Abstract
Some territorial animals exhibit a form of social recognition, commonly termed the "dear enemy effect", in which territory residents display lower levels of aggression toward familiar neighbors compared to unfamiliar individuals who are non-territorial "floaters". Despite the widespread occurrence of territorial social systems and use of acoustic signals for communication in anuran amphibians, only two previous studies have demonstrated vocally mediated dear enemy behavior in a territorial frog. In this study, I conducted neighbor-stranger discrimination playback experiments in a third species of territorial frog, the strawberry dart-poison frog, Dendrobates pumilio (Anura, Dendrobatidae). In the first experiment (n=24), I broadcast the calls of a subject's nearest neighbor and the calls of an unfamiliar individual from the approximate midpoint between the subject's and the neighbor's territories. Although males responded to the stimuli, they did not exhibit differential responses to the calls of neighbors and strangers. In a second experiment (n=22), I broadcast the calls of a neighbor and a stranger to subjects through a speaker located in the approximate center of the neighbor's territory. Males also responded to the playback, although less intensely than in the first experiment, but no discrimination between the calls of neighbors and strangers was found. Thus, territorial males of the strawberry dart-poison frog appear not to discriminate behaviorally between the advertisement calls of neighbors and strangers. Several proximate and ultimate-level hypotheses for this lack of vocally mediated neighbor-stranger discrimination are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals in a number of taxa exhibit the capability to learn to recognize other conspecific individuals based on idiosyncratic phenotypic features (Colgan 1983; Sherman et al. 1997). Social recognition is thought to enhance fitness by providing a mechanism that allows animals to direct appropriate behaviors toward specific individuals during repeated social interactions. One form of social recognition that occurs in territorial species has been variously termed "the dear enemy effect" or "neighbor recognition" (reviews in Ydenberg et al. 1988; Temeles 1994). Evidence for the dear enemy effect typically consists of a relatively lower level of aggression exhibited by territory holders toward established territorial neighbors in comparison to unfamiliar individuals. By exhibiting lower levels of aggression toward their nearby neighbors, territory residents are generally assumed to avoid the costs associated with repeated agonistic encounters with individuals that pose relatively little threat to stable territory ownership.
Dear enemy relationships, however, are not ubiquitous among territorial species, and several studies have reported that territory residents respond similarly to neighbors and strangers under some conditions (reviewed in Temeles 1994). Based on an extensive literature review, Temeles (1994) argued that displays of reduced aggression toward a neighbor depended on the relative threats posed by neighbors and strangers, and not simply on the degree of familiarity between adjacent neighbors. The relative threat of neighbors versus strangers was further hypothesized to depend on the type of defended territory. Temeles (1994) proposed that the costs associated with the long-term defense of multi-purpose, breeding territories favor situations in which neighbors behave as dear enemies, because neighbors, which already posses a territory, represent a potential threat to a resident's ability to acquire mates, whereas a stranger represents potential threats to both mate acquisition and territory ownership (Temeles 1994). To date, however, most of the data supporting Temeles' (1994) hypothesis are derived from studies of birds and mammals. Of the 47 species exhibiting the dear enemy phenomenon that were listed in Temeles' (1994) review, 40 of these (85%) were birds or mammals (40% were songbirds), compared to 3 species of reptiles (6%), 2 species of amphibians (4%), and 2 species of insects (4%). This biased representation of birds (especially songbirds) and mammals probably stems from the expectation that these taxa would exhibit the dear enemy phenomenon, and hence their selection as suitable study organisms (Temeles 1994), and from the difficulty of interpreting and publishing negative results when the dear enemy effect is not observed. Unfortunately, the strong taxonomic bias in previous research on the dear enemy effect makes it difficult to know the general extent to which the costs associated with the long-term defense of multi-purpose, breeding territories are able to select for dear enemy recognition across taxa.
Playback studies show that territorial male songbirds exhibit lower levels of aggression in response to broadcasts of their neighbor's vocalizations from the direction of the neighbor's territory in comparison to broadcasts of the vocalizations of an unfamiliar individual from the same location (reviewed in Stoddard 1996). As in songbirds, vocal communication plays a fundamental role in the social behavior of anuran amphibians (Wells 1977; Gerhardt and Huber 2002), and many species of frogs in different families have mating systems in which males establish and defend a long-term multi-purpose territory that functions as a breeding resource for females, which deposit their eggs in the male's territory (Wells 1977). To date, however, only two studies have reported vocally mediated dear enemy behavior in territorial frogs. Davis (1987) demonstrated that territorial males of the North American bullfrog, Rana catesbeiana (Anura, Ranidae), responded more aggressively to the advertisement calls of an unfamiliar individual than to those of a neighbor when both kinds of calls were broadcast from the direction of the neighbor's territory. Bourne et al. (2001) recently reported similar results for territorial males of the frog Colostethus beebei (Anura, Dendrobatidae).
Here, I report results from a playback study investigating whether territorial males of the strawberry dart-poison frog, Dendrobates pumilio (Anura, Dendrobatidae), discriminate behaviorally between the acoustic signals of territorial neighbors and unfamiliar individuals. D. pumilio is a common and well-studied frog found in lowland forests along the Atlantic coast of Central America from Nicaragua to Panama. As in other dendrobatid frogs (Duellman and Trueb 1986), D. pumilio has a complex mating system that involves prolonged courtship, territorial oviposition, and parental care (Limerick 1980; Weygoldt 1980; Pröhl 1997; Pröhl and Hödl 1999). I chose to examine the dear enemy effect in D. pumilio for several reasons. First, males defend multi-purpose, breeding territories that contain food resources, calling sites, courtship areas, oviposition sites, and perhaps also tadpole-rearing sites (Donnelly 1989a, 1989b, 1991; Pröhl 1997; Pröhl and Hödl 1999; Pröhl and Berke 2001). Second, territorial males commonly have one to three territorial neighbors, and individual males can maintain the same territories for extended periods, from several weeks or months to perhaps as long as a few years (Donnelly 1989a; Pröhl 1997; Pröhl and Hödl 1999; Pröhl and Berke 2001). Third, acoustic communication plays important roles in social behavior and territory defense (Bunnell 1973; Donnelly 1989a; Forester et al. 1993; Pröhl and Hödl 1999; Pröhl 2003). Fourth, Pröhl (2003) recently reported that the dominant frequency, call rate, call duration, call duty cycle, and pulse rate of advertisement calls of male D. pumilio exhibit highly significant among-individual variation. Hence, because the mating system of D. pumilio is characterized by the long-term defense of multi-purpose, breeding territories, and because vocalizations play a role in territory defense and exhibit significant among-individual variation, we might expect that males of this species would exhibit lower levels of aggression in response to the vocalizations of neighbors compared to those of strangers (Temeles 1994). To test this hypothesis, I conducted a neighbor-stranger discrimination playback experiment.
Methods
Between 6 October and 26 November 2000, I conducted two field playback experiments in the Archipelago de Bocas del Toro, Republic of Panama. My study area consisted of a 420-m2 forest plot located near Playa del las Ranas Rohas on Isla Bastimentos. Approximately 30 males held territories in this plot and were observed calling throughout the study. Twenty-eight of these males were captured and individually marked by clipping the toes of the hind feet. Male territories were usually associated with discrete habitat structures (e.g., fallen logs or branches, patches of vegetation). The distances between adjacent calling territorial neighbors ranged from 1.3 m to 4.0 m (mean=2.7 m, n=24 inter-male distances) and were similar to previous reports of territorial spacing in this species (Bunnell 1973; Donnelly 1989a; Pröhl and Hödl 1999). I marked the approximate center of each male's territory with surveyors' flagging after noting the male's calling positions over a period of several days. Although males sometimes foraged away from the center of their territories, calling males were always observed within approximately 1 m of my estimate of the center of the territory. All observations, recordings, and playback tests were conducted during the daily period of peak calling activity between 0730 and 1200 hours (Pröhl 1997).
Playback stimuli
Advertisement calling by male D. pumilio consists of short, pulsed advertisement calls that are repeated in discrete call groups that consist of a few calls to several hundred calls (Fig. 1). During the first 3 weeks of the study, I recorded 5–20 call groups from each of 26 of the territorial males in my study plot, using a Sennheiser ME67 shotgun microphone and a Sony WM D6C portable cassette recorder. Call groups from 13 of these frogs were used to generate 13 different neighbor (N) stimuli for use in subsequent playback tests. I also recorded 5–20 call groups from each of 3 frogs that held territories on the opposite side of the island to generate 3 "stranger" (S) stimuli. These frogs were located approximately 0.5–1.0 km away from my study site, and I assume that they were unfamiliar to the subjects in this study. Five to ten call groups (usually 10) from each of these 16 individuals were digitized (16-bit resolution; sampling rate=22.05 kHz) and then bandpass filtered between 2 and 7 kHz to reduce background noise due to wind, surf, and other signaling animals, using GoldWave 4.02 sound-editing software running on a Dell Inspiron 5000 portable computer. Playback stimuli were generated by editing the call groups of individual males into separate 5-min sequences of ten call groups that approximated the call-group rate of an actively calling frog (1–3 call groups/min; M.A. Bee, unpublished data). In the few instances where fewer than ten call groups were recorded from a frog, I duplicated haphazardly selected call groups from the frog, using the copy/paste functions of the editing software to create a sequence of ten total call groups. I never used fewer than five different call groups from each frog. Using the digital-to-analogue output of the computer, I recorded the digitized stimulus sequences onto high-quality audio cassette tapes with the Sony recorder. Stimuli were broadcast using the Sony recorder and an Optimus AMX 10 amplified speaker at a sound pressure level (SPL) of 67–70 dB (re 20 μPa, fast RMS, flat-weighting) measured at a distance of 50 cm in the field. Playback SPLs were measured following the completion of each playback test. These playback levels approximate the natural SPLs of D. pumilio calls measured at 50 cm (M.A. Bee, unpublished data). Sound pressure levels were measured with a GenRad 1982 sound-level meter.
Playback protocol
I conducted two separate experiments using a within-subjects design, in which each subject was presented with the pre-recorded call groups of one of its adjacent neighbors (N) and a stranger (S). Experiment 1 (n=24) was conducted between 17 October and 12 November, and experiment 2 (n=22) was conducted between 6 and 21 November. The important difference between experiments 1 and 2 was that I placed the playback speaker approximately halfway between the subject and the center of the neighbor's territory in experiment 1, and in the approximate center of the neighbor's territory in experiment 2. In the second experiment, I opportunistically re-tested 22 of the 24 subjects that had been previously tested in experiment 1. Subjects in experiment 2 were re-tested a median of 18 days (range=2–29 days) after they had been tested in the first experiment. Because males experienced only 10 min of playbacks during a test (see below), and because the playbacks in the two experiments were usually separated by several days, I assume that playbacks in experiment 1 had no carryover effects (e.g., long-term habituation) on responses in experiment 2. Previous studies (e.g., Pröhl 1997, 2002) have observed calling by territorial males over a time period that extends beyond that covered in the present study, and I noticed no seasonal changes in calling and territorial behavior; therefore, I also assume that any response differences arising between experiments 1 and 2 were not the result of seasonal changes in aggressive behavior.
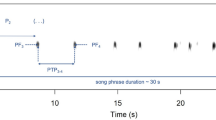
Both experiments consisted of five consecutive 5-min observation periods (Fig. 2). The first observation period was always a pre-stimulus period during which I recorded the subject's baseline behavior (Pre). During the second and fourth periods, I broadcast the N and S stimuli. The N stimulus always consisted of the pre-recorded call groups of a male that held a territory adjacent to that of the subject. In experiment 1, half of the subjects heard the N stimulus during period 2 and the S stimulus during period 4, and the other half heard the opposite stimulus arrangement (Fig. 2). The intervening third period served as a 5-min post-stimulus observation period for the first stimulus (Post). The fifth period was a 5-min post-stimulus observation period for the second stimulus (Post). Observation periods 3 and 5 each served as Post-N and Post-S observation periods for half of the subjects. In experiment 1, tests were completed in four temporal blocks comprising six playback tests. In each block, I used two different stimulus sequences: three subjects were tested with the sequence Pre—N—Post-N—S—Post-S, and three subjects were tested with the sequence Pre—S—Post-S—N—Post-N (see Fig. 2). Each of the three S stimuli was used twice in each block, once in each sequence (4 blocks×2 sequences×3 S stimuli=24 tests). The order in which tests were completed in each block was randomly determined. Subjects were tested in experiment 2 with the same stimulus sequence that had been used in experiment 1. Two subjects that had heard the S stimulus during the second observation period in experiment 1 could not be relocated at the time of the second experiment. Twenty subjects were re-tested with the same N and S stimuli; two subjects, however, were tested with the same S stimulus, but a different N stimulus, because the frog that previously had served as the donor of the N stimulus in experiment 1 was no longer resident on its territory at the time of experiment 2. Two subjects had moved to a new position at the time they were tested in experiment 2 (both approximately 1 m from the original locations in experiment 1). Both of these subjects were tested with the same N stimulus, however, because this neighbor still held an adjacent territory.
For the majority of tests, the neighbor was removed from its territory, temporarily stored in an acoustically opaque plastic container, and returned to the center of its territory after the completion of the test. Neighbors were held captive for, at most, 40 min. In no instances did I observe that a new individual attempted to take over the vacant territory of the neighbor or that a removed individual failed to take possession of its territory after being released. Individuals were never removed from their territory and then later tested on the same day after being released. In the few instances where the neighbor was not removed, I was located in a convenient position to ensure that the neighbor did not call and was not visible to the subject during the test. I waited at least 10 min after positioning the speaker before beginning a playback test. The playback speaker was usually placed on the ground, but in a few instances when a subject's neighbor usually called from an elevated calling site, I mounted the speaker at an appropriate height on a tripod. At the beginning of tests in both experiments, subjects were either actively calling or foraging near the centers of their territories, or they were sitting silently in plain view near the centers of their territories and had been observed calling or foraging just prior to the beginning of the test. I did not begin tests if the subject was actively courting a female that was present on its territory or interacting with a neighbor.
Data analysis
For a male to be included as a subject in the data set, I required that it exhibit a clear behavioral response, in the form of turning to face the playback speaker, during at least one of the playback periods in experiment 1. Eight males initially failed to orient to the speaker during the playback periods and I assumed they were non-responsive. Results from these tests were not analyzed. I successfully re-tested six of these males in experiments 1 and 2 on later dates, and the data from these tests were included in statistical analyses. In anurans, the intensity of aggressive responses varies with the perceived proximity of the calling individual, as indicated by the stimulus SPL (e.g., Schwartz 1989; Wagner 1989), and individuals fail to respond aggressively to broadcasts of calls below a certain threshold SPL (e.g., Brenowitz and Rose 1994; Marshall et al. 2003). Similar results have been found in D. pumilio (Bunnell 1973). Therefore, because subjects in experiment 2 were expected a priori to exhibit less-intense responses to playbacks from the center of the neighbor's territory, in which playback SPLs measured at the subject's calling site were approximately 6 dB less than in experiment 1, I did not apply the same orientation criterion in experiment 2.
In response to acoustic playbacks, male frogs commonly orient to the sound source, increase their calling rate, exhibit positive phonotaxis toward the sound source, repeatedly move about by hopping, walking, or swimming, presumably in search of the intruder, and in some cases produce aggressive signals (e.g., Schwartz 1989; Wagner 1989; Zimmermann 1990; Bee et al. 1999; Bee 2003). Similar behaviors have been reported in D. pumilio (Bunnell 1973; Forester et al. 1993). The response measures that I chose to record during playback tests were based on changes in calling rate and phonotaxis behavior. During each 5-min observation period, I recorded four response variables as measures of aggressive responsiveness to the playbacks. I counted (1) the number of call groups, and (2) the number of movements. A movement was defined as any forward or lateral motion followed by at least a brief motionless period. Rapid sequences of hops in one direction were counted as one movement; a change of position within the same location (i.e., turning around) was not counted as movement. I also determined (3) the subject's closest approach to the speaker, measured from the speaker to the frog, and (4) the maximum absolute distance that the subject approached toward the speaker, measured from the subject's position at the start of the observation period to its position of closest approach during the period. Distances were measured to the nearest 5 cm immediately after a test was completed. I also measured the distance between the subject and the appropriate neighbor following the completion of each test. Male D. pumilio have been reported to use a visual "push-up" display during aggressive encounters (Baugh and Forester 1994). However, I did not observe push-up displays in response to the playbacks in this study. A number of previous studies (e.g., Bunnell 1973; Zimmermann 1990) have also reported the use of aggressive calls by male D. pumilio during territorial encounters. To date, however, there have been no quantitative studies of these signals to describe their usage and how they differ from advertisement calls. Zimmermann (1990) described both advertisement calls and aggressive calls in D. pumilio as "buzz calls", and advertisement calls may grade into aggressive calls, as they appear to in the closely related D. histrionicus (Zimmermann 1990). Thus, because it remains unclear precisely what constitutes aggressive signaling in D. pumilio, I preferred to assess the more robust measures of changes in calling rate and phonotaxis, as opposed to classifying signals as advertisement calls or aggressive calls. Future work will be required to fully characterize aggressive signaling in D. pumilio and assess its potential importance for dear enemy behavior.
All four response variables were log-transformed [Y′=log10(Y+1)] to improve normality. I analyzed the four dependent variables across the five observation periods using repeated measures multivariate analysis of variance (MANOVA). In preliminary analyses, I included stimulus sequence (two levels) as a between-subjects factor. There were, however, no significant effects of stimulus sequence (Ps>0.40) or interactions between stimulus sequence and the repeated measures (Ps>0.55) in these analyses; therefore, I did not include this between-subjects factor in further statistical analyses. After analyzing the data using MANOVA to account for any possible correlations among variables, each dependent variable was subsequently analyzed in a univariate repeated-measures analysis of variance (ANOVA). I used the Greenhouse and Geiser (1959) method to correct the P-values obtained in omnibus tests of repeated-measures effects with greater than a single degree of freedom. I chose to conduct univariate analyses even when the overall MANOVA was non-significant for the purpose of calculating effect sizes for each response variable (see below).
In each experiment, I tested two specific hypotheses, using focused contrasts (Rosenthal and Rosnow 1985) to assign appropriate contrast weights (λ) to different observation periods. The first contrast analysis tested the hypothesis that response magnitudes were greater during the playback periods compared to the pre- and post-stimulus observation periods (λ Pre=λ Post-N=λ Post-S=−2; λ N=λ S=+3). Second, to determine whether males discriminated behaviorally between the vocalizations of neighbors and strangers, I tested the hypothesis that the dependent variables differed in response to the N and S stimuli (λ Pre=λ Post-N=λ Post-S=0; λ N=+1; λ S=−1). I also tested this hypothesis in between-subjects analyses that compared the responses of different groups of subjects to the N and S stimuli in the second observation period, during which each subject heard either the N or the S stimulus. These between-subjects analyses effectively removed all possibility of a confounding effect of stimulus presentation order in the data analysis.
Statistical analyses were performed with Statistica 5.5 and SPSS 11.0. For all univariate statistical tests, I computed effect sizes (partial η 2) and the observed statistical power of the test. Partial η 2, which varies from 0 to 1, represents the proportion of the combined effect and error variance that is attributable to the effect, and thus represents a non-additive "variance-accounted-for" measure of effect size, which serves as an estimate of the extent to which the null hypothesis is false. This measure is a generalization of the more familiar coefficient of determination (r 2) associated with tests of differences between two groups (see Cohen 1988 and Rosenthal and Rosnow 1991 for further discussion). A significance criterion of α=0.05 was used for all statistical tests.
Results
Experiment 1: playbacks from between the territories
The average (±SE) distance between the subject and the speaker at the start of a test was 1.4±0.4 m. This distance was, on average, 54.3±11.1% of the distance from the subject to the neighbor's position at the start of a test. In responses to the playbacks in the first experiment, subjects typically oriented toward the speaker during playbacks of both the N and S stimuli. Subjects commonly left their original calling site and hopped or walked toward the speaker. These subjects appeared to be searching for another frog. During the post-stimulus observation periods, subjects usually either remained silent and motionless or returned to their original calling site. Figure 3 (a–d) depicts the magnitude of responses during the five observation periods of experiment 1. MANOVA revealed significant differences across observation periods (Wilks' λ=0.08; F 16,8=6.04; P=0.0073). Univariate repeated-measures ANOVAs revealed significant differences across the five observation periods for the numbers of call groups and movements, the closest approach, and the maximum approach distance (Table 1).
Results from experiment 1 (a–d) and experiment 2 (e–h) showing means (±SE) for the number of call groups (a, e), the number of movements (b, f), the subject's closest approach toward the speaker (c, g), and the maximum approach distance (d, h) during each observation period of the test. The pre-stimulus observation period (Pre) is depicted by white bars; the neighbor-stimulus (N) and post-neighbor-stimulus (Post-N) observation periods are denoted by black bars; and the stranger-stimulus (S) and post-stranger-stimulus (Post-S) observation periods are denoted by gray bars. Note that the order of observation periods along the x-axes does not necessarily reflect the order of stimulus presentations (see text and Fig. 2). The speaker was located approximately halfway between the center of the subject's territory and the center of the neighbor's territory in experiment 1 and in the center of the neighbor's territory in experiment 2
The contrast analysis for the hypothesis that response magnitudes were greater during playback periods revealed significant effects in the overall MANOVA (Wilks' λ=0.32; F 4,20=10.19; P=0.0001) and in the univariate analyses for call groups, movements, closest approach, and maximum approach distance (Table 1), indicating that subjects were responsive during the two playback periods. However, a direct test of the hypothesis that subjects discriminated behaviorally between the calls of neighbors and strangers failed to reveal any significant differences in the overall MANOVA (Wilks' λ=0.94; F 4,20=0.30; P=0.8740) or in the univariate analyses of the number of call groups, the number of movements, closest approach, or maximum approach distance (Table 1). In between-subjects comparisons of responses to the N and S stimuli in the second observation period, there was no significant difference in an overall MANOVA (Wilks' λ=0.94; F 4,19=0.29; P=0.8861), and there were no significant differences in univariate analyses of the numbers of call groups (F 1,22=0.12, P=0.7317, η 2<0.01, power=0.06) and movements (F 1,22=0.06, P=0.8125, η 2<0.01, power=0.06), the closest approach (F 1,22=0.44, P=0.5131, η 2=0.02, power=0.10), and the maximum approach distance (F 1,22=0.78, P=0.3868, η 2=0.03, power=0.14).
Experiment 2: playbacks from the neighbor's territory
The average distance between the subject and the speaker at the start of a test in experiment 2 was 2.6±0.8 m. Twelve of 22 subjects (55%) failed to orient to the speaker at all during playbacks from the center of the neighbor's territory. Figure 3 (e–h) shows the magnitude of responses during the five observation periods of experiment 2. The overall MANOVA failed to reveal any significant differences across observation periods (Wilks' λ=0.21; F 16, 6=1.39; P=0.3608), and subsequent univariate repeated-measures ANOVAs also did not reveal any significant differences across the five observation periods for the numbers of call groups and movements, the closest approach, and the maximum approach distance. The relatively greater average for maximum approach distance in response to the S stimulus (Fig. 3h) was due to one animal that approached a distance of 1.1 m toward the speaker during the playback of the S stimulus. The median and the mode for maximum approach distance in this observation period were both 0 m.
The contrast analysis for the hypothesis that response magnitudes were greater during playback periods was non-significant in the overall MANOVA (Wilks' λ=0.74; F 4,18=1.58; P=0.2224). In the separate univariate analyses, there was no significant difference for call groups, but there was a significant effect for movements, and marginally significant effects (P<0.10) for closest approach and maximum approach distance (Table 1). These results suggest that subjects were perhaps somewhat responsive to acoustic playbacks during observation periods 2 and 4, but responses during these two periods in experiment 2 were much less intense than those during the equivalent periods of experiment 1 (Fig. 3). In response to the N and S stimuli, contrast analyses failed to reveal any significant differences in the overall MANOVA (Wilks' λ=0.82; F 4,18=1.01; P=0.4292) or in the univariate analyses of call groups, movements, closest approach, or maximum approach distance (Table 1). Between-subjects comparisons of responses to the N and S stimuli in the second observation period were non-significant in an overall MANOVA (Wilks' λ=0.86; F 4,17=0.67; P=0.6233) and in subsequent univariate ANOVAs of the numbers of call groups (F 1,20=0.06, P=0.8084, η 2<0.01, power=0.06) and movements (F 1,20=0.24, P=0.6267, η 2=0.01, power=0.08), the closest approach (F 1,20=0.52, P=0.4789, η 2=0.03, power=0.11), and the maximum approach distance (F 1,20=1.66, P=0.2121, η 2=0.08, power=0.23).
Discussion
In this study, I was unable to detect any difference in the responses of territorial males of D. pumilio to the advertisement calls of strangers and adjacent territorial neighbors. When the playback speaker was located midway between the subject's and the neighbor's territories in experiment 1, males responded equally strongly to both the calls of their neighbor and a stranger. Subjects were less responsive to the stimuli broadcast from within the neighbor's territory in experiment 2, and they did not respond differentially to the calls of neighbors and strangers broadcast from this distance. The data from both experiments are consistent with the null hypothesis that male D. pumilio do not discriminate behaviorally between territorial neighbors and strangers based on individual differences in acoustic communication signals. Studies that report negative results, such as this one, are open to several sources of criticism. Chief among these criticisms are inadequate sample sizes (and hence low statistical power) to detect the hypothesized effect, and inadequate or inappropriate experimental design. Thus, before discussing possible hypotheses for why territorial neighbors in this species may not exhibit vocally mediated dear enemy relationships, three cautionary points concerning methodology are worth considering.
First, the statistical tests of the hypothesis that subjects responded differentially to the calls of neighbors and strangers had consistently low statistical power to detect the observed effect sizes associated with comparisons of responses to the neighbor and stranger stimuli (e.g., power≤0.25). Statistical power, which is the probability of rejecting a false null hypothesis, varies as a function of the effect size and the sample size at a given alpha level (Cohen 1988; Rosenthal and Rosnow 1991). Effect size is a measure of the degree to which the null hypothesis is false (Cohen 1988). The low power of this study most likely resulted from the extremely small effect sizes associated with the response variables chosen to investigate neighbor-stranger discrimination, and not from an inadequate sample size. This study had ample statistical power to detect a behavioral response to the playbacks in experiment 1. The measured effect sizes associated with the discrimination task, however, were considerably smaller than those associated with detecting a behavioral response in general (Table 1). The size of this study (n=22 or 24) is as large or larger than many other studies of the dear enemy effect, suggesting that the effect sizes associated with the vocal discrimination task in D. pumilio were much smaller than those observed in previous studies. There is certainly the possibility that the scope of response variables measured in this study was too narrow to identify differences in responses to neighbors and strangers. For example, I was unable to measure response latency as a dependent variable, and I did not attempt to differentiate between advertisement and aggressive calls. However, the response variables included in this study (i.e., changes in signaling rates and positive phonotaxis) are similar to those commonly measured in other field studies of dear enemy recognition in songbirds, and usually show robust differences in responses to neighbors and strangers.
Second, although the present study found the same null result in two locations, one important limitation of this study is that, unlike several previous songbird studies, I did not map the precise boundaries of each subject's territory prior to conducting playback tests, and I made no effort to place the speaker at or near a particular boundary. Thus, one explanation for the lack of discriminative behavioral responses is that subjects perceived the calls of a neighbor and stranger from midway between the territories as equally threatening, and from the center of the neighbor's territory as equally non-threatening. Perhaps males would discriminate behaviorally between the calls of neighbors and strangers if these were heard vocalizing precisely along established territory boundaries, as may be the case in some songbirds (e.g., Stoddard et al. 1991). This explanation, however, would require that territorial males of D. pumilio discriminate behaviorally between neighbors and strangers over a very restricted range of inter-male distances, namely, some distance shorter than half the distance typically separating two neighboring males. In addition, it is generally assumed that an unfamiliar individual signaling from the direction of a neighbor's territory represents an important change in the status quo of established territory boundaries that should elicit an aggressive response. An experimental approach that might be worthwhile in a future study would be to present the neighbor and stranger stimuli using a two-choice paradigm (e.g., Stoddard et al. 1990; Bourne et al. 2001; Rosell and Bjørkøyli 2002).
Third, readers should bear in mind that this study, as with most previous studies, investigated discrimination in a single sensory modality. Neighbor-stranger discrimination could be mediated by sensory modalities other than audition. For example, vision clearly plays an important role in the social behavior of these diurnal frogs. Forester et al. (1993) reported results from a laboratory experiment indicating that the visual stimulus of a male D. pumilio tethered in another male's territory elicited more intense aggressive responses than did playbacks of advertisement calls to the same subjects. Narins et al. (2003) recently reported that a bimodal stimulus in the form of acoustic playbacks of communication signals coupled with the visual stimulus of a pulsating vocal sac was required to elicit aggression in another dendrobatid frog (Epipedobates femoralis). Summers et al. (1999) also demonstrated that females of D. pumilio can use visual cues to discriminate among potential mates from two different color morphs found in the Archipelago de Bocas del Toro, where the present study was conducted. Thus, perhaps vision also plays some role in discriminating among neighbors and strangers.
Keeping the above caveats in mind, and taking the results of this study at face value, it is worthwhile considering both proximate and ultimate level hypotheses for why males of D. pumilio may not discriminate behaviorally between neighbors and strangers based on individual difference in vocalizations. At a proximate level, several components must be present within a communication system before vocal recognition and discrimination can occur, including individually distinctive signals and an ability of receivers to perceive among-individual differences (Beecher 1990, 1991; Sherman et al. 1997). As in other anurans (Gerhardt 1991; Howard and Young 1998; Bee and Gerhardt 2001a; Bee et al. 2001; Friedl and Klump 2002), several acoustic properties of male D. pumilio advertisement calls exhibit significant among-individual variation (Pröhl 2003). However, with the exceptions of dominant frequency and call duty cycle, all other acoustic properties varied significantly with ambient temperature (Pröhl 2003). Call properties that vary with temperature would probably not be useful acoustic recognition cues unless temperature varied over a very narrow range (as is possible in the tropics) or unless the auditory system could effectively compensate for variation in signals introduced by variation in temperature (Gerhardt 1978; Brenowitz et al. 1985). Hence, the temperature-dependent nature of some call properties may functionally constrain the extent of reliable individual differences in vocalizations.
Recent studies of territorial male bullfrogs, Rana catesbeiana, indicate that spectral or fine-temporal properties related to the fundamental frequency of the signal partially mediate neighbor recognition in this species (Bee and Gerhardt 2001a, 2001b, 2001c, 2002). The fundamental frequency (100 Hz) or low-frequency spectral components (200–400 Hz) of the bullfrog advertisement call are reliably encoded by a temporal code of phase-locked action potentials in the auditory nerve and midbrain (Simmons et al. 1992, 1993, 2000; Simmons and Ferragamo 1993). It is unlikely that male D. pumilio could discriminate among the calls of individuals using a similar mechanism. The call of D. pumilio consists of a single spectral peak that ranges between 4 and 5 kHz (Fig. 1) and almost certainly falls within the hearing range of the basilar papilla in the auditory periphery, which is usually considered to be a resonant organ lacking the tonotopic organization found in the amphibian papilla (reviewed in Gerhardt and Huber 2002). Although dominant frequency exhibits significant among-individual variation (Pröhl 2003), it is unlikely that the basilar papilla could resolve these differences in frequency within the range of 4–5 kHz, independently of differences in intensity, using either a place code or a temporal code of phase-locked discharges (Gerhardt and Huber 2002). Thus, limits on the among-individual variation in signals that can be perceived by receivers is one additional hypothesis for the lack of discrimination reported here.
In light of these potential proximate-level constraints on the evolution of a vocally mediated recognition system, it is also important to ask whether natural selection would favor male D. pumilio that establish dear enemy relationships with neighboring males. Temeles (1994) and Stoddard (1996) have argued convincingly that territory residents should be expected to respond similarly to neighbors and strangers under some conditions, for example, when territory boundaries are in flux, or when neighbors represent a potent threat to residents. Although males defend long-term multi-purpose, breeding territories, several additional observations on the D. pumilio mating system suggest that neighbors might be as threatening as non-territorial floaters. First, the distribution of females is related to the availability of suitable tadpole-rearing sites, a critical resource for successful reproduction, and males appear to compete to establish territories in areas of forest dense with females (Pröhl and Berke 2001; Pröhl 2002). Second, unlike many birds, males and females of this species do not form social pair bonds, and both sexes are polygamous, mating several times with different individuals (Pröhl and Hödl 1999; Pröhl 2002). Females are generally more selective than males and have been observed to apparently assess different males between matings and to reject males during courtship before mating with other nearby males (Pröhl and Hödl 1999). Third, female home ranges are larger than those of males and can encompass the territories of several neighboring males, thereby facilitating assessments of potential mates from a pool of territorial neighbors (Donnelly 1989a, 1989b; Pröhl and Berke 2001). Fourth, because of their role in parental care, females are the limiting sex in reproduction (Pröhl and Hödl 1999; Pröhl and Berke 2001; Pröhl 2002). Consequently, male mating success is both highly variable and highly dependent on the time a male devotes toward calling to attract females (Pröhl and Hödl 1999; Pröhl 2002, 2003). Finally, escalated physical contests are rare, and territorial encounters may be settled primarily through a prior residence effect (Baugh and Forester 1994; Pröhl and Berke 2001). In staged contests between pairs of males in artificial territories, Baugh and Forester (1994) showed that males became dominant and were more aggressive when they played the role of territory resident, compared to males playing the role of intruder. In reciprocal contests, however, males that were formerly dominant as territory residents became subordinate and were less aggressive when they played the role of intruder against the same opponent and the opponent played the resident. Consistent with Baugh and Forester's (1994) observations, Pröhl and Berke (2001; see also Pröhl 1997) reported results from extensive field observations in which only 13 of 75 (17%) aggressive interactions between males of D. pumilio escalated to physical combat. Most encounters were settled with vocal displays at the territory border, with the resident remaining in possession of its territory. In only 2 of 75 (3%) territorial encounters was the intruding male successful in taking over the contested territory. Interestingly, in both cases, the successful intruder was an adjacent neighbor.
Together, these factors suggest that any male, whether territorial neighbor or non-resident floater, that began calling within or near a resident's territory represents a potentially serious threat to that resident's potential mating success, and hence warrants an aggressive response. Because neighbors may represent threats that are equivalent to or even greater than the threat of strangers, residents may receive no net benefit from responding differentially to neighbors and strangers, and may actually risk a loss of mating opportunities and the possession of a territory by failing to respond aggressively to their neighbors. Although the physical costs of escalated combat in this species are unknown, males of D. pumilio are small (19–21 mm in my population) and do not possess weaponry, suggesting that there is a very low risk of sustaining serious injury during an escalated fight. Aposematic coloration probably ameliorates the costs of fighting that might otherwise result from increased attraction of predators or reduced vigilance against predators. Hence, the potential costs associated with escalating a contest are probably low, especially if contests are usually settled using a "prior resident wins" rule. In fact, the prior residence effect could dilute any advantages of establishing dear enemy relationships.
Anurans represent important taxa for better understanding the proximate and ultimate mechanisms of social recognition. For example, in dendrobatid species that exhibit bi-parental care, males and females may establish long-term social pair bonds (Caldwell 1997; Bourne et al. 2001), which could require some form of individual recognition. Moreover, the diversity of mating systems and territorial behaviors exhibited by frogs (Wells 1977) makes them particularly attractive study organisms for understanding the proximate and ultimate mechanisms of the dear enemy effect. For example, although the territories of the two anuran species in which territorial males exhibit dear enemy relationships (Davis 1987; Bourne et al. 2001) can be characterized as multi-purpose, breeding territories (Temeles 1994), there are striking differences between the mating systems of these two species and also that of D. pumilio. On the one hand, the bullfrog mating system is a resource defense polygyny, in which males defend territories that females use as oviposition sites. Some males may defend several different territories over the course of the 3-month breeding season (Emlen 1976; Howard 1978). Females are the limiting sex for reproduction, and males, but not females, can mate numerous times each breeding season (Howard 1978). Males and females do not engage in a prolonged courtship ritual, and neither sex provides parental care of eggs or tadpoles. In contrast, mating and reproduction in the frog Colostethus beebei is characterized by a prolonged courtship ritual and bi-parental care, in which territorial males moisten terrestrial eggs and transport larvae to water-filled bromeliad axils, and females provision developing tadpoles with unfertilized eggs (Bourne et al. 2001). Furthermore, males and females of this species apparently establish long-term social pair bonds (Bourne et al. 2001). Precisely how these differences among mating systems might influence the evolution or expression of dear enemy behavior is not clear and will require additional study. Nevertheless, these studies of anurans suggest that it would be worthwhile to investigate the dear enemy effect in taxa with diverse territorial mating systems in which individuals defend multi-purpose, breeding territories.
In conclusion, my results suggest that territorial males of D. pumilio, which defend long-term multi-purpose, breeding territories, do not discriminate behaviorally among neighbors and strangers based on differences in vocalizations. Additional work will be required to confirm the absence of dear enemy behavior in this species, and readers should bear in mind that a lack of behavioral discrimination does not necessarily imply the absence of a perceptual discrimination. However, a consideration of both the sources of variation in acoustic signals and the capabilities of the auditory system suggests the proximate-level hypothesis, that limits of the acoustic communication system could impose constraints on the evolution of acoustically mediated neighbor recognition in this species. At an ultimate level, however, aspects of the D. pumilio mating system suggest that the costs of escalated encounters are probably low, and that neighbors might sometimes impose high costs on territory residents in terms of lost mating opportunities and territory ownership. Therefore, territory residents in this species may incur no selective advantage by establishing dear enemy relationships with their nearby neighbors. This study should serve to highlight the importance of testing hypothesized relationships between breeding ecology and the dear enemy effect in a broader range of taxa.
References
Baugh JR, Forester DC (1994) Prior residence effect in the dart-poison frog, Dendrobates pumilio. Behavior 131:207–224
Bee MA (2003) Experience-based plasticity of acoustically evoked aggression in a territorial frog. J Comp Physiol A 189:485–496
Bee MA, Gerhardt HC (2001a) Neighbour-stranger discrimination by territorial male bullfrogs (Rana catesbeiana). I. Acoustic basis. Anim Behav 62:1129–1140
Bee MA, Gerhardt HC (2001b) Neighbour-stranger discrimination by territorial male bullfrogs (Rana catesbeiana). II. Perceptual basis. Anim Behav 62:1141–1150
Bee MA, Gerhardt HC (2001c) Habituation as a mechanism of reduced aggression between neighbouring territorial male bullfrogs, Rana catesbeiana. J Comp Psychol 115:68–82
Bee MA, Gerhardt HC (2002) Individual voice recognition in a territorial frog (Rana catesbeiana). Proc R Soc Lond Ser B 269:1443–1448
Bee MA, Owen PC, Perrill SA (1999) Size assessment in simulated territorial encounters between male green frogs (Rana clamitans). Behav Ecol Sociobiol 45:177–184
Bee MA, Kozich CE, Blackwell KJ, Gerhardt HC (2001) Individual variation in advertisement calls of territorial male green frogs, Rana clamitans. Implications for individual discrimination. Ethology 107:65–84
Beecher MD (1990) The evolution of parent-offspring recognition in swallows. In: Dewsbury DA (ed) Contemporary issues in comparative psychology. Sinauer, Sunderland, pp 360–380
Beecher MD (1991) Success and failures of parent-offspring recognition in animals. In: Hepper PG (ed) Kin recognition. Cambridge University Press, Cambridge, pp 94–124
Bourne GR, Collins AC, Holder AM, McCarthy CL (2001) Vocal communication and reproductive behavior of the frog Colostethus beebei in Guyana. J Herpetol 35:272–281
Brenowitz EA, Rose GJ (1994) Behavioral plasticity mediates aggression in choruses of the Pacific treefrog. Anim Behav 47:633–641
Brenowitz EA, Rose GJ, Capranica RR (1985) Neural correlates of temperature coupling in the vocal communication system of the gray treefrog (Hyla versicolor). Brain Res 359:364–367
Bunnell P (1973) Vocalizations in the territorial behavior of the frog Dendrobates pumilio. Copeia 1973:277–284
Caldwell JP (1997) Pair bonding in spotted poison frogs. Nature 385:211
Cohen J (1988) Statistical power analysis for the behavioral science, 2nd edn. Erlbaum, Hillsdale, NJ
Colgan P (1983) Comparative social recognition. Wiley, New York
Davis MS (1987) Acoustically mediated neighbor recognition in the North American bullfrog, Rana catesbeiana. Behav Ecol Sociobiol 21:185–190
Donnelly MA (1989a) Effects of reproductive resource supplementation on space-use patterns in Dendrobates pumilio. Oecologia 81:212–218
Donnelly MA (1989b) Demographic effects of reproductive resource supplementation in a territorial frog, Dendrobates pumilio. Ecol Monogr 59:207–221
Donnelly MA (1991) Feeding patterns of the strawberry poison frog, Dendrobates pumilio, (Anura, Dendrobatidae). Copeia 1991:723–730
Duellman WE, Trueb L (1986) Biology of amphibians. John Hopkins University Press, Baltimore
Emlen ST (1976) Lek organization and mating strategies in the bullfrog, Rana catesbeiana. Behav Ecol Sociobiol 1:283–313
Forester DC, Cover J, Wisnieski A (1993) The influence of time of residency on the tenacity of territorial defense by the dart-poison frog Dendrobates pumilio. Herpetologica 49:94–99
Friedl TWP, Klump GM (2002) The vocal behaviour of male European treefrogs (Hyla arborea): implications for inter- and intrasexual selection. Behaviour 139:113–136
Gerhardt HC (1978) Temperature coupling in the vocal communication system of the gray treefrog, Hyla versicolor. Science 199:992–994
Gerhardt HC (1991) Female mate choice in treefrogs: static and dynamic acoustic criteria. Anim Behav 42:615–635
Gerhardt HC, Huber F (2002) Acoustic communication in insects and frogs. Chicago University Press, Chicago
Greenhouse SW, Geiser S (1959) On the methods in the analysis of profile data. Psychometrika 24:95–112
Howard RD (1978) The evolution of mating strategies in bullfrogs, Rana catesbeiana. Evolution 32:850–871
Howard RD, Young JR (1998) Individual variation in male vocal traits and female mating preferences in Bufo americanus. Anim Behav 55:1165–1179
Limerick S (1980) Courtship behavior and oviposition of the poison-arrow frog Dendrobates pumilio. Herpetologica 36:69–71
Marshall VT, Humfeld SC, Bee MA (2003) Mechanisms and evolution of plasticity of male aggressive signalling in spring peepers (Pseudacris crucifer). Anim Behav 65:1223–1234
Narins PM, Hödl W, Grabul DS (2003) Bimodal signal requirements for agonistic behavior in a dart-poison frog, Epipedobates femoralis. Proc Natl Acad Sci 100:577–580
Pröhl H (1997) Territorial behavior of the strawberry poison dart frog, Dendrobates pumilio. Amphib Reptil 18:437–442
Pröhl H (2002) Population differences in female resource abundance, adult sex ratio, and male mating success in Dendrobates pumilio. Behav Ecol 13:175–181
Pröhl H (2003) Variation in male calling behaviour and its influence on male mating success in the strawberry poison frog (Dendrobates pumilio). Ethology 109:273–290
Pröhl H, Berke O (2001) Spatial distribution of male and female strawberry poison frogs and their relation to female reproductive resources. Oecologia 129:534–542
Pröhl H, Hödl W (1999) Parental investment, potential reproductive rates, and mating system in the strawberry dart-poison frog, Dendrobates pumilio. Behav Ecol Sociobiol 46:215–220
Rosell FB, Bjørkøyli T (2002) A test of the dear enemy phenomenon in the Eurasian beaver. Anim Behav 63:1073–1078
Rosenthal R, Rosnow RL (1985) Contrast analysis: focused comparisons in the analysis of variance. Cambridge University Press, Cambridge
Rosenthal R, Rosnow RL (1991) Essentials of behavioral research: methods and data analysis. McGraw-Hill, New York
Schwartz JJ (1989) Graded aggressive calls of the spring peeper, Pseudacris crucifer. Herpetologica 45:172–181
Sherman PW, Reeve HK, Phennig DW (1997) Recognition systems. In: Krebs JR, Davies NB (eds) Behavioural ecology: an evolutionary approach, 4th edn. Blackwell, Boston
Simmons AM, Ferragamo M (1993) Periodicity extraction in the anuran auditory nerve. I. "Pitch-shift" effects. J Comp Physiol A 172:57–69
Simmons AM, Schwartz JJ, Ferragamo M (1992) Auditory nerve representation of a complex communication sound in background noise. J Acoust Soc Am 91:2831–2844
Simmons AM, Reese G, Ferragamo M (1993) Periodicity extraction in the anuran auditory nerve. II. Phase and temporal fine structure. J Acoust Soc Am 93:3374–3389
Simmons AM, Sanderson MI, Garabedian CE (2000) Representation of waveform periodicity in the auditory midbrain of the bullfrog, Rana catesbeiana. JARO 1:2–24
Stoddard PK (1996) Vocal recognition of neighbors by territorial passerines. In: Kroodsma DE, Miller EH (eds) Ecology and evolution of acoustic communication in birds. Cornell University Press, Ithaca, pp 356–374
Stoddard PK, Beecher MD, Horning CL, Willis MS (1990) Strong neighbor-stranger discrimination in song sparrows. Condor 92:1051–1056
Stoddard PK, Beecher MD, Horning CL, Campbell SE (1991) Recognition of individual neighbors by song in the song sparrow, a species with song repertoires. Behav Ecol Sociobiol 29:211–215
Summers K, Symula R, Clough M, Cronin T (1999) Visual mate choice in a frog. Proc R Soc Lond B 266:2141–2145
Temeles EJ (1994) The role of neighbours in territorial systems: when are they 'dear enemies'? Anim Behav 47:339–350
Wagner WE (1989) Graded aggressive signals in blanchard's cricket frog: vocal responses to opponent proximity and size. Anim Behav 38:1025–1038
Wells KD (1977) The social behaviour of anuran amphibians. Anim Behav 25:666–693
Weygoldt P (1980) Complex brood care and reproductive behavior in captive poison-arrow frogs, Dendrobates pumilio O. Schmidt. Behav Ecol Sociobiol 7:329–332
Ydenberg RC, Giraldeau LA, Falls JB (1988) Neighbours, strangers, and the asymmetric war of attrition. Anim Behav 36:343–347
Zimmermann E (1990) Behavioral signals and reproduction modes in the neotropical frog family Dendrobatidae. In: Hanke W (ed) Biology and physiology of amphibians. Fischer, Stuttgart, pp 61–73
Acknowledgements
I thank John Christy, Luis Mao, Carlos Dixon, and Enrike Downer for logistical support in Panama, Carl Gerhardt for equipment, and Sarah Humfeld, Vince Marshall, Stephanie Palmer, and Sandra Blumenrath for comments on earlier versions of the manuscript. The author was supported by a Doctoral Dissertation Improvement Grant from the National Science Foundation and a Short-term Fellowship from the Smithsonian Tropical Research Institute. The research in this study was approved by the University of Missouri IACUC (protocol no. 3479) and the Republic de Panama Instituto Nacional de Recursos Naturales Renovable (permit no. 26-200) and complies with all of the laws of the United States of America and Panama.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Czeschlik
Rights and permissions
About this article
Cite this article
Bee, M.A. A test of the "dear enemy effect" in the strawberry dart-poison frog (Dendrobates pumilio). Behav Ecol Sociobiol 54, 601–610 (2003). https://doi.org/10.1007/s00265-003-0657-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-003-0657-5