Abstract
Individuals produce advertisement signals with intended purposes and targets. However, these signals can be received by “eavesdroppers,” who may extract information from them and alter their behavior according to the extracted information. In anuran systems, males congregate at breeding sites to produce advertisement calls to attract receptive females and fend off rival males. Both sexes directly assess these calls in dyadic encounters and make decisions based on the call’s characteristics, e.g., frequency. What is unknown is whether bystander males eavesdrop on these same calls to inform their future competitive decisions. Here, we examined whether male green tree frogs (Hyla cinerea) eavesdrop on competing males, assess their competitor’s call frequency, and respond accordingly. We exposed males to playbacks of two competing males that varied in call frequency—high, average, low—and quantified latency to call, time spent calling, and number of calling bouts. We found that males had reduced latency to call and called more when eavesdropping low-frequency competition, but not average or high-frequency competition. Focal male size also influenced how they responded, with larger males being more responsive than smaller males. Our results indicate that male green tree frogs are capable of eavesdropping on nearby male calls and produce behavioral responses accordingly. Further, it appears males are able to alternate between assessment strategies dependent upon the frequency of the eavesdropped competition. These findings indicate that males not only directly assess an opponent’s call in dyadic encounters, but also indirectly through eavesdropping.
Significance statement
Animals produce signals with intended purposes and targets, but which can be received by nearby eavesdroppers. Eavesdropped signals can elicit complex phenotypic changes in the eavesdropper, and lead to significant fitness consequences for the signal producer and eavesdropper. Thus, examining whether and to what extent individuals eavesdrop is an important step in understanding the evolution of signal production and response. Here, we examined whether male green tree frogs, H. cinerea, eavesdrop on nearby competing males and base their own calling behaviors on eavesdropped male’s calling characteristics. Calling behavior was mediated by focal male body size and eavesdropped male’s call frequency. Larger males reduced response latency and called more to nearby low-frequency males, while smaller males reduced response latency to nearby calls of average frequency. These results indicate that males will extract information from their competitive environment through eavesdropping and produce behavioral responses according to the eavesdropped information.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In lek-breeding systems, males gather at breeding sites where they produce advertisement signals in an attempt to attract receptive females (Ryan 2001; Gerhardt and Huber 2002; Bee 2015; Yorzinski et al. 2017). These signals are often considered to contain information regarding the “quality” of the signaler (e.g., size, condition, etc.), which a female may use when making mate selection decisions; the actual information content of these signals is often assumed or not easily quantifiable (Dall et al. 2005; Rendall et al. 2009; Taylor et al. 2011). Ultimately, these signals are designed to elicit a response from potential mates or rivals. However, these signals can be received by nearby individuals, “eavesdroppers,” which can lead to unintended and sometimes significant fitness consequences for the signal producer (Johnstone 2001; Earley and Dugatkin 2002; Peake et al. 2002; Earley 2010; Halfwerk et al. 2014). Eavesdropping is a widespread phenomenon seen in both invertebrate and vertebrate systems where bystanders of the same or different species respond to signals produced by an individual and make behavioral decisions based on the signal received (reviewed in Earley 2010). In crayfish (Procambarus clarkii), females eavesdrop on male-male contests and use sight and smell to recognize the contest winner (Aquiloni and Gherardi 2010). Song sparrows (Melospiza melodia) eavesdrop on neighboring territory holders and alter their vigilance against neighbors who exhibit higher levels of intrusion into a third party’s territory (Akçay et al. 2010). Further, male túngara frogs (Physalaemus pustulosus) produce multimodal, advertisement calls consisting of auditory, visual (vocal sac), and mechanical (water ripples) components, to attract gravid females, but hunting bats (Trachops cirrhosis) use the mechanical component of the frog’s advertisement call to echolocate and consume the signaler (Halfwerk et al. 2014). These examples demonstrate that while potentially detrimental to signalers, eavesdropping can have great benefits for the eavesdroppers themselves.
In the context of competitive interactions, the information gleaned from eavesdropping can impact the eavesdropper’s physiology and future aggressive decisions (Johnstone 2001; Peake et al. 2002; Earley and Dugatkin 2002; Earley 2010; Hirschenhauser et al. 2013; Garcia et al. 2016). Bystanders who observe a contest or are aware of nearby competitors may exhibit a “priming” effect where physiological (i.e., neural gene expression and steroid hormone levels) and aggressive behavioral states are modified (e.g., elevated) in anticipation of a potential aggressive interaction (Burmeister and Wilczynski 2000; Oliveira 2009; Clotfelter and Paolino 2003; Lado et al. 2013; Garcia et al. 2016). If bystanders then engage the individuals they eavesdropped on, they may alter their aggressive behaviors based on the observed contest outcome and/or level of intensity (i.e., escalated vs. non-escalated; Earley and Dugatkin 2002; Peake et al. 2002; Peake and McGregor 2004). For example, in green swordtail fish (Xiphophorus helleri), bystanders reduce their propensity to engage or escalate against previously observed winners and persistent losers (i.e., those who put up a strong fight against their opponent but still lost; Earley and Dugatkin 2002). While bystanders are extracting information from observing the contest, the combatants may also glean information about potential future fights with their audience members through an “audience” effect (Hirschenhauser et al. 2013). In Japanese quail (Coturnix japonica), winners and losers of an unobserved contest exhibit similar spikes in post-contest testosterone levels and their aggressiveness in subsequent contests against naïve opponents was unchanged by previous contest experience. However, when an audience was present, observed losers did not exhibit a spike in post-contest testosterone and were less likely to win subsequent contests (loser effects) while winners still increased post-contest testosterone levels and had an increased probability of winning subsequent contests (winner effects; Hirschenhauser et al. 2013). Ultimately, by paying attention to social surroundings, individuals may gain information regarding their current competitive environment, and produce adaptive physiological and behavioral responses.
Males of many anuran species engage in communal signaling, where males aggregate at ponds and form choruses where they produce advertisement calls for mates (Ryan 2001). These choruses often contain high densities of males, thus producing high levels of competition for prime calling sites and ample opportunities for eavesdropping on competitors (Höbel and Kolodziej 2013; Reichert and Gerhardt 2014; Chuang et al. 2017). Competitive interactions between male frogs often consist of non-physical “dueling bouts,” where males alternate production of advertisement calls; these calls function both to space males in the chorus and attract females (Ryan 2001; Reichert and Gerhardt 2013; Reichert 2014). In green tree frogs, males normally time their calls to avoid overlap with their neighbor and often enter a stable antiphonal call pattern during a bout with their neighbor (Höbel and Gerhardt 2007). Males of some species may escalate contests by switching to an aggressive call, occasionally even leading to physical combat (Marshall et al. 2003; Reichert and Gerhardt 2013, 2014).
Female mate choice has probably been the best-studied aspect of anuran communication (Ryan 2001; Gerhardt and Huber 2002; Schrode et al. 2012; Taylor and Ryan 2013; Laird et al. 2016), but numerous studies have also examined vocal competition among males (Fellers 1979; Bee and Perril 1996; Bee et al. 1998; Bee 2002). In gray tree frogs (Hyla versicolor), males can differentiate between conspecific advertisement calls and heterospecific advertisement calls produced by their sister taxa, H. chrysoscelis, and alter their aggressive behaviors towards either (Reichert and Gerhardt 2014). In olive frogs (Babina adenopleura), territorial males can recognize the calls of their surrounding neighbors and exhibit less aggression towards neighbors compared to unknown individuals (“Dear Enemy”; Chuang et al. 2017). Further, in green frogs (Rana clamitans), males engaging in dyadic, agonistic encounters can directly assess their opponent’s call (e.g., dominant frequency) and alter their calling behaviors (e.g., duration, fundamental frequency, rate) based on the opponent’s call characteristics (Bee and Perril 1996; Bee et al. 1998). While assessment may be prevalent in some anuran systems, it is not ubiquitous among all taxa (see Bee 2002, 2003). While the above studies provide evidence for direct male call assessment, there has yet to be, to our knowledge, an explicit examination of whether male frogs eavesdrop (indirect assessment) on two nearby competing males.
In this study, we examined whether male green tree frogs (Hyla cinerea) eavesdrop on the calls of nearby competing males, assessing the frequency of their competitors’ calls. A previous study showed that male green tree frogs in our focal population average 3.76 g in mass (range = 2.15—5.11 g) and produce short advertisement calls, typically at rates of 60–80 calls/min (Laird et al. 2016). The calls consist of numerous harmonics with two frequency peaks, 0.64–0.96 kHz (low) and 2.34–3.45 kHz (high), at similar amplitudes (Oldham and Gerhardt 1975; Höbel 2010), and exhibit an inverse size-frequency relationship, where larger males produce lower frequency calls (Laird et al. 2016). Female green tree frogs prefer to mate with males with faster call rates and lower frequencies relative to their slower and/or higher frequency counterparts (Laird et al. 2016). Male green tree frogs also engage in alternative reproductive tactics by forgoing calling and instead acting as “satellites,” where non-calling males position themselves next to calling males and attempt to intercept approaching females (Humfeld 2008; Leary and Harris 2013; Leary 2014). Current data suggest that a male’s decision to engage in satellite behavior is condition dependent, with satellite males exhibiting poorer body condition and higher stress levels (Leary and Harris 2013), but the possibility of additional social signals influencing their decision to engage in satellite behaviors is not well understood.
We hypothesize that males will produce calling behaviors—latency to call, time spent calling, and number of calling bouts—based on the call frequency of nearby dueling males. Lower frequency calling males represent potentially greater competitive threats (reviewed in Searcy and Beecher 2009; Reichert and Gerhardt 2014); thus, we predict that playback of low-frequency calls will elicit lower latency to call, more time spent calling, and more calling bouts from our focal males relative to average and higher frequency playbacks. Also, because green tree frog males have been shown to alter their behavioral tactics based on body condition (Leary and Harris 2013), we predict that male response to competitor calls will be dependent on their own body size, with larger males having lower latency to respond to nearby competitors relative to smaller males.
Methods
Animal collection and care
We collected amplectant pairs from Vienna, MD, from 1 July to 31 August 2015 and from 30 May to 31 July 2016. In initial trails, we found that all non-amplectant, “bachelor” males failed to respond to any of our treatments while amplectant males responded about 60% of the time (see below). As such, we only used amplectant males throughout the experiment. We placed pairs into individual plastic bags within a darkened cooler and transported them back to Salisbury University. Collection and transportation in this manner have been previously performed and shown to have minimal impact on individual behavior (Laird et al. 2016). All pairs were housed within the darkened cooler for a minimum of 1 h to ensure their eyesight was dark adapted (Fain et al. 2001). We tested each male once within a night. Afterwards, we collected morphometric data [mass (g) and snout-vent length (SVL; mm)] and unique toe-clippings, reunited the pair, and housed the pair in 0.3 m3 terrarium with a small amount of dechlorinated water. To minimize the impact of collections on population size, we returned the pairs and any deposited eggs to the original collection site the next evening. All experimental procedures were conducted under Salisbury University’s Institutional Animal Care and Use Committee (IACUC Protocol # SU 0036) approval and guidance.
Playback experiment
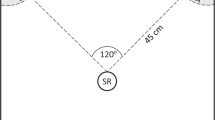
We tested each male using a two-speaker playback test in a 1.6 × 2.5 m hemi-anechoic chamber. We placed Mirage Nanosat speakers (left and right) 1 m away from the male starting point, at a 60° angle relative to the male, and set each to broadcast calls at an amplitude of 86 dB (re. 20 μPa, fast-C weighting) as measured at male starting point. We used a set of three synthetic male calls developed by Laird et al. (2016) as our competitor call treatments (Table 1). Laird et al. (2016) had developed these synthetic male calls based on the average male call characteristics of the Vienna, MD, population. These calls were effective stimuli, mimicking real calls of varying frequencies by differentially eliciting behavioral responses (mate choice) from receptive females. We standardized call playback at ~ 70 calls/min and set playback to mimic a “dueling bout” between two competing males, where calls would alternate back-and-forth between the right and left speakers. For ease of reading, we will refer to competitor’s call playbacks as treatment stimuli. Both speakers were set to playback the same competitor stimuli creating high v. high (HvH), average v. average (AvA), and low v. low (LvL) dueling bouts. We refer to these hereafter as high (H), average (Avg), or low (L) frequency treatments. We observed all trials via an IR video camera (Everfocus EHD 500 IR) mounted on the ceiling of the acoustic chamber. Observers used audio and/or visually confirmation of focal male response (i.e., calling) to treatment.
For each trial, we separated a male from his female and placed him under an acoustically transparent funnel at the designated starting point within the acoustic chamber (sensu Taylor et al. 2008), while the female was used in a separate study. We began broadcasting a dueling bout (H, Avg, or L) chosen haphazardly and left the male under the funnel for 2 min to allow acclimation to the new conditions. Afterwards, we raised the funnel from outside the chamber using a nylon string and allowed the male to freely roam the acoustic chamber and respond to the playback for 15 min. Within the 2 min acclimation and 15 min observation period, we scored (i) the focal individual’s latency to begin calling, (ii) total time spent calling, and (iii) number of calling bouts [a series of repeated calls followed by a brief pause (≥ 10 s) when the male ceased calling, and either rotated himself or moved to a new location]. When the 15-min observation period ended, we quickly re-captured the male and collected morphometric data as described above. For the data set, we excluded focal males who failed to respond to competitor calls within the 17 min trial period (2015: N = 7, 2016: N = 14) and retained individuals that did respond (N = 30 in total; 2015: N = 13, 2016: N = 17). We had an N = 9 for the H treatment, N = 11 for the Avg treatment, and N = 10 for the L treatment. Due to the nature of our experimental design, it was not possible to record behavioral data blind. To reduce observer bias from a single individual, all behavioral observations were simultaneously performed and confirmed by three individuals (MJG, TB, HB).
During each trial, we attempted to record the focal male’s call to examine whether focal male call characteristics had any influence on their responsiveness to the treatment received (H, Avg., L). We recorded focal male calls using a Tascam DR-05 Linear PCM Recorder (TEAC America, Inc. Montebello CA, USA) with a SME-ATR55 shot-gun microphone (Saul Mineroff Electronics, Inc. Elmont, NY, USA). We set the microphone 30 cm away from the male starting point within the acoustic chamber and ran the microphone cable outside of the chamber to allow remote recording operations. Because of the static nature of the microphone and because the focal males were allowed to freely roam the chamber, we were unable to get clear recordings for all focal males. However, we were able to obtain clear recordings for a subset of our focal males (N = 13; 2015: N = 5, 2016: N = 8). For each male call, we measured call rate (call/min), call duration (ms), low-peak frequency (kHz), and high-peak frequency (kHz). Call duration and rate were measured using the program SoundRuler, while low- and high-peak frequencies were measured using AviSoft SASLab Pro software (AviSoft Bioacoustics Berlin, Germany). Individual responsible for call data collection (AC) was blind to the experimental treatment of each individual.
Statistical analyses
We performed all statistical analyses using JMP Pro (version 13.0.1, SAS Institute Inc., Cary, NC, USA). We examined the relationships between focal male morphology (mass and SVL) and call characteristics (low-peak frequency, high-peak frequency, and call duration) using pairwise correlations. We then used an analysis of covariance (ANCOVA) to analyze whether treatment (H, Avg, or L) had any effect on focal male latency to call, total time spent calling, and number of calling bouts. We included treatment (H, Avg, L) and year as fixed effects, and mass as a covariate. From our pairwise correlations, we found that call frequency was negatively correlated with both mass and SVL; that is, male call frequency decreased as male size increased (Fig. 1a, b). Considering this and the fact that we had size data for all individuals compared to having a partial dataset for call characteristics, we elected to only use mass as our covariate. To determine what drove any significant effects involving the covariate, we performed post hoc, linear regressions.
Results
From our call analyses, we found male advertisement calls had a mean duration of 104 ms ± 11 SD (N = 13) and were produced at an average rate of 77 calls/min ± 10 SD (N = 13). Male calls exhibited the stereotypic two primary energy bands, high-frequency and low-frequency peaks, which averaged 3.51 kHz ± 0.22 SD and 1.16 kHz ± 0.06 SD (N = 13), respectively. Our pairwise comparisons revealed significant, negative correlations between male body size (mass and SVL), low-peak frequency, and high-peak frequency (mass v. low-peak: r2 = 0.67, N = 13, p = 0.0006; mass v. high-peak: r2 = 0.72, N = 13, p = 0.00062; SVL v. low-peak: r2 = 0.48, N = 13, p = 0.009; SVL v. high-peak: r2 = 0.47, N = 13, p = 0.0094; Fig. 1a, b). We saw that as male body size increased, their call frequency (low- and high-peak) decreased. Our ANCOVA’s revealed significant treatment × mass interaction effects on latency to call and total time spent calling, but not on number of calling bouts (Table 2). Treatment, year, and focal male mass alone did not have significant effects on any male calling behavior (all p > 0.05). Average male mass for all treatments was 3.31 (± 0.11 SE) g and SVL was 50.28 mm (± 1.70 SE). Although there were a range of male sizes, neither mass nor SVL differed significantly among playback treatments (ANOVA; mass: F2,30 = 0.21, p = 0.81; SVL: F2,30 = 0.19, p = 0.83). From our post hoc regressions, we saw that when eavesdropping on a low-frequency treatment, focal male latency to call decreased as their mass increased (r2 = 0.60, N = 10, p = 0.009; Fig. 2a). Larger males were quicker to respond to low-frequency treatment relative to smaller males, but this trend did not hold for males eavesdropping on high and average frequency treatment (high: r2 < 0.001, N = 9, p = 0.99, average: r2 = 0.29, N = 11, p = 0.14; Fig. 2b, c). Although not statistically significant, we also saw an opposing trend when focal males faced average frequency stimuli, with latency to call increasing with focal male mass (Fig. 2b). Further, we saw that when eavesdropping on low-frequency treatment, focal males spent more time calling as their mass increased (r2 = 0.61, N = 10, p = 0.007; Fig. 3a). Larger males tended to spend more time calling against low-frequency treatment compared to smaller males. This trend did not hold for males eavesdropping on high and average frequency treatments (high: r2 = 0.02, N = 9, p = 0.69, average: r2 = 0.09, N = 11, p = 0.44; Fig. 3b, c).
Regression analyses examining relationship between focal male mass and latency to call when pitted against a low-frequency competitor call (r2 = 0.60, N = 10, p = 0.009), b average frequency competitor call (r2 = 0.29, N = 11, p = 0.14), and c high-frequency competitor call (r2 < 0.001, N = 9, p = 0.99)
Regression analyses examining relationship between focal male mass and total time calling when pitted against a low-frequency competitor call (r2 = 0.61, N = 10, p = 0.007), b average frequency competitor call (r2 = 0.09, N = 11, p = 0.44), and c high-frequency competitor call (r2 = 0.02, N = 9, p = 0.69)
Discussion
Eavesdropping can have substantial benefits within a competitive environment, whereby eavesdroppers may gain information about a potential competitor’s competitive ability and modify their behavior accordingly (Johnstone 2001; Earley 2010). For anurans that engage in communal signaling, there are substantial opportunities for individuals to eavesdrop on their competition, which could inform their competitive decisions. In the green tree frog, low-frequency males tend to be larger in size and more attractive to receptive females compared to their average and high-frequency counterparts (Laird et al. 2016). As such, we predicted that males given the chance to eavesdrop on competitors would consider low-frequency callers to be a greater competitive threat (both in physical combat and mate attraction) and would thus respond faster and more vigorously against them. Our results support this prediction, showing that eavesdropping males were significantly more likely to respond to duels between perceived, competitively superior individuals, dependent on the size of the eavesdropper itself. Further, the condition of a green tree frog male is known to mediate their decision of whether to engage in the alternative reproductive tactic of satellite behavior (Humfeld 2008; Leary and Harris 2013). We thus predicted that males that were larger or in better condition would be more prone to respond to competitors relative to smaller or poorer condition males. Our results, in part, supported this prediction, demonstrating that larger males when eavesdropping on perceived, low-frequency opponents reduced latency to respond and called more relative to their smaller counterparts. However, we also found that smaller males, when eavesdropping mid-size competitors, showed a non-significant reduction in latency to respond. Our findings lend support to the idea that male frogs eavesdrop on and assess their competitive environment based on their own body size and the call characteristics of surrounding males. Further, our results seem to indicate that males may switch between assessment strategies dependent upon the call characteristics of nearby males.
Before engaging in a contest, individuals should critically evaluate the potential costs and benefits associated with engaging in an agonistic encounter; otherwise, they may face significant fitness consequences (e.g., energy loss and predation risk; Emerson 2001; Halfwerk et al. 2014). However, preempting an opponent and being quick to engage can have potential benefits. In green anole lizards (Anolis carolinensis), individuals who initiated a contest improved their chances of winning the contest, even if they were smaller than their opponent (Garcia et al. 2012, 2014); a trend that is not ubiquitous across taxa (see Moretz 2003). Based on previous research (e.g., Gingras et al. 2012; Laird et al. 2016) and our own findings, we know that in anuran species, call frequency is a reliable indicator of individual size and, ultimately, competitive threat. As such, eavesdropping on and being able to assess one’s opponent prior to engaging may assist an individual in making faster and better agonistic decisions. Here, we found individuals alter their willingness to engage potential competitors based on the combination of nearby competitor call frequency and their own body size. When eavesdropping high-frequency competitors, focal males did not show any alteration in their willingness to engage regardless of their own size. High-frequency males are likely assessed as smaller, competitively inferior, and not warranting a rapid response. However, this trend changes as the perceived competitive prowess of the competition increased. Although a non-significant trend, we found that willingness to engage changed when males eavesdrop on average frequency competitors, with smaller males being faster to engage relative to larger males. We hypothesize that smaller males may assess average frequency males as a competitive threat and, as such, are quicker to respond to that threat. As noted earlier, prior works have shown that competitors with lower latency to initiate a contest have a higher likelihood of winning a contest, regardless of size (Garcia et al. 2012, 2014). Larger males on the other hand likely assess average frequency competitors as a minimal threat and thus will delay or forgo their engagement. Further, we found that when eavesdropping on perceived, low-frequency competitors, larger males engaged faster than smaller males. Here, we suspect that smaller males may assess their chances of winning any potential contests against low-frequency males as non-existent and, ultimately, are less willing to engage, while larger males appear to assess their opponent as a greater but not unbeatable competitive threat, and thus show greater willingness to engage. Cumulatively, it appears that males can indirectly assess the competitive ability of nearby males through eavesdropping and use that information when deciding whether and how quickly they respond to nearby competitive threats.
While initiation is the first decision an individual will make in an agonistic contest, it is the decision to persist and/or retreat that dictates the outcome of a contest. Contest persistence and, ultimately, contest outcome are dictated by the competing individual’s resource holding potential (RHP), the culmination of factors (e.g., size, experience, weaponry, etc.) which contributes to an individual obtaining and retaining fitness-related resources (Maynard-Smith and Price 1974; Parker 1974; Briffa and Elwood 2009). When deciding whether to persist or forfeit a match, individuals may employ (i) a self-assessment strategy where they base their decisions solely on their own RHP and irrespective of their opponents’ (Arnott and Elwood 2008; Briffa 2008; Briffa and Elwood 2009; but see Payne 1998), (ii) a mutual-assessment strategy when they monitor asymmetries between their and their opponents RHP through gradually escalating contests (e.g., Enquist et al. 1990), or (iii) a combination of both (Hsu et al. 2008). These assessment strategies are usually determined by how long individuals persist in a contest, a “giving-up” threshold, which our experimental design did not measure. However, recent theoretical work has indicated that assessment strategies can be analyzed using playback tests by analyzing the relationship between focal individual RHP (e.g., body size) and their persistence time (total time calling in our study) when facing low- or high-RHP opponents (Reichert 2014). Self, mutual, or mixed-assessment can be determined by regressing individual RHP against their persistence time when facing high- or low-RHP opponents and then comparing the slope and intercepts from the fitted lines. When pitted against low- and high-RHP opponents, positive slopes with equal intercepts would indicate pure self-assessment, while flat slopes with unequal intercepts would indicate pure mutual-assessment. We found that larger focal males persisted longer against low-frequency competitors relative to smaller focal males. These results would suggest that male green tree frogs are able to assess their own competitive prowess, i.e., self-assessment. However, we also found that when focal males were pitted against high-frequency opponents, there was no relationship between focal male RHP and their total time spent calling, which would indicate the use of a mutual-assessment strategy. It thus appears that green tree frogs may adjust their assessment strategies dependent on the perceived competitive ability of their opponent. Similar results have been seen in the mangrove rivulus fish (Kryptolebia marmoratus), where competitors can switch between assessment strategies (mutual to self) when contests escalated from non-physical to physical (Hsu et al. 2008). Our findings seem to indicate that males may switch between assessment strategies based on the perceived strength of their opponent, using mutual-assessment when facing competitively inferior opponents but switching to self-assessment when faced with more challenging competition.
An individual’s advertisement signal may provide information about the producing individual such as their location, behavioral motivation, size, and condition (Searcy and Beecher 2009; Halfwerk et al. 2014; Linhart and Fuchs 2015). Much of this information would benefit an eavesdropper as it could indicate an easy meal (Halfwerk et al. 2014), an area to avoid (Höbel and Kolodziej 2013), or whether or not to engage in an agonistic contest (Earley and Dugatkin 2002). In frogs, previous work has shown that males engaged in an agonistic contest can directly assess their opponent’s competitive abilities (Bee and Perril 1996; Bee et al. 1998), but whether they could also indirectly assess nearby competition, i.e., eavesdropping, was unclear. Here, we have shown that green tree frog males are capable of eavesdropping on nearby competing male, and were quicker to respond to and persisted longer against opponents based on their own size and the perceived competitive ability of nearby males. This eavesdropping-acquired, competitive information may factor into a male’s decision to engage in alternative reproductive tactics (i.e., satellite behavior: Humfeld 2008; Leary and Harris 2013). Previous work has shown stressed and poor condition male green tree frogs were more likely to engage in satellite behavior compared to non-stressed and better-conditioned males (Leary and Harris 2013). It was shown that while satellite behavior is a less successful reproductive strategy relative to calling (Humfeld 2008), it is an energetically inexpensive strategy which may still provide individuals the opportunity to mate without the energetic costs involved with calling. Our data, combined with previous studies, suggest that an individual’s decision to satellite may be multi-faceted, with body condition and information on competitive environment acting synergistically to mediate the employment of particular reproductive tactics.
For male frogs, producing advertisement calls is energetically taxing (Taigen and Wells 1985; Emerson 2001; Leary and Harris 2013) and potentially dangerous (e.g., predation risk; Halfwerk et al. 2014). Because of the costs associated with calling, it should benefit an individual to gauge their competitive ability and condition against the abilities and conditions of their potential opponent before producing any behavioral response (Maynard-Smith and Price 1974; Parker 1974; Briffa and Elwood 2009). Here, we saw in male green tree frogs that call frequency is negatively correlated with size, generating a useful metric by which males can use for their competitive decisions. Further, males appear capable of gauging their own competitive prowess against their competitors and assess whether to engage/persist in a contest. Previous research has shown that within a dyadic agonistic encounter, male frogs are able to directly assess the calls of their opponent and alter their behavior according to the opponent’s call characteristics (Bee and Perril 1996; Bee et al. 1998). However, this study is one of the first to show that male frogs are also capable of eavesdropping on nearby competitors and use the information gleaned from eavesdropping when making their own behavioral decisions. These findings open exciting new possibilities for research on male signal assessment. While call frequency was the main signal characteristic we focused on here, additional signal characteristics, such as call rate, may be assessed by males. Whether males attend to these additional signal components remains an open question.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Akçay Ç, Reed V, Campbell S (2010) Indirect reciprocity: song sparrows distrust aggressive neighbours based on eavesdropping. Anim Behav 80:1041–1047
Aquiloni L, Gherardi F (2010) Crayfish females eavesdrop on fighting males and use smell and sight to recognize the identity of the winner. Anim Behav 79:265–269
Arnott G, Elwood R (2008) Information gathering and decision making about resource value in animal contests. Anim Behav 76:529–542
Bee MA (2002) Territorial male bullfrogs (Rana catesbeiana) do not assess fighting ability based on size related variation in acoustic signals. Behav Ecol 13:109–124
Bee MA (2003) A test of the “dear enemy effect” in the strawberry dart-poison frog (Dendrobates pumilio). Behav Ecol Sociobiol 54:601–610
Bee MA (2015) Treefrogs as animal models for research on auditory scene analysis and the cocktail party problem. Int J Psychophysiol 95:216–237
Bee MA, Perril SA (1996) Responses to conspecific advertisement calls in the green frog (Rana clamitans) and their role in male-male communication. Behaviour 133:283–301
Bee MA, Perril SA, Owen PC (1998) Size assessment in simulated territorial encounters between male green frogs (Rana clamitans). Behav Ecol Sociobiol 45:177–184
Briffa M (2008) Decisions during fights in the house cricket, Acheta domesticus: mutual or self assessment of energy, weapons and size? Anim Behav 75:1053–1062
Briffa M, Elwood RW (2009) Difficulties remain in distinguishing between mutual and self-assessment in animal contests. Anim Behav 77:759–762
Burmeister S, Wilczynski W (2000) Social signals influence hormones independently of calling behavior in the treefrog (Hyla cinerea). Horm Behav 38:201–209
Chuang MF, Kam YC, Bee MA (2017) Territorial olive frogs display lower aggression towards neighbours than strangers based on individual vocal signatures. Anim Behav 123:217–228
Clotfelter ED, Paolino AD (2003) Bystanders to contests between conspecifics are primed for increased aggression in male fighting fish. Anim Behav 66:343–347
Dall SRX, Giraldeau LA, Olsson O, McNamara JM, Stephens DW (2005) Information and its use by animals in evolutionary ecology. Trends Ecol Evol 20:187–193
Earley RL (2010) Social eavesdropping and the evolution of conditional cooperation and cheating strategies. Phil Trans R Soc B 365:2675–2686
Earley RL, Dugatkin LA (2002) Eavesdropping on visual cues in green swordtail (Xiphophorus helleri) fights: a case for networking. Proc R Soc Lond B 269:943–952
Emerson SB (2001) Male advertisement calls: behavioral variation and physiological processes. In: Ryan MJ (ed) Anuran communication. Smithsonian Institution Press, Chicago, pp 36–44
Enquist M, Leimar O, Ljungberg T, Mallner Y, Segergahl N (1990) A test of the sequential assessment game: fighting in the cichlid fish Nannacara anomala. Anim Behav 40:1–14
Fain GL, Matthews HR, Cornwell MC, Koutalos Y (2001) Adaptation in vertebrate photoreception. Physiol Rev 81:117–151
Fellers G (1979) Aggression, territoriality, and mating behavior in North American treefrogs. Anim Behav 27:107–119
Garcia MJ, Paiva L, Lennox M, Sivaraman B, Wong SC, Earley RL (2012) Assessment strategies and the effects of fighting experience on future contest performance in the green anole (Anolis carolinensis). Ethology 118:821–834
Garcia MJ, Murphree J, Wilson J, Earley RL (2014) Mechanisms of decision making during contests in green anole lizards: prior experience and assessment. Anim Behav 92:45–54
Garcia MJ, Williams J, Sinderman B, Earley RL (2016) Ready for a fight? The physiological effects of detecting an opponent’s pheromone cues prior to a contest. Physiol Behav 149:1–7
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans: common problems and diverse solutions. University of Chicago Press, Chicago
Gingras B, Boeckle M, Herbst CT, Fitch WT (2012) Call acoustics reflect body size across four clades of anurans. J Zool 289:143–150
Halfwerk W, Jones PL, Taylor RC, Ryan MJ, Page RA (2014) Risky ripples allow bats and frogs to eavesdrop on a multisensory sexual display. Science 343:413–416
Hirschenhauser K, Gahr M, Goymann W (2013) Winning and losing in public: audiences direct future success in Japanese quail. Horm Behav 63:625–633
Höbel G (2010) Interaction between signal timing and signal feature preferences: causes and implications for sexual selection. Anim Behav 79:1257–1266
Höbel G, Gerhardt HC (2007) Sources of selection on signal timing in a tree frog. Ethology 113:973–982
Höbel G, Kolodziej RC (2013) Wood frogs (Lithobates sylvaticus) use water surface waves in their reproductive behaviour. Behaviour 150:471–483
Hsu Y, Lee S-P, Chen M-H, Yang SY, Cheng KC (2008) Switching assessment strategy during a contest: fighting in killifish Kryptolebias marmoratus. Anim Behav 75:1641–1649
Humfeld SC (2008) Intersexual dynamics mediate the expression of satellite mating tactics: unattractive males and parallel preferences. Anim Behav 75:205–215
Johnstone RA (2001) Eavesdropping and animal conflict. Proc Natl Acad Sci USA 98:9177–9180
Lado WE, Zhang D, Mennigen JA, Zamora JM, Popesku JT, Trudeau VL (2013) Rapid modulation of gene expression profiles in the telencephalon of male goldfish following exposure to waterborne sex pheromones. Gen Comp Endocrinol 192:204–213
Laird KL, Clements P, Hunter KL, Taylor RC (2016) Multimodal signaling improves mating success in the green tree frog (Hyla cinerea), but may not help small males. Behav Ecol Sociobiol 70:1517–1525
Leary CJ (2014) Close-range vocal signals elicit a stress response in male green treefrogs: resolution of an androgen-based conflict. Anim Behav 96:39–48
Leary CJ, Harris S (2013) Steroid hormone levels in calling males and males practicing alternative non-calling mating tactics in the green treefrog, Hyla cinerea. Horm Behav 63:20–24
Linhart P, Fuchs R (2015) Song pitch indicates body size and correlates with males’ response to playback in a songbird. Anim Behav 103:91–98
Marshall VT, Humfeld SC, Bee MA (2003) Plasticity of aggressive signaling and its evolution in male spring peepers, Pseudacris crucifer. Anim Behav 65:1223–1234
Maynard-Smith J, Price GR (1974) The logic of animal conflict. Nature 246:15–18
Moretz J (2003) Aggression and RHP in the Northern swordtail fish, Xiphophorus cortezi: the relationship between size and contest dynamics in male-male competition. Ethology 109:995–1008
Oldham RS, Gerhardt HC (1975) Behavioral isolating mechanisms of the treefrogs Hyla cinerea and H. gratiosa. Copeia 1975:223–231
Oliveira RF (2009) Social behavior in context: hormonal modulation of behavioral plasticity and social competence. Integr Comp Biol 49:423–440
Parker GA (1974) Assessment strategy and the evolution of fighting behaviour. J Theor Biol 47:223–243
Payne R (1998) Gradually escalating fights and displays: the cumulative assessment model. Anim Behav 56:651–662
Peake TM, McGregor PK (2004) Information and aggression in fishes. Learn Behav 32:114–121
Peake TM, Terry AMR, McGregor PK, Dabelsteen T (2002) Do great tits assess rivals by combining direct experience with information gathered by eavesdropping? Proc R Soc Lond B 269:1925–1929
Reichert MS (2014) Playback tests and studies of animal contest dynamics: concepts and an example in the gray tree frog. Behav Ecol 25:591–603
Reichert MS, Gerhardt HC (2013) Gray tree frogs, Hyla versicolor, give lower-frequency aggressive calls in more escalated contests. Behav Ecol Sociobiol 67:795–804
Reichert MS, Gerhardt HC (2014) Behavioral strategies and signaling in interspecific aggressive interactions in gray tree frogs. Behav Ecol 25:520–530
Rendall D, Owren MJ, Ryan MJ (2009) What do animal signals mean? Anim Behav 78:233–240
Ryan (2001) Anuran communication. Smithsonian Institution Press, Washington DC
Schrode KM, Ward JL, Vélez A, Bee MA (2012) Female preferences for spectral call properties in the western genetic lineage of Cope’s gray treefrog (Hyla chrysoscelis). Behav Ecol Sociobiol 66:1595–1606
Searcy WA, Beecher MD (2009) Song as an aggressive signal in songbirds. Anim Behav 78:1281–1292
Taigen TL, Wells KD (1985) Energetics of vocalization in an anuran amphibian. J Comp Physiol B 155:163–170
Taylor RC, Ryan MJ (2013) Interactions of multisensory components perceptually rescue túngara frog mating signals. Science 341:273–274
Taylor RC, Klein B, Stein J, Ryan MJ (2008) Faux frogs: multimodal signaling and the value of robotics in animal behavior. Anim Behav 76:1089–1097
Taylor RC, Klein B, Ryan MJ (2011) Inter-signal interaction and uncertain information in anuran multimodal signals. Curr Zool 57:153–161
Yorzinski JL, Patricelli GL, Bykau S, Platt ML (2017) Selective attention in peacocks during assessment of rival males. J Exp Biol 220:1146–1115
Acknowledgments
We thank Salisbury University for providing funding for equipment and field vehicles. The Henson School of Science Guerrieri summer fellowship program provided support for TB and HB. We also wish to thank Matthew Murphy, Caitlin Minton, and Shelby Nicole Ferrell for their assistance with field collections. We are grateful to two anonymous reviewers for improving the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
Handling and toe clipping were performed in accordance with The American Society of Ichthyology and Herpetologists’ “Guidelines for Use of Live Amphibians and Reptiles in Field and Laboratory Research.” All experiments were conducted under Salisbury University’s Institutional Animal Care and Use Committee (IACUC Protocol # SU 0036).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by K. Summers
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garcia, M.J., Cronin, A., Bowling, T. et al. Dueling frogs: do male green tree frogs (Hyla cinerea) eavesdrop on and assess nearby calling competitors?. Behav Ecol Sociobiol 73, 21 (2019). https://doi.org/10.1007/s00265-018-2632-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2632-1