al. 2004; Careau et al. 2008; Burton et al. 2011). Such residual variation in baseline metabolic rate had been considered noise around populations’ or species’ means and treated analytically as measurement error (Careau et al. 2008), but researchers have since demonstrated that individual differences in baseline metabolic rate can persist over the lifetime of individuals (Nespolo and Franco 2007; Burton et al. 2011; White et al. 2013). Current work illuminates the ecological and evolutionary consequences of CIDs in behavioral (Sih et al. 2012) and energetic traits (Burton et al. 2011), although the maintenance of variation in these traits within populations remains unresolved (Careau and Garland 2012).
Links between CIDs in behavior and energetics could shed light on the maintenance of individual-level variation in these traits (Careau et al. 2008; Biro and Stamps 2010; Careau and Garland 2012), although empiricists must first contend with potential methodological (or artifactual) relationships (Careau et al. 2008). Measurements of baseline metabolic rate (e.g., O2 consumption via respirometry) assume that animals are at rest within metabolic chambers designed to restrict movement, yet individual animals often vary in the amount of activity or stress exhibited during the measurement of metabolic rate. Active individuals are expected to consume more O2 than resting individuals, and so measurements from excessively active individuals are often discarded and metabolic rate re-measured at another time (Careau et al. 2008). Nevertheless, individual differences in behavior expressed during the measurement of metabolic rate are potentially repeatable (i.e., a behavioral type) and correlated with other behaviors (i.e., a behavioral syndrome). Therefore, removal of excessively active individuals can bias the population sample. In contrast to baseline measures of metabolic rate, routine metabolic rate includes metabolic rate measurements when individuals are both inactive and spontaneously active (Careau et al. 2014b). Therefore, individual activity expressed during metabolic rate measurement should covary with routine metabolic rate due to a methodological relationship (Dall 1986; Careau et al. 2008).
Functional links between CIDs in behavioral and energetic traits have also been hypothesized (Careau et al. 2008; Biro and Stamps 2010; Careau and Garland 2012). First, the increased-intake model posits that individuals with an intrinsically higher rate of metabolism should exhibit behaviors to support greater energetic demands (e.g., frequent or intense foraging activity), driving a positive relationship between baseline metabolic rate and behavioral traits related to energy acquisition (Careau and Garland 2012). This relationship is expected in part due to the finding that conspecific individuals sometimes differ in the relative size of metabolically active organs (e.g., intestine, liver, and heart), with organ size explaining a substantial portion of individual-level variation in baseline metabolic rate (Biro and Stamps 2010). The positive link between individual behavior and energetics (i.e., the increased-intake model) has received the most empirical attention, in part because it is potentially part of a broader covariation of behavioral, physiological, and life-history traits referred to as the “pace-of-life” syndrome (Biro and Stamps 2008; Réale et al. 2010). The pace-of-life syndrome hypothesis predicts that conspecifics range from a reactive to a proactive pace of life; reactive individuals are characterized by lower activity, boldness, and aggression, and this lifestyle is associated with reduced energy expenditure, growth, and fecundity. In contrast, proactive individuals are characterized by the opposite traits and pace of life. Covariation of these traits could be maintained by common hormonal control (e.g., testosterone: Buchanan et al. 2001) or correlational selection (Galliard et al. 2013).
Second, an allocation or compensation model predicts that behavior and baseline metabolic rate compete for finite energy reserves, driving a negative relationship between these traits at the individual level. This relationship is particularly expected during periods of food resource limitation (Careau et al. 2008; Careau and Garland 2012). Studies of birds (Deerenberg et al. 1998; Wikelski et al. 1999) and rodents (Wiersma and Verhulst 2005) provide support for an allocation model, although these studies generally induce activity by altering the animal’s environment (e.g., by increasing workload or testosterone). Activity also varies naturally among conspecifics as a behavioral type (Careau et al. 2008), and therefore the utility of the allocation model in explaining relationships between CIDs in behavior (e.g., behavioral types) and energetics requires further attention.
Support for functional relationships between CIDs in behavioral and energetic traits at the individual level has been mixed (Bouwhuis et al. 2014), although adequate tests are few (Careau and Garland 2012). In a recent review, Killen et al. (2013) suggested the importance of environmental stressors (i.e., abiotic and biotic factors that “challenge” individuals to adjust their behavior or physiology in order to cope) in mediating behavior-energetic relationships, and potentially explaining this mixed support. Environmental stressors can modify the relationship between CIDs in behavior and energetics by modifying the distribution of one or both of these types of traits, and having differential effects on the traits of certain phenotypes (i.e., different individual reaction norms). Studying the context dependence of energetic-behavior relationships is important because it can yield insight into the mechanisms behind relationships, as well as the effects of environmental change on these relationships (Burton et al. 2011; Killen et al. 2013).
In the present study, we tested for relationships between individual behavior and metabolic rate in a geographically widespread and abundant marine invertebrate, the common mud crab (Panopeus herbstii). We measured individual crab behavior both in metabolic rate chambers (activitychamber) and in mesocosms (activitymesocosm) that recreated the crab’s natural oyster (Crassostrea virginica) reef habitat. In addition, we measured both routine metabolic rate (RMR) and standard (i.e., baseline) metabolic rate (SMR) of these same crabs. This allowed us to test for methodological and functional activity-metabolic rate relationships. We further examined how activity-metabolic rate relationships were affected by the presence of threat from a major predator of mud crabs, the toadfish (Opsanus tau). Previous work in this study system demonstrated that toadfish predation threat reduces crab activitymesocosm (Griffen et al. 2012; Toscano et al. 2014), but individual crabs differ in the magnitude of their response to threat (Griffen et al. 2012). Therefore, a functional relationship between activitymesocosm and SMR in P. herbstsii could be altered in the presence of predation threat (Killen et al. 2013). Predation is a powerful agent of selection (Svanbäck and Eklöv 2011; Siepielski et al. 2014), and the threat of predation in the form of visual or chemical cues is a widespread biotic stressor (Lima and Dill 1990). Nevertheless, the effects of predation threat on the relationship between CIDs in behavioral and energetic traits are unclear (Killen et al. 2013).
Methods
We first tested for CIDs in crab activitymesocosm and RMR by measuring the repeatability (i.e., proportion of phenotypic variation due to between-individual variation) of these traits. We then tested for a methodological relationship between crab activitychamber and RMR, as well as a functional relationship between crab activitymesocosm and SMR. We tested for both methodological and functional relationships in the absence and presence of predation threat from toadfish (O. tau). In doing so, we were also able to examine the responses of behavioral and energetic traits to predation threat, in order to investigate mechanisms behind potential context dependence. Experiments were conducted from May through August 2013 in the wet laboratory at the Baruch Marine Field Laboratory (Georgetown, SC, USA), which is adjacent to North Inlet estuary (33° 20′ N, 79° 10′ W). All animals used in the study were collected by hand from the highest intertidal portion of oyster reefs in North Inlet.
Repeatability of activitymesocosm and RMR
Repeatability of activitymesocosm and RMR was measured for separate groups of crabs (n = 38 crabs for activitymesocosm, n = 24 crabs for RMR) from the same size range (mean carapace width [CW] ± 1 SE, 29.03 ± 0.24 and 28.53 ± 0.28 mm, respectively). We exclusively used male crabs to remove the potential influence of sex on both activitymesocosm (Toscano et al. 2014) and metabolic rate (Niewiarowski and Waldschmidt 1992). To determine the repeatability of activitymesocosm and RMR, these traits were measured twice per crab with 48 h between measurements. All crabs were fed with hard clams (Mercenaria mercenaria) and starved for 24 h before the measurement of both activitymesocosm and RMR to standardize hunger levels.
Individual crab activitymesocosm was measured following similar methods to those used in previous studies of P. herbstii individual behavior (Griffen et al. 2012; Toscano et al. 2014; Toscano and Griffen 2014). Each crab was housed in a plastic mesocosm (43 cm length × 31 cm width × 18 cm height) during the measurement of activitymesocosm. Mesocosms were set up to mimic the physical structure of a natural oyster reef; each mesocosm received sand substrate (1 cm deep) and a matrix of loose oyster shell (6 cm deep) that had been dried and cleaned to remove epifauna. Activitymesocosm was measured at night from approximately 2000 to 2300 h, and mesocosms received flow-through, unfiltered seawater throughout the measurement of activitymesocosm. The activitymesocosm of crabs was observed under red light and behind a blind to minimize disturbance to crabs (Griffen et al. 2012; Toscano et al. 2014).
To measure activitymesocosm, we first placed crabs in mesocosms and allowed them to acclimate for 15 min. After this acclimation period, the behavior of crabs was observed once every 9 min over 3 h (20 observations in total per crab). During each observation, we recorded whether crabs were active and moving (usually on top of the oyster shell matrix) or inactive (usually within the matrix). Crab activitymesocosm was calculated as the proportion of observations where crabs were observed active out of 20 total observations.
We quantified the repeatability of P. herbstii RMR to examine CIDs in the energetic requirements of crabs. Prior to all measurements, crabs were individually housed in small tackle box compartments (3 cm length × 3 cm width) to prevent excessive activity. We measured RMR at night (generally from 2100 to 2400 h) and under red light to minimize disturbance to crabs. RMR was measured in a climate-controlled room to maintain water temperatures between 20 and 22 °C throughout the duration of measurements.
We began trials by placing individual crabs in 0.7-L acrylic chambers (14 cm length × 7 cm width × 7 cm height) filled with 1-μm filtered, air-bubbled seawater (100 % O2 saturation). Each container received a magnetic stir bar to ensure mixing. Chambers were then sealed and a Clark-type dissolved oxygen sensor (HANNA, model HI-9146; HANNA instruments, USA) was inserted. We recorded the O2 concentration in chambers every 10 min for 60 min. Preliminary trials revealed that crabs normalized their O2 consumption rate after less than 10 min in the chamber; therefore, only data collected after 10 min was used in statistical analyses. Trials were terminated early if oxygen concentration dropped below 70 % of O2 saturation level. After each trial, we measured the water volume in chambers with a graduated cylinder. We also measured the O2 concentration in two crab-free chambers to control for background changes in dissolved O2. We calculated individual O2 consumption from the average of the five 10-min interval measurements. We standardized all metabolic rate measurements in our study by crab dry weight, although doing so did not qualitatively alter our results. Crabs were dried for 48 h at 70 °C after experiments to obtain dry weight.
We analyzed the repeatability of activitymesocosm and RMR using the methods of Nakagawa and Schlereth (2010) and the associated rptR package in the statistical software R (R Core Team 2012). Repeatability is the proportion of total phenotypic variation due to between-subject, as opposed to within-subject variation (Lessells and Boag 1987; Bell et al. 2009). Due to the proportional measurement of activity level, we used a generalized linear mixed model (GLMM) with a binomial error distribution to calculate the repeatability of activitymesocosm. Confidence intervals (95 %) of the repeatability of activitymesocosm were estimated using parametric bootstrapping with 1000 resamplings. Mass-specific RMR data met the assumption of normality (Shapiro-Wilk normality test: W = 0.973, p = 0.330), and therefore, we calculated the repeatability of RMR using the ANOVA method in the rptR package.
Testing for relationships between crab behavior and metabolic rate
Next, we tested for a methodological relationship between crab activitychamber and RMR, and potential functional relationships between crab activitymesocosm and SMR. We further examined how both methodological and functional relationships were affected by predation threat from toadfish in the form of waterborne chemical cues. By measuring behavioral and energetic traits in the absence and presence of predation threat, this experiment also allowed us to examine how predation threat affected these traits (i.e., mechanisms behind potential context dependence). Activitymesocosm and RMR were measured as described previously in the “Repeatability of activitymesocosm and RMR” section. In addition, we recorded the activity of crabs in metabolic rate chambers (activitychamber) during the measurement of RMR. Specifically, we recorded whether crabs were moving or still (i.e., resting) at 10-min intervals corresponding with regular measurements of O2 concentration in chambers (six observations per crab, including the 0-min observation). Activitychamber was quantified as the proportion of observations where crabs were observed moving. Furthermore, to quantify SMR, we used the lowest single metabolic rate measurement for each crab (from metabolic rate measurements used to measure RMR), if the crab was inactive during this metabolic rate measurement.
This experiment was run in a randomized complete block design (n = 8 crabs per block) using a different group of crabs (mean CW ± 1 SE, 30.22 ± 0.22 mm) from those used in the repeatability experiments. Three blocks were run in total (i.e., n = 24 crabs), and each block lasted 5 days. Crab activitychamber was recorded during half of the first block as well as the last two blocks (i.e., n = 40 coupled activitychamber and RMR measurements, including measurements both in the absence and presence of predation threat), and so our analysis of a potential methodological relationship used these 40 coupled measurements. In addition, SMR could only be obtained from 19 RMR measurements because a number of crabs were active during the entire metabolic rate measurement period, particularly in the presence of predation threat.
The following describes our procedure for a single block. On the first day, we collected eight male crabs from North Inlet. Crabs were housed in tackle box compartments (3 cm length × 3 cm width) between behavioral and energetic trait measurements. Crabs were fed daily with M. mercenaria and then starved for 24 h to standardize hunger levels at 24 h before all measurements. On the second day, the RMR of these eight crabs was measured either in the absence or presence of predation threat (this order was alternated between blocks). RMR in the absence of predation threat was measured as described previously (see the “Repeatability of activitymesocosm and RMR” section). To measure RMR in the presence of toadfish predation threat, we first treated 1-μm filtered seawater by soaking a single toadfish (25 cm total length [TL]) in 5.65 L of water for 1.5 h. We then sieved the treated water through a 10-μm filter bag to remove minimal traces of fish slime and aerated it to saturate with O2. This water was otherwise treated in the same way as seawater used to measure RMR in the absence of predation threat. The RMR of crabs was then measured in this treated water.
On the third day, we measured the activitymesocosm of these eight crabs. We measured the activitymesocosm of four crabs in the absence of predation threat and the activitymesocosm of the other four in the presence of threat. Activitymesocosm in the absence of predation threat was measured as described previously (see the “Repeatability of activitymesocosm and RMR” section). Activitymesocosm in the presence of predation threat was measured the same as in the absence of threat, except that seawater which fed mesocosm tanks was first directed through a holding chamber that contained a single toadfish (30 cm TL). On the fourth day, the activitymesocosm of this same group of eight crabs was measured again, but in the alternate treatment (e.g., crabs receiving the predation threat treatment received the predation threat absent treatment). Finally, on the fifth day, the RMR of all eight crabs was measured again but under the alternate predation threat treatment level.
We used two linear mixed models (LMM) to examine relationships between (1) crab activitychamber and RMR (i.e., a methodological relationship; n = 40 RMR measurements), and (2) crab activitymesocosm and SMR (i.e., a functional relationship; n = 19 SMR measurements). These models included toadfish predation threat and crab carapace width as additional fixed factors because behavioral and energetic traits were measured in the absence and presence of predation threat, and crab size (carapace width) could additionally influence mass-specific metabolic rate measurements. We also tested for an interaction between the main predictor trait (activitychamber or activitymesocosm) and toadfish predation threat in each model, to test whether trait relationships were altered by the presence of predation threat (i.e., context dependence). Crab identity and experimental block were treated as random factors to control for pseudoreplication in both models. We tested for the significance of terms in LMM by dropping fixed factors (while retaining random factors) and comparing nested models using F tests (Crawley 2009). We additionally examined relationships between plasticity (i.e., changes in trait values in the presence versus absence of predation threat) in energetic and behavioral traits. We found no evidence for these relationships and therefore do not present these results.
Visual inspection of data suggested a curvilinear relationship between activitychamber and RMR. Therefore, we further used polynomial regression to test whether the significant relationship between activitychamber and RMR was in fact nonlinear. We also tested whether activitychamber was related to activitymesocosm measured in a more natural setting (i.e., a behavioral syndrome) using a generalized linear mixed model (GLMM). Activity (proportional data) was modeled with a binomial error distribution and logit link (Bolker et al. 2009). For the GLMM, we used z-values (i.e., the Wald statistic) and associated p values to examine whether factor coefficients were significantly different from zero.
We used GLMMs to examine the direct effects of predation threat on activitychamber and activitymesocosm, and a LMM to examine the direct effect of predation threat on RMR. We could not test for the effect of predation threat on SMR because SMR was measured in both contexts (predation threat absent and present) for only four crabs; this was due to the generally high activitychamber of crabs in the presence of predation threat. While these trait comparisons across contexts lacked true controls (i.e., individuals that were repeatedly subjected to the same predation treatment level), the order of predation treatment levels that individual crabs received was alternated between blocks for activitychamber and RMR measurements, and within blocks for activitymesocosm measurements. Therefore, to test whether predation threat or treatment order was responsible for trait changes, we also included treatment order as a fixed factor. Again, crab identity and experimental block were treated as random factors to control for pseudoreplication. All statistical analyses were conducted using R (R Core Team 2012).
Results
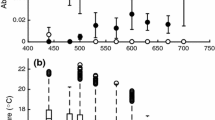
Both individual activitymesocosm (r ± 1 SE = 0.231 ± 0.081, 95 % confidence interval (CI) 0.068–0.386, p = 0.002; Fig. 1a) and RMR (r ± 1 SE = 0.533 ± 0.148, 95 % CI 0.227–0.838, p = 0.005; Fig. 1b) were repeatable over time, indicating temporal consistency in these traits.
Individual activitymesocosm (a) and routine metabolic rate (RMR) (b) of mud crabs (Panopeus herbstii) measured twice per crab over a period of 3 days with 48 h between measurements. Dashed lines indicate a 1:1 relationship (i.e., perfect consistency) for comparison to the distribution of data points
Individual crab activitychamber (observed during the measurement of crab RMR) was positively related (effect of activitychamber ± 1 SE = 10.073 ± 1.942) to crab RMR (F test: model with activitychamber as a fixed factor vs. null model [random factors only], p < 0.001; Fig. 2). The relationship between crab activitychamber and RMR was best described by a second-order polynomial model (F test: polynomial term, F 2,37 = 17.630, p = 0.025; Fig. 2). Activitychamber and threat did not interact to predict crab RMR (F test: LMM with activitychamber × threat interaction vs. model with independent factors, p = 0.448; Fig. 2).
In contrast, crab activitymesocosm, observed under more natural conditions, was negatively related to crab SMR (F test: LMM with activitymesocosm as a fixed factor vs. null model [random factors only], p = 0.036; Fig. 3). This negative effect of activitymesocosm was consistent across the absence and presence of predation threat (F test: LMM with activitymesocosm × threat interaction vs. model with independent factors, p = 0.793; Fig. 3), although SMR was measured in both the absence and presence of predation threat for just four crabs. Crab activitychamber did not predict activitymesocosm (GLMM: effect of activitychamber ± 1 SE = −0.359 ± 0.765, z = −0.470, p = 0.638), either in the absence or presence of toadfish predation threat (GLMM: effect of activitychamber × predation threat interaction ± 1 SE = 1.192 ± 0.812, z = 1.470, p = 0.142).
Predation threat increased crab activitychamber (GLMM: effect of threat ± 1 SE = 1.610 ± 0.357, z = 4.516, p < 0.001; Fig. 4a), but reduced activitymesocosm (GLMM: effect of threat ± 1 SE = −1.183 ± 0.186, z = −6.376, p < 0.001; Fig. 4b). Predation threat had no effect on RMR (F test: LMM with threat as a fixed factor vs. null model [random factors only], p = 0.788; Fig. 4c). The order of predation treatment levels that crabs received had no effect on any of the three trait measurements (p > 0.222).
Discussion
In the present study, we found repeatable individual-level variation (i.e., CIDs) in both crab activitymesocosm and mass-specific RMR measured in the absence of predation threat. Although activity level is typically less repeatable than other behaviors (e.g., aggression or exploration; Bell et al. 2009), the repeatability of crab activity level detected here qualifies this trait as a behavioral type of P. herbstii. Furthermore, in line with other studies of CIDs in mass-specific metabolic rate (reviewed in White et al. 2013), our data indicate that mass-specific crab RMR is a temporally consistent trait at the individual level.
Our data supported the hypothesis that crab activitychamber would be positively related to RMR (i.e., a methodological relationship; Careau et al. 2008), suggesting that crabs which moved more in metabolic rate chambers also respired more. This relationship was best described by a polynomial model which peaked at approximately 20 μmol/h/g DW oxygen consumption. This suggests an upper limit to mass-specific RMR in our study beyond which additional activitychamber had little effect on RMR. Methodological relationships between behavior and metabolic rate make it difficult to effectively separate the effects of individual behavioral variation from baseline metabolic rate (Careau et al. 2008). Preventing animal movement via restraint could eliminate individual differences in movement, although this would likely induce further stress and thereby modify metabolic rates in unrealistic ways. A better method for minimizing direct effects of activity on metabolic rate is to measure metabolic rate over a longer time period, which should allow animals to calm and eventually become inactive (Careau et al. 2008). In either case, it is critical that activity exhibited during the measurement of metabolic rate is observed, in order to account for the direct effect of activity on respiration. While other studies have failed to detect a methodological relationship between behavior and energetics (e.g., Bouwhuis et al. 2014), this likely depends on the behavioral response of the particular study species to the method of metabolic rate measurement. The method used in the present study is commonly used to measure the metabolic rates of aquatic invertebrates (e.g., Widdows and Staff 2006; Sarà et al. 2013), and so future studies using this method should consider this source of variation in individual metabolic rates.
We accounted for this methodological relationship by quantifying SMR (i.e., baseline metabolic rate) as the lowest metabolic rate measurement per crab, if the crab was inactive during this metabolic rate measurement. This allowed us to test for functional relationships between activity measured in a more natural setting (activitymesocosm) and baseline metabolic rate. We detected an overall negative relationship between activitymesocosm and SMR, although it is worth noting that our sample size used to examine this relationship was relatively low: We could only extract 19 SMR measurements from 40 total RMR measurements in which activitychamber was measured. Furthermore, this relationship was measured over a short duration for crabs (at most 48 h), and whether such a relationship between activitymesocosm and SMR remains over longer time periods is unknown.
While the precise mechanism behind this negative relationship is unclear and beyond the scope of the present study, such a relationship can be explained by an allocation model that predicts individual activity and baseline metabolic rate compete for limited energy reserves (Careau et al. 2008; Careau and Garland 2012). Under such a scenario, crabs that exhibit consistently high activity under relatively natural conditions have less available energy to maintain baseline metabolic rate. We starved crabs for 24 h before all trait measurements throughout our study, and this level of food limitation could have driven energy competition between activitymesocosm and SMR. While we found no evidence of context dependence (predation threat) in this study, an allocation model suggests that the relationship between activitymesocosm and SMR depends on crab hunger level or resource availability. Thus, an additional experiment in which this relationship is examined across different crab hunger levels could be used to test whether an allocation model is responsible for the negative relationship between activitymesocosm and SMR. In such an experiment, we would expect that increased food levels act to mitigate the negative relationship activitymesocosm and SMR (Burton et al. 2011).
Although studies testing for functional relationships between CIDs in behavior and energetics are increasing (Biro and Stamps 2010; Careau and Garland 2012), relatively few have provided evidence for negative functional relationships (Careau and Garland 2012; Bouwhuis et al. 2014; Careau et al. 2014a; Gifford et al. 2014; Guenther et al. 2014; Killen 2014). Evidence in support of an allocation model has mostly come from studies that manipulate animal activity and measure the effects on baseline metabolic rate (Deerenberg et al. 1998; Wiersma and Verhulst 2005; Wikelski et al. 1999). Thus, support for an allocation model in our study is unique in that activity varied naturally among individual crabs, and this variation in individual activity was consistent over time (i.e., a behavioral type).
Crab activitychamber was not related to crab activitymesoscosm, and this could help explain the positive methodological relationship between activitychamber and RMR versus the negative functional relationship between activitymesocosm and SMR. The increase in activitychamber and decrease in activitymesocosm with predation threat further indicates that these activity measures represent fundamentally different behaviors. Specifically, we attribute these different behavioral responses to predation threat to the lack of refuge habitat in the containers where crab metabolic rate was measured and presence of refuge habitat in mesocosms. Increased activitychamber in the presence of predation threat likely reflects attempts to hide or escape the metabolic rate chamber (i.e., stress). In contrast, crabs that reduced activitymesocosm in the presence of threat often took refuge under oyster shells, consistent with other studies on the effects of predation threat on P. herbstii behavior (Grabowski 2004; Griffen et al. 2012; Toscano et al. 2014). Reduced activity is a taxonomically widespread response to predation threat and an effective mechanism of predator avoidance (Krupa and Sih 1998; Anholt et al. 2000; Grabowski 2004; Toscano et al. 2010).
Our study is one of few to test for functional relationships between CIDs in behavior and energetics in an invertebrate species (Careau and Garland 2012; Krams et al. 2013). Krams et al. (2013) found that mealworm beetles (Tenebrio molitor) with lower metabolic rates also displayed higher durations of immobility, a behavior that reduces predation risk, providing support for a pace-of-life syndrome. Compared to endotherms, ectothermic invertebrates could be expected to show different individual-level relationships between behavior and energetics, and context dependence in these relationships, due to fundamentally different energetic strategies (Kralj-Fišer and Schuett 2014; Careau et al. 2014b). For example, activity (e.g., Briffa et al. 2013) and metabolic rate (e.g., Clarke and Johnston 1999) in ectotherms covary with temperature due to the need to satisfy maintenance requirements, while endotherms can maintain high activity under decreased temperatures through thermoregulation (Careau et al. 2014b). We suggest that additional research on CIDs in the behavioral and physiological traits of invertebrates will provide insight into the commonness of individual-level behavior-energetics relationships across taxonomic groups and therefore the mechanisms behind relationships. This information is critical in refining our understanding of the maintenance of individual-level variation in behavioral and energetic traits in natural populations.
References
Anholt BR, Werner E, Skelly DK (2000) Effect of food and predators on the activity of four larval ranid frogs. Ecology 81:3509–3521
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77:771–783
Bell AM, Sih A (2007) Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol Lett 10:828–834
Biro PA, Stamps JA (2008) Are animal personality traits linked to life-history productivity? Trends Ecol Evol 23:361–368
Biro PA, Stamps JA (2010) Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol Evol 25:653–659
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Bouwhuis S, Quinn JL, Sheldon BC, Verhulst S (2014) Personality and basal metabolic rate in a wild bird population. Oikos 123:56–62
Briffa M, Bridger D, Biro PA (2013) How does temperature affect behaviour? Multilevel analysis of plasticity, personality and predictability in hermit crabs. Anim Behav 86:47–54
Buchanan KL, Evans MR, Goldsmith AR, Bryant DM, Rowe LV (2001) Testosterone influences basal metabolic rate in male house sparrows: a new cost of dominance signalling? Proc R Soc B 268:1337–1344
Burton T, Killen S, Armstrong J, Metcalfe N (2011) What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc R Soc B 278:3465–3473
Careau V, Buttemer WA, Buchanan KL (2014a) Developmental stress can uncouple relationships between physiology and behaviour. Biol Lett 10:20140834
Careau V, Garland T Jr (2012) Performance, personality, and energetics: correlation, causation, and mechanism. Physiol Biochem Zool 85:543–571
Careau V, Killen SS, Metcalfe NB (2014b) Adding fuel to the “fire of life”: energy budgets across levels of variation in ectotherms and endotherms. In: Martin LB, Ghalambor CK, Woods HA (eds) Integrative organismal biology. Wiley-Blackwell, Hoboken, pp 219–233
Careau V, Thomas D, Humphries M, Réale D (2008) Energy metabolism and animal personality. Oikos 117:641–653
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68:893–905
Crawley MJ (2009) The R book. John Wiley and Sons, West Sussex
Dall W (1986) Estimation of routine metabolic rate in a penaeid prawn, Penaeus esculentus Haswell. J Exp Mar Biol Ecol 96:57–74
Deerenberg C, Overkamp G, Visser G, Daan S (1998) Compensation in resting metabolism for experimentally increased activity. J Comp Physiol B 168:507–512
Galliard JF, Paquet M, Cisel M, Montes‐Poloni L (2013) Personality and the pace-of-life syndrome: variation and selection on exploration, metabolism and locomotor performances. Funct Ecol 27:136–144
Gifford ME, Clay TA, Careau V (2014) Individual (co) variation in standard metabolic rate, feeding rate, and exploratory behavior in wild-caught semiaquatic salamanders. Physiol Biochem Zool 87:384–396
Gosling SD (2001) From mice to men: what can we learn about personality from animal research? Psychol Bull 127:45–86
Grabowski JH (2004) Habitat complexity disrupts predator-prey interactions but not the trophic cascade on oyster reefs. Ecology 85:995–1004
Griffen BD, Toscano BJ, Gatto J (2012) The role of individual behavior type in mediating indirect interactions. Ecology 93:1935–1943
Guenther A, Finkemeier M-A, Trillmich F (2014) The ontogeny of personality in the wild guinea pig. Anim Behav 90:131–139
Killen SS (2014) Growth trajectory influences temperature preference in fish through an effect on metabolic rate. J Anim Ecol 83:1513–1522
Killen SS, Marras S, Metcalfe NB, McKenzie DJ, Domenici P (2013) Environmental stressors alter relationships between physiology and behaviour. Trends Ecol Evol 28:651–658
Kralj-Fišer S, Schuett W (2014) Studying personality variation in invertebrates: why bother? Anim Behav 91:41–52
Krams I, Kivleniece I, Kuusik A, Krama T, Freeberg TM, Mänd R, Vrublevska J, Rantala MJ, Mänd M (2013) Predation selects for low resting metabolic rate and consistent individual differences in anti-predator behavior in a beetle. Acta Ethol 16:163–172
Krupa JJ, Sih A (1998) Fishing spiders, green sunfish, and a stream-dwelling water strider: male-female conflict and prey responses to single versus multiple predator environments. Oecologia 117:258–265
Lessells C, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956
Nespolo RF, Franco M (2007) Whole-animal metabolic rate is a repeatable trait: a meta-analysis. J Exp Biol 210:2000–2005
Niewiarowski P, Waldschmidt S (1992) Variation in metabolic rates of a lizard: use of SMR in ecological contexts. Funct Ecol 6:15–22
Core Team R (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio PO (2010) Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil Trans R Soc B 365:4051–4063
Sarà G, Palmeri V, Montalto RA, Widdows J (2013) Parameterisation of bivalve functional traits for mechanistic eco-physiological dynamic energy budget (DEB) models. Mar Ecol: Prog Ser 480:99–117
Siepielski AM, Wang J, Prince G (2014) Nonconsumptive predator-driven mortality causes natural selection on prey. Evolution 68:696–704
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378
Sih A, Cote J, Evans M, Fogarty S, Pruitt J (2012) Ecological implications of behavioural syndromes. Ecol Lett 15:278–289
Speakman JR, Król E, Johnson MS (2004) The functional significance of individual variation in basal metabolic rate. Physiol Biochem Zool 77:900–915
Svanbäck R, Eklöv P (2011) Catch me if you can—predation affects divergence in a polyphenic species. Evolution 65:3515–3526
Toscano BJ, Griffen BD (2014) Trait-mediated functional responses: predator behavioural type mediates prey consumption. J Anim Ecol 83:1469–1477
Toscano BJ, Fodrie FJ, Madsen SL, Powers SP (2010) Multiple prey effects: agonistic behaviors between prey species enhances consumption by their shared predator. J Exp Mar Biol Ecol 385:59–65
Toscano BJ, Gatto J, Griffen BD (2014) Effect of predation threat on repeatability of individual crab behavior revealed by mark-recapture. Behav Ecol Sociobiol 68:519–527
White CR, Schimpf NG, Cassey P (2013) The repeatability of metabolic rate declines with time. J Exp Biol 216:1763–1765
Widdows J, Staff F (2006) Biological effects of contaminants: measurement of scope for growth in mussels. ICES Tech Mar Environ Sci 40:1–30
Wiersma P, Verhulst S (2005) Effects of intake rate on energy expenditure, somatic repair and reproduction of zebra finches. J Exp Biol 208:4091–4098
Wikelski M, Lynn S, Breuner J, Wingfield J, Kenagy G (1999) Energy metabolism, testosterone and corticosterone in white-crowned sparrows. J Comp Physiol A 185:463–470
Acknowledgments
This study was supported by a NSF Graduate Research Fellowship (under Grant No. DGE-0929297) and a SPARC Graduate Research Fellowship (University of South Carolina) to BJT, and a GAANN Fellowship (Fellowship No. P200A090301) to CJM. Partial funding was further provided by NASA (NNX11AP77G). This is contribution number 81 in Ecological Forecasting from the University of South Carolina. We thank J.M. Harding and P.D. Kenny for technical assistance, and B.D. Griffen, K. Niitepõld, J.L. Dudycha, and anonymous reviewers for helpful suggestions that improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Breithaupt
Rights and permissions
About this article
Cite this article
Toscano, B.J., Monaco, C.J. Testing for relationships between individual crab behavior and metabolic rate across ecological contexts. Behav Ecol Sociobiol 69, 1343–1351 (2015). https://doi.org/10.1007/s00265-015-1947-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-1947-4