Abstract
Many species gather in choruses to advertise and search for mates, creating noisy social environments that may impair effective communication. These challenges may be further compounded in mixed-species aggregations, where signals from different species overlap and mate-searching females may perceive heterospecific signals in more attractive relative timing positions. I conducted playback experiments with green treefrogs (Hyla cinerea) to test the effects of cross-species call interference on sexual communication and found that the sexes responded differently to conspecific and heterospecific calls. Females differentiated between call types and preferentially approached the conspecific call, indicating that they were not negatively affected by cross-species call interference. By contrast, males did not differentiate between conspecific and heterospecific calls irrespective of the presented call type and males avoided call overlap and called shortly after the offset of any interfering call. I suggest that the observed sex difference is a function of the time frames that the individual has to evaluate the call before initiating a behavioral response. Slower behavioral responses, like phonotaxis that allows for a repeated sampling of a call during approach, may facilitate finer discrimination. Fast behavioral responses, like timing one’s call relative to the call of a rival, may limit processing and result in higher permissiveness. As a result, similar call timing behavior in response to conspecific and heterospecific signals may be an artifact of strong selection for fast and precise call timing in the conspecific context and may essentially trap males into wasting time and energy interacting with heterospecifics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Successful mate choice relies on the ability of females to assess and respond to male displays (Bradbury and Vehrencamp 1998). Yet, whenever many individuals gather to display simultaneously, such as in leks or choruses, they create “noisy” social environments in which signal interference may reduce the ability of females to accomplish these tasks (Wollerman 1999; Schwartz et al. 2001a; Brumm and Slabbekoorn 2005). For example, a major problem when communicating in a frog chorus arises when high densities of displaying males, often compounded by males producing calls at high rates, results in acoustic interference (Wollerman 1999; Schwartz et al. 2001b). Two distinct forms of acoustic interference can be recognized, each with different consequences for communication. First, call overlap, either with individual calls of rival males or the background chorus as a whole, may obscure pertinent call characters, thus impairing discrimination and potentially leading to errors in communication (Wiley 1994, 2006; Langemann and Klump 2005). Indeed, females often discriminate against overlapping calls in favor of calls without such interference (e.g., Schwartz 1987; Bosch and Marquez 2000) and males alternate their calls such that instances of call overlap occur less often than would be expected by chance (e.g., Rosen and Lemon 1974; Grafe 1996). Second, even if calls do not overlap completely, the timing at which a male’s call is perceived relative to the call of another male can still strongly influence female preferences. This is because females of many species prefer the leading of two calls in close temporal succession (i.e., Dyson and Passmore 1988; Greenfield 1994; Grafe 1996; Höbel and Gerhardt 2007; but see Schwartz and Wells 1984; or Grafe 1999 for rare examples where females prefer lagging calls). Males generally avoid producing calls that would fall in unattractive positions; i.e., when females show leader preferences, males avoid producing lagging calls (Greenfield 1994; Grafe 1996; Höbel and Gerhardt 2007). Such call timing behavior has been observed in several taxa, including cicadas, katydids, and frogs (reviewed in Greenfield 1994, 2005) but is most well studied in anurans, where call timing serves as the most significant behavioral mechanism by which males reduce acoustic interference (Schwartz 1987; Klump and Gerhardt 1992; Grafe 1996; Höbel and Gerhardt 2007).
Challenges arising from conspecific call interference have received the most attention so far, yet mixed-species aggregations are common in nature (Hödl 1977; Aichinger 1987; see also Gerhardt and Schwartz 1994). While some studies have investigated effects of cross-species call overlap (Schwartz and Gerhardt 1989; Narins 1992; Wollerman 1999), detailed studies of the impact of heterospecific interference on female call timing preferences and male call timing behavior are rare (but see Schwartz and Wells 1984; Marshall et al. 2006). This is surprising, given the high stakes involved in interactions that can result in mating mistakes with heterospecifics.
I investigated cross-species call interference in green treefrogs (Hyla cinerea). This species displays in dense, often multi-species choruses (Oldham and Gerhardt 1975), and relies on long-range acoustic signals for mate choice (Gerhardt 1987), suggesting that they commonly encounter cross-species call interference. I tested three hypotheses about cross-species call interference, focused on female and male behavior, respectively. First, I tested the hypothesis that interference with heterospecific calls negatively affects mate choice in females. This hypothesis makes two predictions: (i) females should have difficulties recognizing or orienting towards the conspecific call if it is overlapped by a heterospecific one, and (ii) females should not approach the conspecific call if it is perceived in unattractive lagging position relative to a heterospecific call. Second, I tested the hypothesis that interference from heterospecific calls changes male calling behavior. This hypothesis also makes two predictions: (i) males should avoid call overlap with heterospecific calls, and (ii) males should engage in call timing adjustments with heterospecific calls, and how they do it should depend on the call timing preferences of females. If the preference that females show for leading conspecific calls extends also to heterospecific calls (i.e., if heterospecific calls become attractive when perceived in leading position), then males should avoid placing calls in the unattractive lagging position relative to both conspecific and heterospecific calls. On the other hand, if species recognition is not affected by leader preferences, then males confronted with heterospecific call interference should not show the precise call timing they typically exhibit when interacting with conspecific calls. Third, the full factorial design of my experiments also allowed me to evaluate the hypothesis that strong selection in the conspecific context carries over to responses to heterospecific calls. This hypothesis makes the prediction that any behavior shown by males and females to conspecific call interference should be similar to those shown in response to heterospecific call interference.
Because I had tested both males and females, I was able to examine whether the sexes differ in their response to cross-species acoustic interference. Males and females often react differently when confronted with the same social signals. For example, the sexes may differ in which signal feature they pay most attention to (i.e., body posture vs motion displays; Martins et al. 2005; Nava et al. 2009) or which signal type elicits a stronger behavioral response (i.e., conspecific vs heterospecific; Searcy and Brenowitz 1988; Bernal et al. 2007). Whether the sexes also differ in how strongly they are affected by acoustic interference of those social signals has, to my knowledge, never been tested. Given that signal interference is an inevitable challenge of communal signaling (Greenfield 1994, 2005), the existence and type of such sex differences may help further elucidate the sources of selection acting on signal timing behavior.
Finally, because I had tested frogs from two populations, one of which had an evolutionary history with the heterospecific calls used in this study (i.e., sympatric distribution) and one that did not (i.e., allopatric distribution), I was also able to examine the geographic variation in how frogs respond to cross-species call interference and whether a history of interaction with the heterospecific has selected for stronger responses.
Material and methods
Study species and study sites
Green treefrogs (H. cinerea) have been well studied in terms of their acoustic preferences. Females prefer average and low frequency calls over high-frequency calls (Gerhardt 1974; Höbel and Gerhardt 2003), louder calls, longer calls, and calls produced at higher rates (Gerhardt 1987). Females also show strong call timing preferences. If two calls overlap, females prefer the leading call, but as soon as there is a short silent interval of about 25 ms between consecutive calls, relative call timing no longer influences female choice (Klump and Gerhardt 1992; Höbel and Gerhardt 2007). Leading call preferences are strong (100 % of female will approach the leading of two identical calls; Höbel and Gerhardt 2007), but preferences for leading calls can interact with preferences for other call traits such as call amplitude, duration, or frequency (Höbel 2010). For example, the normally greater attractiveness of longer calls is lost if the longer call is presented in lagging position relative to a shorter but leading call (Höbel 2010); by contrast, a more attractive lower-frequency call remains attractive even when presented in lagging position (Höbel 2010). Male green treefrogs are very good at timing their calls such that they do not fall in the unattractive lagging position (Klump and Gerhardt 1992; Höbel and Gerhardt 2007; Höbel 2011).
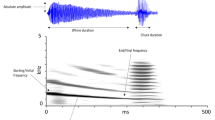
Green treefrogs, the focal species, and barking treefrogs (Hyla gratiosa), the heterospecific example used in this study, are broadly distributed in the southeastern part of the USA (Conant and Collins 1998). The vocal repertoires of these sister species (Wiens et al. 2010) are structurally and functionally similar, with calls mainly differing in frequency content (Oldham and Gerhardt 1975) (see Fig. 1(a, b) for sonograms of calls used as a basis for this study). Study ponds were located at Welder Wildlife Refuge (28.121823, -97.442929), San Patricio County, Texas (thereafter TX), and Hobcaw Barony (33.364020, -79.227008), Georgetown Co., South Carolina (thereafter SC). The population at the SC site is sympatric with H. gratiosa, while the one at the TX site is allopatric with H. gratiosa (Höbel and Gerhardt 2003). The majority of the experiments took place during the 2000 breeding season (May–August), but I returned to TX in 2001 to increase the sample size for the male playback trials (n = 5).
Spectrograms (top traces) and oscillograms (bottom traces) of a call of Hyla cinerea (a) and H. gratiosa (b) as well as spectrograms and oscillograms of the conspecific (c) and heterospecific (d) call stimuli used during playback trials. The three frequency components of the conspecific call stimulus (Hyla cinerea) were 0.9 + 2.7 + 3.0 kHz (c, top trace), and those of the heterospecific call stimulus (H. gratiosa) were 0.5 + 1.5 + 2.0 kHz (d, top trace). Temporal properties were identical (c, d, bottom traces)
Female preferences
Stimulus design
To test the effect of cross-species call overlap on female preferences, I used a custom-written program (courtesy of J.J. Schwartz) to generate conspecific and heterospecific call stimuli. The conspecific stimulus was modeled after a H. cinerea advertisement call, with acoustic properties set close to the mean values of the species (0.9 + 2.7 + 3.0 kHz; Gerhardt 1987) (Fig. 1(c), top trace). The heterospecific stimulus was modeled after a H. gratiosa advertisement call, with acoustic properties set close to the mean values of this species (0.5 + 1.5 + 2.0 kHz; Murpy and Gerhardt 1996) (Fig. 1(d), top trace). Both stimuli had a duration of 150 ms, a rise time of 25 ms (inverse exponential), and a fall time of 50 ms (inverse exponential; Fig. 1(c, d) bottom traces). I used editing software (Cool Edit 96; Syntrilium Software Corp., Phoenix, AZ, USA) to copy and paste stimuli with the appropriate spacing between consecutive signals.
For the female choice trials, I generated stereo files and set the signal period (time from the start of call to the start of following call) on both channels to 800 ms (see below for information on the relative timing of the presented alternatives). I conducted two trials in which I tested for the presence of a leader preference when females are confronted with identical calls: (i) the conspecific trial (CC50 %) presented two identical conspecific call stimuli whose relative timing was set to overlap by 50 %, and (ii) the heterospecific trial (HH50 %) presented two heterospecific call stimuli, again set to overlap by 50 % (see Fig. 2(a)).
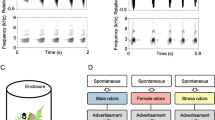
Effect of species call type on call timing preferences of female H. cinerea. a When given a choice between identical calls (both either conspecific or heterospecific), females in both study sites prefer the leading call. b When conspecific and heterospecific calls are presented together, females always prefer the conspecific one, irrespective of the relative timing relationship between the calls. Black symbols/bars and C denote conspecific calls, and gray symbols/bars and H denote heterospecific calls. Numbers above graphs indicate the sample size and P values of two-tailed binomial tests
To test for cross-species call interference, I conducted three trials in which I presented females with one conspecific stimulus and one heterospecific stimulus, presented at varying degrees of call overlap: (i) in the HCsyn trial, the two stimuli were presented synchronously (i.e., completely overlapping); (ii) in the HC50 % trial, the two stimuli overlapped by 50 %, with the heterospecific stimulus in leading position; and (iii) in the HCalt trial, the timing of the two stimuli was set so that they perfectly alternated with each other; i.e., in this trial, the conspecific stimulus and heterospecific stimulus were separated by equal amounts of silence between them (see also Fig. 2(b)). The HCsyn trial served to test the call overlap hypothesis, the HC50 % trial served as the test for the call timing hypothesis, and the HCalt trial served as the control test whether females indeed prefer conspecific calls if they are free from cross-species call interference. I did not conduct a CH50 % trial, because previous studies on both call timing (Höbel and Gerhardt 2007) and species recognition (Höbel and Gerhardt 2007) suggested that a leading, conspecific call would be more attractive than a lagging, heterospecific one; this trial would thus have constituted at best another control trial but would not have provided additional information for testing the cross-species call interference hypothesis.
Experimental procedure
I conducted the choice trials in a portable playback arena (area, 1 × 2 m; sides, 50 cm high) set up in the field (quiet location >500 m from the chorus). The sides of the arena were lined with black cloth (opaque, but acoustically transparent). The floor was made of plywood and had a 10 × 10 cm grid painted on it to aid in observing the movement of the females within the arena. Two speakers (RadioShack Optimus XTS-40; RadioShack Corporation, Fort Worth, TX, USA) were placed 2 m apart, facing each other along the central long axis and just outside the arena (i.e., speakers were not visible to the frogs). I used a laptop computer to broadcast acoustic stimuli at a sampling rate of 44 kHz and equalized the sound pressure level (SPL) of each stimulus to 85 dB SPL (re, 20 μPa) using a Lutron SL-4001 sound level meter (C weighting, fast response; Lutron Electronic Enterprise, Taipei, Taiwan).
I collected pairs in amplexus to ensure that all tested females would be sexually responsive. Pairs were collected during peak chorus activity (between 21:00 and 00:00 hours), and playback trials were conducted thereafter (i.e., between 01:00 and 04:00 hours on the night of capture). Before commencing the evening testing, I separated the females from their mates. Males were released immediately, and females were housed individually in small containers (8 × 8 × 5 cm) placed within a larger, sound-attenuated container (large cooler) until testing. I tested females sequentially, presenting one females with each of the five two-choice stimulus combinations before starting to test the next female. Individual trials lasted up to 5 min (the maximum allotted time; the average (±SD) response time was 64 ± 50 s; data not shown), and there was a rest period of at least 5 min between consecutive trials. All females were released at the site of capture within 1 day of being tested. Although I did not individually mark the released frogs, I am confident that I tested every female only once: female green treefrogs generally only mate once per season, and the few that remate take on average 3 weeks to produce another clutch (Perrill and Daniel 1983). Since I worked at each site for less than this time frame, the chances of recapturing a female were negligible.
For testing, I placed a female in a release box (small round hardware cloth cage, 10 cm in diameter) midway between the loudspeakers. After starting the playback of the experimental call stimuli, I waited until three repetitions had been presented to the females before removing the lid of the release box by pulling a string so that the female could move freely. The presence of the observer did not disturb the frogs, as they always oriented towards the speakers and did not appear to focus on the observer. I observed the frogs under dim red light to minimize disturbance and scored a positive response if a female touched the arena screen in front of the speaker, after having shown directed movement towards the stimulus.
Statistical analysis
I report the proportion of females that responded to each alternative in the two-choice trials. To test whether females preferred the leading of two identical calls (CC and HH trials) and to test whether females preferred a lagging conspecific over a leading heterospecific call (HC trials), I calculated two-tailed binomial tests (Rohlf and Sokal 1995). To test for geographic variation in female call timing preferences, I used a model with the proportions of females approaching the leading or lagging call stimulus (arcsine transformed), as the test variable, and site (TX, SC), trial (CC/HH), stimulus timing (lead/lag), and their interactions, as fixed effects. To test for geographic variation in cross-species call timing preferences, I used a model with the proportions of females approaching the conspecific or heterospecific call stimulus (arcsine transformed), as the test variable, and site (TX, SC), timing trial (HC trials), stimulus type (con/het), and their interactions, as fixed effects. Although I ran complete models with three terms and all their interactions, for brevity, I only report the results for (i) the three-way interaction terms (site × trial × timing or site × trial × call type, respectively), which test for geographic variation in cross-species signal timing, and (ii) for the timing/call type term, which corroborate the results of the binomial trials. Models were calculated in JMP 8.0 (SAS Institute, Cary, NC, USA).
Male calling behavior
To test the effect of cross-species call interference on male calling behavior, I presented focal males with playbacks of conspecific and heterospecific call stimuli and scored (i) whether they overlapped their calls with the playbacks and (ii) how they timed their calls relative to the playback stimuli.
Stimulus design
To examine the call timing behavior of male H. cinerea during call interactions with conspecific and heterospecific call playbacks, I generated conspecific and heterospecific call stimuli as described for female choice trials (above; Fig. 1(c, d)). Then, I used editing software (Cool Edit 96) to generate mono files that repeated the call stimuli (either conspecific or heterospecific). Periods of silence between successive calls ranged from 180 to 1800 ms (in 10 steps) in random order. I recorded the sound files onto cassette tapes, which I later used during field playback trials.
Experimental procedure
The playback setup consisted of a RadioShack Optimus XTS-40 speaker, driven by a Sony WM-D6C recorder and amplified by a RadioShack Optimus XL-50 Stereo Amplifier (RadioShack Corporation, Fort Worth, TX, USA). The signal from the playback recorder was split so it could be recorded on one channel of a Sony TC-D5M stereo tape recorder via the line input (Sony Corporation, Tokyo, Japan), while the other channel recorded the focal male using an Audio-Technica U.S. ATR55 microphone (Audio-Technica U.S., Inc., Stow, OH, USA). I later digitized (at 22.5 kHz) the recordings with Cool Edit 96.
I avoided adjusting playback levels in situ since this would have resulted in too much disturbance of the focal male. Rather, before starting trials each evening, I used a Lutron SL-4001 sound level meter (C weighting, fast response) to adjust the output gain setting of the playback recorder so that the call stimuli measured an amplitude of 80 dB SPL at 2-m distance from the speaker.
At the pond, I selected an isolated male (nearest neighbor >10 m) that was calling regularly and placed the speaker and the microphone, each mounted on tripods, 2 m away from it. I started experimental playbacks once the males resumed normal calling. I elicited male vocal responses with playbacks of synthetic call stimuli and presented each male with 100 stimulus repetitions per call type (conspecific and heterospecific); given the duration of the call stimuli and average intercall intervals (varied between 180 and 1800 ms, see above), each playback trial lasted about 100 s. Trials with different call types were presented in random order, with a rest period of 5 min, during which males resumed normal calling activity. After completing trials with a given male, I measured the actual stimulus amplitude at the location of the focal frog as well as the air temperature (to the nearest 0.1 °C). To verify that my experimental playbacks were louder than the local background noise, I measured the relative amplitudes of the playback stimuli and the natural background noise from each focal male’s experimental recordings (measured from the microphone-recorded track).
I presented call stimuli to 10 males from Texas and 17 males from South Carolina. The average SPL ± SD of the noise stimuli was 80 ± 2 dB, the average difference ± SD between call stimulus and natural chorus background noise at the location of focal males was 36 ± 5 dB, and the average temperature ± SD at the calling site was 24 ± 1 °C. There was no significant difference in stimulus amplitude (t test: t = 0.27, degree of freedom (DF) = 10.56, P = 0.79), stimulus to natural noise difference (t test: t = 0.40, DF = 11.12, P = 0.70), and temperature (t test: t = 0.36, DF = 11.94, P = 0.72) between sites.
Data analysis
Because males did not always respond to every presented call stimulus, the sample size per treatment (conspecific/heterospecific) varied between 9 and 95 (mode = 55) responses per male. I analyzed this data in terms of call overlap avoidance and call timing. To examine call overlap avoidance, I calculated the proportion of overlapping lagging calls, i.e., the calls that started after the onset of a playback stimulus such that they overlapped with that stimulus; I focused on overlapped lagging calls because these are discriminated against by females (Höbel and Gerhardt 2007). To examine call timing, I measured the latencies of all calls given after a playback stimulus (i.e., time from the end of stimulus to the beginning of male call, Fig. 3a); I focused on the stimulus offset because a previous study had determined that male H. cinerea use the offset of interfering stimuli to time their own calls (Höbel and Gerhardt 2007). From this latency data, I determined (i) the duration of post-stimulus suppression (i.e., the time period after an interfering stimulus during which calling is suppressed; duration is indicated by the timing of the earliest call the male gave after the end of a stimulus) and (ii) the modal call latency (i.e., the time period after an interfering stimulus at which interacting males trade calls; duration is indicated by the timing at which the male placed most of its calls) (see Fig. 3 for details). I chose the mode rather than the mean to describe call timing behavior because it more realistically reflects male call timing behavior (see Höbel and Gerhardt 2007; see also histograms in Fig. 4a). I consider suppression and modal latency to be different traits, because the timing of the first call after a stimulus might be due to a physiological, stimulus-induced suppression response, whereas the timing of most calls could be due to a male choosing a particular timing relationship between the stimulus and his call. Note that although individual males may have contributed up to 95 call latency measures per treatment, for the final analysis, I obtained only one post-stimulus suppression value and one modal call latency value per treatment per focal male.
Analysis of calling behavior in response to call stimulus playbacks. a The call latency measure; b which calls would be designated as overlapped calls, and how post-stimulus suppression and modal call latency are obtained (see “Material and methods”)
Call timing relative to conspecific (black) or heterospecific (gray) call stimuli. a Representative examples of call delay histograms from one males from TX and one male from SC. Shown is the frequency of calls that began within each of 40 consecutive 20-ms bins, with bin 1 starting at the onset of the playback stimulus (indicated by white oval). Calling was inhibited during and for a short time after the offset of the stimulus. Males from an allopatric population (TX, left panel) and a sympatric population (SC, right panel) showed similar call timing behavior in response to conspecific and heterospecific stimuli. (a–d). However, TX males overlapped more calls (b), showed longer post-stimulus suppression (c), and longer modal call latencies (d). Shown are means ± standard deviation
Statistical analysis
Because data from TX males was collected in two different years, I first confirmed that there was no effect of year (nonsignificant year terms for call overlap: F 1,8.8 = 2.14; P = 0.18; suppression: F 1,8.8 = 2.14, P = 0.18; and latency: F 1,8.2 = 1.37, P = 0.27). Thereafter, I focused on the effects of site, treatment, and their interaction on call timing behavior. To test whether the treatment stimulus type (conspecific/heterospecific) affected call overlap avoidance and call timing behavior, I used mixed models, implementing the restricted maximum likelihood method (REML). To test whether call overlap avoidance was different in response to interference by conspecific and heterospecific calls, I entered the proportion of overlapped calls (arcsine transformed), as the test variable, and playback treatment (con/het), study site, and their interactions, as fixed factors. To test whether call timing behavior was different in response to interference by conspecific and heterospecific calls, I entered poststimulus call suppression or modal call latency, respectively, as the test variable, and playback treatment (con/het), study site, and their interactions, as fixed factors. In all tests, I also entered focal male identity as a random factor to account for the fact that each male provided two data points to the analysis (response to conspecific and heterospecific call treatment, respectively). A significant treatment effect indicates that males time their calls differently in response to interference from conspecific and heterospecific calls, and a significant site effect indicates a geographic variation in call timing. A significant site × treatment interaction indicates that males from allopatric and sympatric sites respond differently to heterospecific call interference, suggesting that an evolutionary history with this heterospecific may have affected their call timing behavior. All statistical tests were performed using JMP 8.0 (SAS Institute, Cary, NC, USA).
Results
Female preferences
When given a choice between identical calls (both either conspecific or heterospecific) that differed only in relative timing, females preferred the leading call [in all but one cases, P = 0.02; in one case (HH trial in SC), females also preferred the leader, but not significantly so (P = 0.1); see Fig. 2(a) for sample sizes and exact P values]. However, presented with a choice between conspecific and heterospecific calls, females always preferred the conspecific call, independent of relative timing (in all cases, P < 0.02; see Fig. 2(b) for sample sizes and exact P values). This suggests that heterospecific call overlap did not reduce a female’s ability to recognize and localize conspecific calls.
There was no geographic variation in female call timing preferences; in both sites, females preferred the leading of two identical calls (significant effect of timing: F 1,1 = 550.3, P = 0.03, corroborating the result from the binomial tests; see Fig. 2(a)), and this pattern was similar across sites (nonsignificant effect of site × trial × timing: F 1,1 = 46.1, P = 0.09). More importantly, there was also no geographic variation in how females responded to cross-species call overlap; in both sites, females preferred the conspecific call irrespective of its position relative to the heterospecific one (significant effect of call type: F 1,1 = 135.6, P = 0.05, corroborating the result from the binomial tests; see Fig. 2(b)), and this pattern was similar across sites (nonsignificant effect of site × trial × call type: F 1,2 = 1.0, P = 0.58).
Male call timing adjustments
Males avoided call overlap equally well with conspecific and heterospecific call stimuli (Fig. 4a). The proportion of overlapped calls was quite low (mean 2.9 % in SC and 10.0 % in TX; see Fig. 4b), and there was no difference in the amount of calls overlapped with conspecific and heterospecific calls (nonsignificant treatment term: F 1,28.4 = 0.004, P = 0.95). The call delay histograms (Fig. 4a) show that calling was inhibited during stimulus presentation and, for a short time afterwards, with average post-stimulus suppression periods of 80 ms in TX and 31 ms in SC (see Fig. 4c) and average modal call latencies of 195 ms in TX and 51 ms in SC (see Fig. 4d). Treatment stimulus (conspecific or heterospecific call) had no effect on post-stimulus suppression (nonsignificant treatment term: F 1,25.1 = 2.14, P = 0.16) or modal call latency (nonsignificant treatment term: F 1,24.9 = 0.65, P = 0.43).
Males showed geographic variation in many aspects of their call timing behavior. Males from TX (allopatry) overlapped significantly more calls than males from SC (sympatry) (10 vs 2.9 %, significant site term: F 1,30.7 = 64.55, P < 0.0001, Fig. 3b), and they had longer post-stimulus suppression periods (80 vs 31 ms; significant site term: F 1,27.4 = 38.62, P < 0.0001, Fig. 4c), longer modal call latencies (195 vs 51 ms; significant site term: F 1,26.4 = 203.23, P < 0.0001, Fig. 4d), and generally less precise call timing behavior (see representative example of one male from each populations in Fig. 4a). These differences, however, did not constitute differential responses to conspecific and heterospecific stimuli (nonsignificant site × treatment interaction terms for call overlap: F 1,28.4 = 0.39, P = 0.54; suppression: F 1,25.1 = 3.28, P = 0.08; and latency: F 1,24.9 = 0.61, P = 0.44).
Discussion
I tested several hypotheses dealing with cross-species call interference in a treefrog and found that females were not negatively affected by cross-species call interference: they correctly approached the conspecific call, irrespective of whether it was overlapped or perceived in unattractive lagging position. Males, on the other hand, showed similar responses to conspecific and heterospecific call interference, i.e., avoiding call overlap with both types of calls and showing similarly high levels of call timing precision. Thus, there was a pronounced sexual difference in response to cross-species call interference: females clearly differentiated between both types of calls, while males responded to both call types in the same manner.
Effects of cross-species call interference on female phonotaxis and male calling behavior
Although mixed-species aggregations are common in nature, to my knowledge, only four frog species have been examined for effects of cross-species call overlap on female preferences, and the present study is the first to examine both the effects of cross-species call overlap and those of call timing behavior. Whether cross-species call overlap has a negative effect on call recognition varies among species: heterospecific call overlap did not affect the ability of female H. cinerea (this study) and Hyla chrysoscelis (Marshall et al. 2006) to respond to conspecific calls, while female Hyla versicolor (Marshall et al. 2006) and Dendropsophus (Hyla) ebraccatus (Schwartz and Wells 1984) made more recognition errors when conspecific calls were overlapped by those of another species. H. cinerea’s apparent immunity to cross-species call overlap may have its origin in the species’ single-note call structure and reliance on spectral call components for species and mate recognition (Gerhardt 1987). Because of the tuning of the anuran auditory organ, frequencies not corresponding to the conspecific call should be perceived as less intense (Capranica and Rose 1983), thus lowering the masking effect of heterospecific call overlap for species focusing on spectral call traits. By contrast, species with pulsed calls that often rely on temporal call traits for species and mate recognition should be, in general, more susceptible to masking or degradation of the relevant call structures during call overlap (Marshall et al. 2006). For example, both H. versicolor and H. chrysoscelis have pulsed calls, but the fast-pulsed H. chrysoscelis seems to be able to extract sufficient pulse rate information from short portions of nonoverlapped calls, thus making them less susceptible to negative effects of heterospecific call overlap, while the longer-pulsed H. versicolor appears not be able to achieve this (Marshall et al. 2006). Previous results from D. ebraccatus are more difficult to interpret, because this species has a composite call consisting of pulsed introductory notes that are usually followed by one or more click notes. Those click notes are preferred by females (Wells and Schwartz 1984). When testing the effect of heterospecific call overlap, Wells and Schwartz (1984) presented females with a conspecific call without click notes that was overlapped by a heterospecific call with click notes. Consequently, it is unclear whether the observed higher error rate was due to heterospecific call overlap obscuring the conspecific calls’ pulse pattern or whether females were attracted to the (unobscured) click notes of the heterospecific call. Nevertheless, most of the available data suggest that there is indeed a role for call structure in determining the severity of call overlap effects on call recognition.
To my knowledge, this is the first study to examine how a species with documented call timing preferences in the conspecific context deals with a situation in which a heterospecific call is perceived in the attractive leading position. In H. cinerea, the conspecific call is preferred even if it is presented in the unattractive lagging position relative to the heterospecific one; i.e., species recognition is robust with respect to call timing preferences. Whether this is due to the species’ single-note call structure and higher reliance on spectral call traits (see above) will remain unanswered until comparative data from a wider range of species (incl. more single-note as well as pulsed-call species) becomes available.
Male frogs call in response to both conspecific and heterospecific call stimuli (i.e., evoked calling) and adjust the temporal placement of their calls relative to a range of acoustic stimuli (i.e., call timing) (Schwartz and Wells 1984; Grafe 1999; Marshall et al. 2006; Amézquita et al. 2011; Penna and Velásquez 2011). Male H. cinerea avoided the call overlap equally well with heterospecific as well as conspecific calls. On the one hand, this is surprising because the frequencies of the heterospecific call used in the present study did not completely overlap with the critical frequency band used for communication in H. cinerea (only lower two thirds of call frequencies overlapped), which may have allowed males to be less strict in their overlap avoidance during heterospecific call interference. On the other hand, the results reported here are in line with data from other studies that report males avoiding call overlap with calls or tone bursts of different frequencies, even frequencies well outside the conspecific range (Zelick and Narins 1983; Grafe 1996).
Call overlap avoidance is one outcome of call timing behavior, which, depending on the species-specific rules employed, can also result in the production of leading or lagging calls (Greenfield 1994). Surprisingly, few studies have attempted to compare whether there is a difference in calling and call timing behavior during conspecific and heterospecific interactions, and because these studies involve species in which females either do not have strong call timing preferences (H. chrysoscelis; Klump and Gerhardt 1992) or have a lagging call preference (D. ebraccatus; Wells and Schwartz 1984), they are not straightforward to compare with the results from the present study with H. cinerea (where females prefer leading calls, i.e., discriminate against lagging ones). Male H. cinerea are equally good at avoiding placement of their calls in the unattractive lagging position relative to a conspecific or heterospecific call. Because both conspecific and heterospecific calls used here are similarly short (150 ms), the required reaction times to achieve successful call timing with either call type is also equally fast (in the tenths of millisecond range; see Fig. 4c), suggesting that the heterospecific call timing documented here is likely an artifact of strong selection for fast call timing in the conspecific context. By comparison, in D. ebraccatus, where males do differentiate when interacting with conspecific and heterospecific calls (Schwartz and Wells 1984), calls are about 1 s long and average delays of synchronized calls are in the range of 250 ms (Reichert 2012), i.e., an order of magnitude slower than the ones involved in call timing in H. cinerea.
In conclusion, despite the fact that mixed-species choruses are common in nature and the widespread observation of female call timing preferences and male call timing behavior (Greenfield 1994, 2005), we still lack sufficient data to elucidate the importance of cross-species call timing interactions for mate choice and sexual selection. Particularly interesting questions for future work would be to test what makes some species apparently more immune to cross-species interference than others (i.e., further tests of the signal structure hypothesis) or whether species that are more immune to cross-species call interference share ponds more frequently than species that show a high rate of interference-related mistakes in call identification.
Sex differences in response to cross-species call interference
The present study provides the first documentation of sex differences in response to conspecific and heterospecific call timing. Sex differences in other behavioral responses to social signals have been found in a variety of taxa. For example, female red-winged blackbirds give more courtship calls and solicitation displays in response to conspecific calls, while males show similar degrees of aggressive call responses to both conspecific calls and mockingbird imitation calls (Searcy and Brenowitz 1988). Male Sceloporus lizards pay more attention to stilted body posture displays, whereas females are more attentive to dynamic head bob displays (Martins et al. 2005). Male túngara frogs (Physalaemus pustulosus) give evoked vocal responses to a larger range of heterospecific and ancestral mating calls than females will approach during playback trials (Bernal et al. 2007).
Two main hypotheses have been proposed to explain sex differences in response to social signals. First, because of the different costs of recognition errors, theory predicts that males should be the more permissive sex and respond to a wider range of signals than females (Wiley 1994). This pattern has been found in frogs (Bernal et al. 2007, 2009), but results in birds are mixed, with males being more permissive in some species (Brenowitz 1982; Searcy and Brenowitz 1988; Danner et al. 2011), but females being more permissive in others (Vicario et al. 2001; Nelson and Soha 2004).
Second, in many animals, males and females perform different behaviors in response to the same social signal, and consequently, the type of behavior performed more so than the sex performing it may underlie those sexual differences (Bernal et al. 2009). In frogs, for example, females respond to a call by approaching it (phonotaxis), while males respond to a call by calling back (evoked vocal response) or, as in the present study, by adjusting the timing of their calls. This difference in behavioral tasks complicates direct comparisons between the sexes in permissiveness to social signals, but in the few cases in which species were amenable to test male and female responses using the same biological assay (either male and female phonotaxis or male and female evoked vocal response), studies found different patterns of sex differences than the one observed when assessing responses using sex-typical assays. For example, in both frogs and birds, males are generally the more permissive sex when sex differences are evaluated based on different behaviors, yet if the sexes are evaluated using the same behavior, male túngara frogs are not more permissive than females (Bernal et al. 2009), and female birds were actually found to be more permissive than males (Vicario et al. 2001; Nelson and Soha 2004).
Here, I propose an extension to this “behavioral task” hypothesis: sex differences may arise from different time scales at which behavioral decisions are made in response to a social signal, with faster responses (i.e., shorter processing times) resulting in higher permissiveness. For example, females generally use social signals to evaluate and localize mates, a process that can take considerable time and often allows females to sample several repetitions of the signal. This may allow them to discriminate between signals at a finer scale, which will manifest in lower permissiveness. By contrast, males often respond to a rival’s signal with their own, frequently trading signals in quick succession or quickly and precisely timing their signals relative to those of a rival. Response times involved in overlap avoidance and signal timing, for example, are often in the range of only tenths of milliseconds (Greenfield 1994, 2005; Höbel and Gerhardt 2007). Such short processing times may allow only for coarse “on/off” algorithm (i.e., calling during silence/not calling while an acoustic stimulus is perceived), which results in similar responses to a wide range of stimuli. This hypothesis makes two predictions: first, the sex that performs the slower response should be the more discriminating sex. Because females perform phonotaxis or courtship solicitation displays, while males respond to social signals with evoked calling and call timing, females are generally found to be the more discriminating sex (Brenowitz 1982; Searcy and Brenowitz 1988; Bernal et al. 2007; Danner et al. 2011). Second, a faster response should lead to higher permissiveness, both in intrasexual and intersexual comparisons. With the available data, this prediction cannot be evaluated for birds, because studies comparing response calling between male and female birds do not provide response time data but use the number of calls given as their assay (Vicario et al. 2001; Nelson and Soha 2004). However, this prediction is supported for call timing in frogs, with H. cinerea males with very short call delays (this study) being more permissive than D. ebraccatus males with longer call delays (Reichert 2012). Consequently, differences in the evaluation times involved in a behavioral response not only explain the existence of sex difference but also why females are generally found to distinguish between conspecific and heterospecific displays, while males are not. A corollary of this idea is that the term “permissive” does not really capture the evolutionary consequence of cross-species call timing behavior in H. cinerea males. Rather, the requirement for fast call timing adjustments in the conspecific context may generate an evolutionary trap that has males wasting time and energy interacting vocally with heterospecifics without gaining fitness advantages via female choice, because females would discriminate against those heterospecific calls anyway.
Geographic variation
An earlier study on cross-species effects on H. cinerea communication behavior had documented a pattern of reproductive character displacement (RCD) in the strength of preference for the conspecific call in females as well as differences in the calls and the call perches of males (Höbel and Gerhardt 2003). The present study extends this investigation to include cross-species call timing and found no evidence for RCD in call timing.
Females in both the allopatric and sympatric population preferred the conspecific call even in unattractive lagging position. It is possible that presenting a leading heterospecific call against a lagging conspecific one at equal amplitude (as done here) may not have been challenging enough to reveal RCD in call timing preferences. Future studies should successively attenuate the lagging conspecific call until females start to approach the (now louder) leading heterospecific call. Such a test may reveal that the amplitude difference at which the preference for the lagging conspecific call is lost is larger in sympatry compared to allopatry (which would constitute evidence for RCD).
Males showed pronounced geographic variation in call timing behavior, but this involved variation in how they responded to acoustic interference in general, not differences between allopatric and sympatric males in their response to conspecific and heterospecific calls. Two additional lines of evidence support the assertion that RCD is not involved in this variation in call timing behavior. First, male H. cinerea show a similar pattern of geographic variation (i.e., more precise timing in western compared to eastern sites) when vocally interacting with conspecific calls (Höbel and Gerhardt 2007; Höbel 2011). Höbel and Gerhardt (2007) suggest that this is related to geographic differences in other call traits, particularly call repetition rate. Since both high call rate (Gerhardt 1987) and precise call timing (Höbel and Gerhardt 2007) are attractive to females, males in different populations may pursue different strategies, with western frogs opting for fast but imprecise calling and eastern frogs opting for slow but precise timing. Second, gap detection, that is, the ability to place calls within short lulls in background noise, also did not show RCD—males in allopatric and sympatric populations showed similarly fast gap detection in conspecific or heterospecific background noise (Höbel 2014). A potential explanation for the lack of RCD in male call timing may be that strong selection for precise call timing in the conspecific context (Höbel and Gerhardt 2007; Höbel 2010), combined with the fast response times required for effective call timing in general (Höbel and Gerhardt 2007; Höbel 2011; see above), precludes any further accentuation of cross-species call timing in sympatry.
References
Aichinger M (1987) Annual activity patterns of anurans in a seasonal neotropical environment. Oecologia 71:583–592
Amézquita A, Flechas SV, Lima AP, Gasser H, Hödl W (2011) Acoustic interference and recognition space within a complex assemblage of dendrobatid frogs. Proc Natl Acad Sci U S A 108:17058–17063
Bernal XE, Rand AS, Ryan MJ (2007) Sex differences in response to nonconspecific advertisement calls: receiver permissiveness in male and female túngara frogs. Anim Behav 73:955–964
Bernal XE, Rand AS, Ryan MJ (2009) Task differences confound sex differences in receiver permissiveness in túngara frogs. Proc R Soc Lond B 276:1323–1329
Bosch J, Marquez R (2000) Acoustical interference in the advertisement calls of the midwife toads Alytes obstetricans and Alytes cisternasii. Behaviour 137:249–263
Bradbury JW, Vehrencamp SL (1998) Principles of animal communication. Sinauer Associates Inc, Sunderland
Brenowitz EA (1982) The active space of red-winged blackbird song. J Comp Physiol A 147:511–522
Brumm H, Slabbekoorn H (2005) Acoustic communication in noise. Adv Study Behav 35:151–209
Capranica RR, Rose G (1983) Frequency and temporal processing in the auditory system of anurans. In: Huber F, Markl H (eds) Neuroethology and behavioral physiology. Springer, Berlin, pp 136–152
Conant R, Collins JT (1998) A field guide to reptiles and amphibians: eastern and central North America, vol 12. Harcourt, Houghton Mifflin
Danner JE, Danner RM, Bonier F, Martin PR, Small TW, Moore IT (2011) Female, but not male, tropical sparrows respond more strongly to the local song dialect: implications for population divergence. Am Nat 178:53–63
Dyson ML, Passmore NI (1988) Two-choice phonotaxis in Hyperolius marmoratus (Anura: Hyperoliidae): the effect of temporal variation in presented stimuli. Anim Behav 36:648–652
Gerhardt HC (1974) The significance of some spectral features in mating call recognition in the green treefrog (Hyla cinerea). J Exp Biol 61:229–241
Gerhardt HC (1987) Evolutionary and neurobiological implications of selective phonotaxis in the green treefrog, Hyla cinerea. Anim Behav 35:1479–1489
Gerhardt HC, Schwartz JJ (1994) Interspecific interactions in anuran courtship. In: Heatwole H, Sullivan BK (eds) Amphibian biology. Vol. 3. Social behaviour. Surrey Beatty and Sons, Chipping Norton, pp 603–632
Grafe TU (1996) The function of call alternation in the African reed frog (Hyperolius marmoratus): precise call timing prevents auditory masking. Behav Ecol Sociobiol 38:148–158
Grafe TU (1999) A function of synchronous chorusing and a novel female preference shift in an anuran. Proc R Soc Lond B 266:2331–2336
Greenfield MD (1994) Synchronous and alternating choruses in insects and anurans: common mechanisms and diverse functions. Am Zool 34:605–615
Greenfield MD (2005) Mechanisms and evolution of communal sexual displays in arthropods and anurans. Adv Study Behav 35:1–62
Höbel G (2010) Interaction between signal timing and signal feature preferences: causes and implications for sexual selection. Anim Behav 79:1257–1266
Höbel G (2011) Variation in signal timing behavior: implications for male attractiveness and sexual selection. Behav Ecol Sociobiol 65:1283–1294
Höbel G (2014) Effect of temporal and spectral noise features on gap detection behavior by calling green treefrogs. Behav Process 108:43–49
Höbel G, Gerhardt HC (2003) Reproductive character displacement in the acoustic communication system of green treefrogs (Hyla cinerea). Evolution 57:894–904
Höbel G, Gerhardt HC (2007) Sources of selection on signal timing in a treefrog. Ethology 113:973–982
Hödl W (1977) Call differences and calling site segregation in anuran species from Central Amazonian floating meadows. Oecologia 28:351–363
Klump GM, Gerhardt HC (1992) Mechanisms and function of call-timing in male-male interactions in frogs. In: McGregor PK (ed) Playback and studies of animal communication. Plenum, New York, pp 153–174
Langemann U, Klump GM (2005) Perception and acoustic communication networks. In: McGregor PK (ed) Animal communication networks. Cambridge University Press, Cambridge, pp 451–480
Marshall VT, Schwartz JJ, Gerhardt HC (2006) Effects of heterospecific call overlap on the phonotactic behaviour of grey treefrogs. Anim Behav 72:449–459
Martins EP, Ord TJ, Davenport SW (2005) Combining motions into complex displays: playbacks with a robotic lizard. Behav Ecol Sociobiol 58:351–360
Murpy CG, Gerhardt CH (1996) Evaluating the design of mate-choice experiments: the effect of amplexus on mate choice by female barking treefrogs, Hyla gratiosa. Anim Behav 51:881–890
Narins PM (1992) Evolution of anuran chorus behavior: neural and behavioral constraints. Am Nat 139:S90–S104
Nava SS, Conway MA, Martins EP (2009) Sex-specific visual performance: female lizards outperform males in motion detection. Biol Lett 5:732–734
Nelson DA, Soha JA (2004) Male and female white-crowned sparrows respond differently to geographic variation in song. Behaviour 141:53–69
Oldham RS, Gerhardt HC (1975) Behavioural isolation of the treefrogs Hyla cinerea and Hyla gratiosa. Copeia 1975:223–231
Penna M, Velásquez N (2011) Heterospecific vocal interactions in a frog from the southern temperate forest, Batrachyla taeniata. Ethology 117:63–71
Perrill SA, Daniel R (1983) Multiple egg clutches in Hyla regilla, H. cinerea and H. gratiosa. Copeia 1983:513–516
Reichert MS (2012) Call timing is determined by response call type, but not by stimulus properties, in the treefrog Dendropsophus ebraccatus. Behav Ecol Sociobiol 66:433–444
Rohlf FJ, Sokal RR (1995) Statistical tables. Freeman, New York
Rosen M, Lemon RE (1974) Vocal behavior of spring peepers, Hyla crucifer. Copeia 1974:940–950
Schwartz JJ (1987) The function of call alternation in anuran amphibians: a test of three hypotheses. Evolution 41:461–471
Schwartz JJ, Gerhardt HC (1989) Spatially mediated release from auditory masking in an anuran amphibian. J Comp Physiol A 166:37–41
Schwartz JJ, Wells KD (1984) Interspecific acoustic interactions of the neotropical treefrog, Hyla ebraccata. Behav Ecol Sociobiol 14:211–224
Schwartz JJ, Buchanan BW, Gerhardt HC (2001a) Female mate choice in the gray treefrog (Hyla versicolor) in three experimental environments. Behav Ecol Sociobiol 49:443–455
Schwartz JJ, Buchanan BW, Gerhardt HC (2001b) Acoustic interactions among male gray treefrogs, Hyla versicolor, in a chorus setting. Behav Ecol Sociobiol 53:9–19
Searcy WA, Brenowitz EA (1988) Sexual differences in species recognition of avian song. Nature 322:152–154
Vicario DS, Naqvi NH, Ranksin JN (2001) Sex differences in discrimination of vocal communication signals in a songbird. Anim Behav 61:805–817
Wells KD, Schwartz JJ (1984) Vocal communication in a neotropical treefrog, Hyla ebraccata: advertisement calls. Anim Behav 32:405–420
Wiens JJ, Kuczynski CA, Hua X, Moen DS (2010) An expanded phylogeny of treefrogs (Hylidae) based on nuclear and mitochondrial sequence data. Mol Phylogenet Evol 55:871–882
Wiley RH (1994) Errors, exaggeration and deception in animal communication. In: Real LA (ed) Behavioral mechanisms in evolutionary ecology. University of Chicago Press, Chicago, pp 157–189
Wiley RH (2006) Signal detection and animal communication. Adv Study Behav 36:217–247
Wollerman L (1999) Acoustic interference limits call detection in a Neotropical frog Hyla ebraccata. Anim Behav 57:529–536
Zelick R, Narins PM (1983) Intensity discrimination and the precision of call timing in two species of neotropical treefrogs. J Comp Physiol A 153:403–412
Acknowledgments
This work was supported by grants from the German Academic Exchange Service and Graduiertenförderung des Landes Baden-Württemberg, Germany. I thank G. Ehret and H.C. Gerhardt for logistic support and R. L. Rodriguez and two anonymous reviewers for their helpful suggestions in improving the manuscript.
Ethical standards
All experiments reported in this article comply with the current laws of the country in which they were performed and were approved by the Animal Care and Use Committee of the University of Missouri (ACUC protocol no. 1910).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Gibbons
Rights and permissions
About this article
Cite this article
Höbel, G. Sexual differences in responses to cross-species call interference in the green treefrog (Hyla cinerea). Behav Ecol Sociobiol 69, 695–705 (2015). https://doi.org/10.1007/s00265-015-1880-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-1880-6