Abstract
In recent years, research on insect motor behaviour―locomotion in particular―has provided a number of important new insights, many of which became possible because of methodological advances in motion capture of unrestrained moving insects. Behavioural analyses have not only backed-up neurophysiological analyses of the underlying mechanisms at work, they have also highlighted the complexity and variability of leg movements in naturalistic, unrestrained behaviour. Here, we argue that the variability of unrestrained motor behaviour should be considered a sign of behavioural flexibility. Assuming that variation of movement-related parameters is governed by neural mechanisms, behavioural analyses can complement neurophysiological investigations, for example by (i) dissociating distinct movement episodes based on functional and statistical grounds, (ii) quantifying when and how transitions between movement episodes occur, and (iii) dissociating temporal and spatial coordination. The present review emphasises the importance of considering the functional diversity of limb movements in insect behaviour. In particular, we highlight the fundamental difference between leg movements that generate interaction forces as opposed to those that do not. On that background, we discuss the spatially continuous modulation of swing movements and the quasi-rhythmic nature of stepping across insect orders. Based on examples of motor flexibility in stick insects, we illustrate the relevance of behaviour-based approaches for computational modelling of a rich and adaptive movement repertoire. Finally, we emphasise the intimate interplay of locomotion and near-range exploration. We propose that this interplay, through continuous integration of distributed, multimodal sensory feedback, is key to locomotor flexibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Like humans, animals make use of their limbs with great flexibility regarding the behavioural requirements for movement speed, accuracy, force and/or coordination among limbs. To some degree, insects and other arthropods meet the different requirements on limb function by dedicating one or more limb pairs to certain movement types of their behavioural repertoire. For example, the jumping-legs of locusts, raptorial legs of praying mantids, or digging-legs of mole crickets have evolved as specialised limb pairs that strongly differ from the other legs, both functionally and morphologically. Nevertheless, almost all insect species have walking-legs that are fairly unspecialised in that they are recruited in various locomotion and manipulation behaviours, including climbing (e.g., Theunissen et al. 2014), grooming (e.g., Berkowitz and Laurent 1996; Seeds et al. 2014), searching (e.g., Berg et al. 2013), and feeding. The flexible use of limbs in diverse behaviours requires both adaptive modification of the step cycle and context-dependent activation of distinct movement types.
Locomotor flexibility is the foundation of several “higher-order” behaviours such as communication and navigation: During a waggle dance, a bee needs to encode the direction and distance of the food source (c.f. Riley et al. 2005) by shaking its body during walks with well-controlled body orientation and intermittent body turns. For navigating in a nearly featureless environment, desert ants integrate distance cues through an enigmatic step odometer during locomotion, in order to compute a straight path back to their nest (e.g., Wehner 2003; Wittlinger et al. 2006). Other examples of flexible locomotion behaviours include walking and running on inclines (e.g., Weihmann and Blickhan 2009; Wöhrl et al. 2017), on verticals or even upside down (e.g., Cruse 1976; Duch and Pflüger 1995; Goldman et al. 2006), pulling of loads (Pfeffer et al. 2016), carrying conspecifics or food (Moll et al. 2013; Pfeffer and Wittlinger 2016), climbing across gaps (e.g., Bläsing and Cruse 2004; Pick and Strauss 2005) or negotiating obstacles (e.g., Harley et al. 2009). In all of these examples, the observed locomotion behaviour is flexible in that the coordination of at least 18 leg joints in six legs must be adjusted by the animal in order to serve a particular goal. Once the goal changes, the control of each joint and the coordination among them often changes, too.
Behavioural analysis of insect locomotion has a long tradition, particularly with regard to the analysis of inter-leg coordination, or gaits (e.g., von Buddenbrock 1920; von Holst 1943; Hughes 1952), for review see (Wilson 1966). This has been complemented by the analysis of local proprioceptive reflexes (e.g., Wendler 1964; for review see Cruse et al. 2009). Much of the progress made was only possible because of electrophysiological studies on restrained animals that allowed well-controlled probing of the underlying neural reflex circuitry (e.g., reviews by Bässler 1983; Büschges and Gruhn 2007; Büschges 2012) and endogenous rhythmic activity in the ventral nerve cord (e.g., Ikeda and Wiersma 1964; see reviews by Delcomyn 1980; Bässler and Büschges 1998). A hallmark of these analyses is the experimental control of as many parameters as possible, allowing clear-cut and comprehensive interpretation of the neural mechanisms at work. This often comes at the cost of reduced behavioural relevance, in that tethered animals or so-called “reduced preparations” potentially execute movements differently than how they would naturally. One reason for this is that restrained animals often are prevented to generate certain movements, thus raising the chances for untypical sensory input and movement output (e.g., an animal that is tethered at an untypical height or body inclination will have to generate step cycles with untypical joint angle time courses). Another reason is that the animal may experience unphysiological parameter ranges (e.g., too low or too high joint torques on slippery surfaces or on spherical treadmills, respectively). Without good knowledge of the natural variation of movement types and/or movement-related parameters, it is hard to infer which part of the experimentally observed variation is functionally relevant and which is not. Since a common argument against the relevance of variability is that it is mostly caused by functionless random processes that confound the experimental analysis (i.e., noise in a strict sense), the first objective of this review is to emphasise the benefits of investigations that allow for behavioural variation at the cost of some experimental control. In sections 1 and 2, we will argue that behavioural variability may be a sign of transient effects of multiple neural control mechanisms and, potentially, of changes in behavioural goal. Assuming that both of these effects—distinct control modes and changes in goals—are factors that drive and support adaptive and context-dependent changes in motor behaviour, we argue that understanding variability can be key to a better understanding of motor flexibility, flexible locomotion in particular. Based on a selection of examples from behavioural analyses of walking, climbing and searching stick insects, section 3 will discuss the opportunities and limitations of modern behavioural analysis in reducing the complexity of the problem without hampering behavioural relevance and, thus, in complementing neurophysiological findings.
A second objective of this review is to illustrate how recent results highlight the compelling similarity between mechanically uncoupled leg movements in different behavioural contexts (e.g., walking, climbing, searching and reaching) and to discuss the role of sensory-induced transitions among apparently distinct movement types. This will lead to the claim that the step cycle of a walking-leg should be considered the result of a quasi-rhythmic alternation of two control modes, rather than the product of a sensory modulated central rhythm. An important argument supporting this claim comes from insights from computational modelling studies: neuro-mechanical models grounded on neurophysiological results on the one hand (e.g., Ekeberg et al. 2004; Szczecinski et al. 2014; Toth and Daun 2017), and behaviour-based models grounded on behavioural experiments on the other (Cruse et al. 1998; Schilling et al. 2013a). In section 4, we will discuss how the explanatory power of these models mirrors their level of description and explain why two essential aspects of flexible motor behaviour are (i) the control of physical interaction with the substrate (or an object) and (ii) spatial coordination of limbs.
As the third objective, section 5 will highlight the mutual interdependence of locomotion and near-range exploration. Once again, examples from stick insects will be used to show that active exploratory movements strongly depend on locomotor activity of the walking-legs. In addition, we will show how the novelty or saliency of a perceptual cue may shift the behavioural goal and lead to a fast adaptation of the ongoing movements of the walking-legs. Because of these mutual influences, we will argue that the common view of “sensing-for-acting” must be complemented by a notion of “acting-for-sensing” in order to account for the flexibility of insect motor behaviour.
Assuming that the fundamental principles of insect motor behaviour are the same in all insect orders that walk and climb, this review will mainly use kinematic analyses of stick insects and discuss the implications on flexible control of insect locomotion behaviour in general. Regarding detailed behavioural analysis of locomotion, stick insects have the advantage that many species are obligatory walkers in that they cannot jump or fly. Thus, the behavioural relevance of legged locomotion, climbing in particular, is high. Moreover, stick insects are relatively large and long-legged, so that animals can be equipped with markers on all leg and body segments relevant to locomotion. Together, these properties make them ideal for the application of fast and precise motion capture of whole-body coordination during unrestrained walking and climbing (e.g., Theunissen et al. 2015), even in combination with measurements of ground reaction forces (Dallmann et al. 2016). Nevertheless, the emphasis on any particular animal taxon introduces a certain bias with regard to this taxon’s biology. In the case of stick insects, this concerns the facts that they are relatively slow walkers, generally do not walk very long distances, have a more posterior centre of mass than most insects, and climb a lot.
Behavioural goal determines the function of limbs and the relevance of sensory feedback
It is important to realise that the function of a limb is determined by the overall behavioural goal of the animal. In locomotion, for example, the goal is to move the body at a given speed into a given direction. This requires the control of at least two parameters: the acceleration of the centre of mass (propulsion or thrust) and the rotation about the vertical body axis (steering). In addition, when the centre of mass is raised above the ground, as in walking or running, the (static or dynamic) stability of body pitch and roll is required to maintain balance and clearance. In other words, locomotion requires the control of those forces and torques that cause the body to move as desired. An important corollary of this consideration is that locomotion neither requires limbs to move rhythmically nor in a stable pattern of coordination, as in a persistent gait. A leg that is to contribute to propulsion and steering has to push against the ground in a certain direction (stance movement). However, contrary to a wheel, a leg can do that only within its limited working range. As a consequence, maintaining a desired movement speed and direction requires each leg to alternate between powerful stance and power-recovering swing movements. Still, from the standpoint of physics this does not imply that alternating step cycles be rhythmical! However, empirical investigations show that they generally are at least quasi-rhythmical in that subsequent step cycles tend to be similar for as long as speed and direction do not change.
From a behavioural point of view, the above considerations are helpful because they highlight that the function of the legs in locomotion is to move the body from one place to another at a given speed, not to move rhythmically. Nevertheless, owing to the physical constraints of legged locomotion, it may be energy-efficient to move the limbs in a persistently coordinated, rhythmical pattern. For example, the fact that specific gaits in running quadrupeds are persistent throughout preferred speed ranges (despite the fact that they do not need to be), is typically explained as a consequence of minimised energy cost (see Alexander 2003). Saving energy by rhythmic storage and release of energy in elastic tissue is particularly important if the inertia of the body is high, as in large and heavy animals, and/or whenever accelerations are high, as in running animals. Since insects generally are small and lightweight animals, saving energy through a rhythmic gait may be relevant only at high speed.
Indeed, biomechanical analyses of fast running cockroaches suggest that the rhythmic tripod gait allows the description of the movement of the centre of mass by a spring-loaded inverted pendulum that rhythmically stores and releases energy, with two alternating tripods acting as functional units (e.g., Blickhan and Full 1993). Yet, insects do not always run at high speeds imposing alternating tripods (see Fig. 1a, d) or even intermittent flight phases. Many species often move slowly, particularly when walking or climbing through their natural environment. As we will discuss in more detail below, virtually all recent gait analyses of unrestrained insect locomotion suggest considerable variability of gaits. If persistent rhythmicity of a gait were a sign of energy optimisation, then variability of a quasi-rhythmic gait should indicate that a criterion other than energy cost is more relevant to the behaviour in which the gait is observed. One reason could be that the behavioural goal of the animals under study was not favouring minimal cost of energy, another could be that energy optimisation through efficient use of elastic energy storage is not of the same relevance to insects as it is to mammals.
The gait continuum in insect locomotion. a Velocity dependence of the two step-cycle phases, swing and stance, in unrestrained walking stick insects, Carausius morosus. Animals walked on the plane floor of a circular arena of 1.2 m diameter. Symbols show median duration of retraction (stance) and protraction (swing) of the middle leg femora of all steps per animal for a given 10 mm/s bin. Different colours denote different animals. Using marker-based motion capture, the protraction/retraction angles of all six femora, forward and sideward translation of the body axis, and yaw rotation of the body axis were measured (see also insert to Fig. 2a). Only steps of straight walking episodes were considered (yaw rotation less than 10 deg./s). At least three steps per bin and animal. Solid lines show the grand median among up to seven animals per bin (not all animals contributed to all bins). The retraction duration decreases hyperbolically with walking speed, as was first observed by Wendler (1964). Based on the grand medians of slow (5 mm/s), normal (45 mm/s) and fast (95 mm/s) walking animals (stance: grey bars; swing: white bars), idealised stepping patterns were generated in d. b The inter-leg coordination of stick insects may be described well by behaviourally derived coordination rules that specify how the current state of a sender leg (green) affects the stance-to-swing transitions in neighbouring receiver legs (blue). Each box denotes a single-leg controller (L1 to L3: left front, middle and leg; R1 to R3: right legs). Arrows indicate the direction of coordination rules 1 and 2 (e.g., Cruse et al. 1995). c Schematic of the effects of coordination rules 1 and 2 for the coloured sender/receiver leg pairs in b. Each leg can be in one of two mutually exclusive states (swing or stance). If the sender leg is in swing, rule 1 inhibits lift-off of the receiver leg. Once the sender leg touches down and begins stance, rule 2 excites lift-off of the receiver leg. d Three idealised stepping patterns drawn from the continuum of swing stance durations from a and arranged to meet coordination rules 1 and 2 as indicated in b and c, assuming that all leg pairs have equal swing and stance durations. Arrows indicate rear-to-front metachronal waves of swing movements. Boxes label alternating tripods of a pair of ipsilateral front and hind legs with the contralateral middle leg
Given that locomotion is about accelerating the centre of mass, appropriate forces must be generated through mechanical interaction of a leg with the substrate. That is, the control of propulsion relies on the control of the stance movements. As a matter of fact, it has been known for a long time that stance duration (and duty cycle) of a leg strongly varies with the speed of the centre of mass, whereas the swing duration generally does little or not. Figure 1a shows this dependence for straight walking episodes of freely walking stick insects. Since the duration of stance is a measure of time, not of force, important additional insights were obtained from measuring the (very small) interaction forces of the legs with the ground during stance (e.g., cockroach: Full et al. 1991; stick insect: Dallmann et al. 2016; ant: Reinhardt et al. 2009). These ground reaction force measurements show fairly consistently across species that some legs may generate interaction forces that decelerate the centre of mass (e.g., a front leg, soon after it touches down), or cancel each other out over time (e.g., laterad forces of the two middle legs; e.g., see Ting et al. 1994). This indicates that leg muscles not only serve as motors that accelerate the body, but may also serve as brakes that decelerate or struts that simply transmit forces (e.g., Ahn and Full 2002; for review, see Dickinson et al. 2000). Interaction forces have been studied mainly in different walking and running contexts (e.g., stick insect: Cruse 1976; cockroach: Goldman et al. 2006; ant: Wöhrl et al. 2017). However, very little is known about how insects adjust and control interaction forces in other behaviours, for example when manipulating objects as in feeding, catching prey, holding on to another insect, grooming or digging.
Depending on the behavioural goal, it is likely that insects need to monitor distinct force components: the normal force that acts perpendicular to the manipulated surface and generates pressure onto the surface, and tangential forces that mainly affect grip or slip. Several studies have demonstrated that campaniform sensilla of insects can monitor interaction forces as strains in their exoskeleton (e.g., Zill et al. 2012, 2015) and grip (e.g., Zill et al. 2010, 2014). Moreover, rhythmic stimulation of campaniform sensilla can entrain centrally generated patterns of motor activity (Borgmann et al. 2009) and potentially contribute to inter-leg coordination (e.g., Zill et al. 2009; Dallmann et al. 2017). Still, very little is known about how insects make use of information about interaction forces in flexible control of distinct movement types.
Whereas interaction forces are particularly relevant for locomotion and manipulation behaviours, a number of behaviours involve contact-free use of a leg. For example, searching movements in near-range exploration behaviour (e.g., Dürr 2001), signalling movements in arthropod communication behaviour (e.g., in jumping spiders, Elias et al. 2012), or so-called grooming-movements (that may not involve body contacts, e.g., Dürr and Matheson 2003) all depend on control of posture rather than force. Two types of sensory organs involved in the control of posture are chordotonal organs (Field and Matheson 1998) and proprioceptive hair fields near the joints (e.g., Markl 1962). Recent demonstration of their relevance in postural control include the re-adjustment of limb posture control after an experimentally induced shift of chordotonal organ output in locust grooming behaviour (Page and Matheson 2009), and the role of a hair plate in the control of targeted searching movements in stick insects (Berg et al. 2013; see also section 3).
In summary, the so-called walking legs of arthropods are used for much more than just for walking. Whenever the behavioural relevance of a leg movement critically depends on load or force—as in propulsion—afferent load/force feedback will supply the most appropriate information about current interaction forces. Likely providers of such information are campaniform sensilla (for review, see Zill et al. 2004). If, in contrast, the behavioural relevance critically depends on kinematic variables like position or velocity—as in searching, grooming, or reaching—afferent postural feedback will supply the most appropriate information. This can be provided by hair fields and chordotonal organs. With regard to the flexible use of limbs according to changes in context or goal, this means: As different sensory cues become more (or less) relevant to the behaviour, leg movement control should become more (or less) sensitive to afferent feedback of the corresponding sensory organs.
Flexible inter-leg coordination and free gait
Apart from distinct movement types of single legs, animals can change the pattern of coordination among legs, too. A persistent issue in studies of insect locomotion is the analysis of gaits. This goes back to the classification of stereotyped patterns of inter-leg coordination called tripod, tetrapod and wave (or pentapod) gaits (e.g., Wilson 1966). In mammals, gait classification is based on phase relationship between step cycles of different leg pairs. Moreover, it includes transitions from walking to running, the latter exhibiting flight phases with no legs in ground contact. In insects, the nomenclature of gaits does not include the transition to flight phases and does not account for fixed phase relationships either. Instead, classification is based on the minimum number of feet on the ground at any one time (three: tripod; four: tetrapod; more than four: wave gait; see Fig. 1d). Cockroaches (Wilson 1966), stick insects (Wendler 1964) and other insects are well known to transition among these gaits as a function of speed, with the tripod being the fastest and the wave gait being the slowest. Regarding the question as to whether insect gaits switch distinctly, much like the gaits of large vertebrates do (e.g., walk, trot and gallop in horses), already the classic study by Wendler (1964) lead to the conclusion that the gaits of a stick insect must form a speed-dependent continuum. The main evidence for this conclusion is that stance, that is the part of the step cycle that generates propulsion, continuously varies with speed, whereas swing varies little. Figure 1a replicates the result of Wendler (1964) for free walking stick insects. The speed-dependent change in phase relationship among legs can be explained by a combination of the speed-dependent shortening of the stance period and a small set of “leg coordination rules” (e.g., Schilling et al. 2013a). Originally, these rules were derived from behavioural experiments on stick insects and crayfish and introduced by Cruse (1990). Sometimes, they are referred to as “Cruse rules.” They describe how the timing of lift-off, the location of touch-down and the coordination of force depend on the states of neighbouring legs (Cruse et al. 1998). Despite the fact that the Cruse rules already give rise to considerable adaptiveness of behaviour, including a change in speed and curvature (Dürr et al. 2004) and transition of gaits (Müller-Wilm et al. 1992; Schilling et al. 2013a), the coupling strength exerted by the rules undergoes leg- and context-specific changes (Dürr 2005). Figure 1c illustrates the effect of a subset of two Cruse rules that couple the legs shown in Fig. 1b. Irrespective of walking speed, these rules will induce a rear-to-front sequence of swing movements on both sides of the animal, and also influence the phase lag between the contralateral waves. They describe how a “sender leg” affects the likelihood of the stance-to-swing transition in an anterior or contralateral “receiver leg”: rule 1 ensures that a leg will not lift off while its posterior neighbour is in swing (light green arrows with “-” in Fig. 1c), whereas rule 2 increases the likelihood of lift-off soon after touch-down of that posterior neighbour (darker arrows with “+” in Fig. 1c). Figure 1d shows idealised stepping patterns, assuming fixed swing and stance durations as measured for three walking speeds highlighted in Fig. 1a (grey and white bars Fig 1a, d are drawn to scale) and strong coupling through rules 1 and 2. The resulting gaits are a slow wave gait, a “normal” tetrapod gait that covers most of the speed range of stick insects, and the tripod gait that occurs in very fast walking. Since only the tripod gait can be described by alternating tripods that comprise the front and hind legs of one side and the middle leg of the other side, it is often treated as a particular gait. However, owing to the effects of the coordination rules, all insect gaits—including the tripod gait—can be described by metachronal waves (slanted red arrows in Fig. 1d). Thus, the tripod gait falls at the end of the continuum, where swing and stance durations are nearly equal. To date, leg coordination rules have only been experimentally demonstrated for stick insects. As yet, all insect species appear to (i) vary stance duration a lot more than swing duration, and (ii) vary stance duration continuously with speed, rather than in discrete speed ranges (e.g.: Drosophila: Berendes et al. 2016; stick insect: Wendler 1964, and Fig. 1; ant: Wahl et al. 2015).
Together with the speed-dependent continuum of stance duration, Cruse’s leg coordination rules allow two predictions about insect gaits: First, there is not one fixed pattern of inter-leg coordination that justifies the classification of distinct gaits. Instead, insects should show what engineers have called a “free gait”, that may even be aperiodic (e.g., McGhee and Iswandhi 1979; Pal and Jayarajan 1990). Second, owing to slight variation in sensory input, the emerging gait should be quasi-rhythmic not only as overall walking parameters such as speed and heading fluctuate, but also during steady-state episodes throughout which these parameters remain nearly constant. This is because Cruse’s rules crucially depend on sensory information about ground contact, interaction force and leg posture.
Indeed, experimental estimation of inter-leg coupling strength in straight and curve-walking stick insects revealed that stepping patterns may fluctuate strongly even within relatively short walking episodes of nearly constant forward velocity (Dürr 2005, Fig. 2a, 7.5 s straight walking, followed by 15 s of turning). Furthermore, stepping patterns may vary considerably between trials with very similar walking paths. This suggests that very similar behavioural performance may be caused by fairly different, quasi-rhythmic stepping patterns. However, since these data had been acquired from tethered walking insects that had to carry their own weight, but also had to produce substantially larger forces than normal during turning (owing to the momentum of the spherical treadmill), it was not clear whether the observed variability was a consequence of unnaturally high joint torques during turning. What was lacking was an experimental test of how stable stick insect gaits are in completely unrestrained locomotion. Indeed, Grabowska et al. (2012) confirmed that the standard gait classification fails in stick insects for substantial fractions of the walking time, consistent with a continuously fluctuating “free gait”. Similarly, several recent studies have reported that even in smaller and faster insects like ants and fruit flies – which were traditionally considered as “tripod-only walkers” (Strauss and Heisenberg 1990; Zollikofer 1994)—the tripod gait is not a rigid pattern. Behavioural studies show consistently across insect orders that gaits are variable rather than fixed patterns, irrespective of what method of gait analysis is chosen (e.g.: gait template matching in stick insects: Grabowska et al. 2012; estimation of triplet overlap in Drosophila: Wosnitza et al. 2013; counting of legs on ground in ants: Wahl et al. 2015; Hidden-Markov model analysis of Drosophila: Isakov et al. 2016).
Stepping patterns vary for similar behavioural output. a Two examples of walking paths and corresponding stepping patterns of Carausius morosus walking on a spherical treadmill. The treadmill was surrounded by black-and-white stripes (see insert: red, blue and grey arrows indicate measured sideward translation, forward translation and yaw rotation, respectively). After an episode of 7.5 s, the stripe pattern began to rotate at constant velocity, inducing an optomotor turning response. Reconstructed paths show body axis and head every 200 ms, plotted on a 10 × 10 cm grid. White and black symbols indicate walking during stationary and rotating visual stimulation, respectively. Stepping patterns fluctuate with time and differ considerably, despite the fact that walked paths are very similar. Boxes mark intermittent short steps (see also Fig. 3b). Slanted lines indicate metachronal waves of swing movements, vertical arrows and arrow heads label an episode of nearly tripod stepping [Adapted from Dürr 2005]. b Two trials of unrestrained, planar and straight walking trials of the same animal, with nearly the same median forward velocity (heavy blue line in mid panel) but differing strongly in lateral translational velocity (red lines in mid panel). See Supplementary Videos 1 and 2 for straight and wiggly walks, respectively. Top panels: Animals walked in a circular arena of 1.2 m diameter (same experiment as in 1A). Inserts show 25 × 25 cm top view area with body axes drawn every 200 ms. In the left trial, a rhythmic sideward swaying movement was superimposed on forward motion. As a result, the drawn body axes come to lie on two straits. Wiggly grey arrows show an idealised trajectory of the head (red points) next to it. Mid panels: Forward (blue) and sideward (red) translational velocity of the body axis calculated every 20 ms, median-filtered with a half width of 60 ms (thin lines) or 1 s (heavy blue line). Yaw rotation not shown. All velocity components fluctuate as each leg contributes to propulsion and/or sideward sway. Bottom panels: Corresponding stepping patterns fluctuate a lot and differ between trials, despite very similar forward walking speed. Note that the leg kinematics must differ considerably for normal straight walking and sideward swaying animals
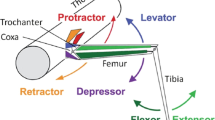
Another important issue to consider about locomotion is the kinematic flexibility of single steps, i.e., of intra-leg coordination of the joints. During unrestrained, spontaneous walking, a leg is not only protracted and retracted in a cyclic fashion: At least two further joints have to be coordinated per leg. This becomes evident in behaviours where locomotion strongly deviates from pure forward translation of the centre of mass, for example, by pronounced yaw rotation during turning, or emphasised sideward translation. Figure 2b illustrates the latter situation by juxtaposing two trials of the same individual that strongly differ in sideward translational velocity (swaying; see Suppl. Videos 1 and 2) of the body while being very similar in both mean forward translation velocity (thrust) and path straightness (low rotational velocity, or yaw turning). In both trials, the stepping patterns fluctuate but there is no obvious difference between them that relates to the dramatic difference in sideward swaying. The reason for this is that the classic definition of a gait only depends on stance/swing sequences that are dominated by the retraction/protraction rhythm while neglecting the effects of other degrees of freedom of the limb (levation/depression; supination/pronation; extension/flexion). Since the rhythmic swaying-movement in the left trial of Fig. 2b is caused by rhythmic in-phase extension/flexion of all three ipsilateral legs (counter-phase for contralateral leg pairs), it is concealed in gait analysis despite the potential behavioural relevance of this conspicuously different behaviour.
In summary, a wide range of recent behavioural analyses suggests that insect gaits vary continuously rather than in discrete classes. Continuous (free) gait transitions and quasi-rhythmic deviations from a strict pattern of coordination can be explained by a combination of two things: (i) a small set of rules that determine how pair-wise couplings among legs depend on sensory information, and (ii) distinctly different dependencies of the swing and stance phase durations on walking speed, with the swing duration being much less speed-dependent than the stance duration. Whereas the latter indicates profound differences in the underlying neural control of stance and swing movements, the former predicts flexible switching between stance and swing control. In the following, we will expand this view from planar walking to climbing and searching behaviours.
Beyond planar walking: short steps, spatial coordination, and intermittent searching
Given the fact that unrestrained locomotion is very variable, an important aspect of modern motion capture techniques is the possibility to acquire large sample sizes. Only they permit a description of the “natural statistics” of various locomotion-related parameters, including the analysis of relatively rare events. An example of this is the occurrence of unusually short steps during walking and climbing. When assuming a regular gait, and even more so when assuming a single mechanism for the generation of a step, a unimodal distribution would be expected for all main kinematic parameters of a step—such as length, height or direction. For example, a normal distribution might be used to describe the typical step length variation of straight walking episodes with a limited speed range. However, since insects may take very short steps when facing gaps or barriers, the question arises whether such short steps are drawn out of the lower tail of a normal distribution or rather form a distinct mode of the step length distribution. The first quantitative description of short steps was given by Bläsing and Cruse (2004) who found that the step length of the stick insect Aretaon asperrimus was humped or even bimodal in all three leg pairs, just before the animals climbed across very large gaps. The same animals showed a unimodal step length distribution during “normal” straight walking, with few very short outliers only. With regard to these few outliers it remained unclear whether or not the short steps were specific to the particular climbing paradigm used. Recently, Theunissen and Dürr (2013) found that the stick insect Carausius morosus showed the same kind of bimodal distribution of step length as reported by Bläsing and Cruse. However, owing to a larger sample size and a set of four experimental conditions that varied in climbing effort (Fig. 3a), they could show that (i) short steps also occur during “normal” straight walking (Fig. 3b, left), although rarely and to a different degree in the three leg pairs. Also, (ii) the proportion of short steps increased as a function of climbing effort (Fig. 3b, middle). The distributions of short and long steps were best described by distinct probability functions, gamma and logistic distributions, respectively (normal distributions made rather bad fits). Moreover, short steps were found to occur most frequently at the edges of the setup (Fig. 3b, right), as would be expected for correction steps in response to slip and/or inappropriate foothold. Since short and long steps differed not only statistically (as if drawn from distinct distributions) but also in nature (e.g., by different directional tuning and lift-off location along the stance trajectory), Theunissen and Dürr (2013) proposed they be functionally distinct step types, possibly generated by different underlying mechanisms. An experimental reduction of tarsus grip through ablation of the tarsal claws or the terminal tarsomere significantly increased the proportion of short steps in the operated leg. Thus, it was further suggested that short steps serve as correction steps in response to sensory cues about inappropriate interaction forces between foot and substrate. Meanwhile, short steps have been described for three stick insect species with similar functional properties, despite leg-type- and species-related differences in the distribution (Theunissen et al. 2015). It will be interesting to see whether or not this “differential recruitment of two distinct step types” occurs in similar situations in other insect groups, too. If so, current models of step cycle generation should integrate it: either by (i) coordinating two competing mechanisms, one for each step type, or by (ii) modulating one mechanism so as to produce steps with bimodal length distributions.
Short steps, spatial coordination and intermittent searching. a Sequences of unrestrained walking and climbing stick insects on two of four setup variants that differed in the height of two stairs (left: no stairs; right: 48 mm stairs). b Left: Step length histogram of hind leg steps reveals two modes, separating short (red) and long steps (blue). Middle: The proportion of short steps increases with climbing height on the flat surface or on low (8 mm), middle-sized (24 mm) and high (48 mm) stairs. Right: Lowest 10% quantile of steps (red; short steps only) clusters around the edges (arrows), whereas the 50–60% quantile (blue; long steps only) forms parallel straits on the planar surfaces (here: 24 mm stairs; hind legs). c Side views of right foot trajectories reveal strong similarities between leading and trailing legs (R1, R2, R3: front, middle and hind leg). Middle: Spatial congruence of average middle leg lift-off locations for 3 × 3 11% quantiles of the XZ-distributions (red, side view) and corresponding average hind leg touch-down locations (black). Green lines connect corresponding points. Blue lines delimit the working range of the hind leg. Right: As before, but after ablation of the trochanteral hair plate of the right middle leg. Spatial congruence of hind-leg touch-down locations deteriorates as touch-down locations no longer form a 3 × 3 grid. d Left: Side view of foot trajectories during planar walking, before (top) and after (bottom) ablation of the trochanteral hair plate. After ablation, the operated leg often shows intermittent searching movements. Middle: Cumulative probability of swing height before (solid line) and after (dashed line) ablation. Ablation leads to substantially higher swing movements and more frequent intermittent searches. Right: percentage of steps terminating in searching movements before (intact), immediately after (day 1) and 24 h after (day 2) ablation. Different symbols for five animals. [Adapted from Theunissen and Dürr 2013, and Theunissen et al. 2014]
Another important functional aspect of insect locomotion that, until today, has been studied predominantly in stick insects is the spatial coordination of footfall patterns. A well-studied example of spatial coordination among legs is the so-called targeting-mechanism that was first described by Cruse (1979). Originally, Cruse varied the standing position of a middle leg on a test platform when walking was initiated. He observed that the touch-down location of the ipsilateral hind leg was systematically affected by the standing position of its anterior neighbour. Later, this effect was shown to occur between all ipsilateral pairs of walking legs (Dean and Wendler 1983), to depend on input from proprioceptive hair fields (Cruse et al. 1984; Dean and Schmitz 1992), and on the turning direction of the animal (Ebeling and Dürr 2006). Recently, Theunissen et al. (2014) showed that spatial coordination among ipsilateral leg pairs works in all three spatial dimensions, and that spatial congruence breaks down only near the border of the leg’s working range (Fig. 3c, middle). Figure 3c (left) shows an example of three-dimensional foot trajectories of an ipsilateral set of front-, middle- and hind-legs, as seen from the side. Examples like this suggest that the middle leg can take advantage of the previous foothold of a front leg. As a result, a hind foot trajectory may take a very similar course as that of its leading middle foot (see arrow in Fig. 3c). Given that the two legs are of different lengths, insert at relatively distant locations on the thorax, and move at different times, similar foot trajectories in space suggest that postural information may be stored for a short time. This would require a surprisingly accurate coordinate transfer among legs. Theunissen et al. (2014) also showed that the spatial coordination among middle and hind legs relies on the trochanteral hair field of the middle leg. This proprioceptive hair field monitors the levation of the trochanterofemur relative to the coxa. Ablation of this hair field strongly hampers the spatial congruence of middle leg lift-off and hind-leg touch-down locations (Fig. 3c, right), suggesting that it is required for appropriate coordinate transfer from the middle leg to the hind leg. Moreover, it causes a strong increase of the maximum swing height of the middle leg (Fig. 3d, middle). Therefore, this proprioceptor must also be involved in the control of the step cycle of the middle leg itself. The coordinate transfer between neighbouring legs can be modelled by means of a reasonably small Artificial Neural Network (Dean 1990), and some candidate interneurons have been identified that may be involved in this coordinate transfer (Brunn and Dean 1994). However, much of the underlying neural mechanism remains elusive.
Intriguingly, the same proprioceptor that is involved in the control of the swing movement and spatial coordination of neighbouring legs is also involved in the formation of a short-term position memory in another stick insect species (Medauroidea extradentata = Cuniculina impigra): Berg et al. (2013) showed that a mechanical interruption of a cyclic searching movement of a front leg leads to a systematic shift in the levation of the trochanterofemur. This shift may last for a few seconds, corresponding to the time of several loops of the search trajectory. Ablation of the trochanteral hair field strongly affects this behaviour. Thus, it appears that the joint angle control during a swing movement relies on the same proprioceptive input as exploratory searching movements do. Consistent with these effects, ablation of the trochanteral hair field also raises the likelihood of intermittent searching movements in walking stick insects (Theunissen et al. 2014), i.e., the occurrence of one or more terminal loops of the foot trajectory before touch-down (Fig. 3d, left and right). Such intermittent searching movements are rare in intact animals (Fig. 3d right, “intact”). After ablation, their likelihood is raised only in the treated leg. As they occur, the other five legs maintain their normal stepping pattern. These multiple effects of a single joint-angle proprioceptor on swing and searching movements, spatial coordination and short-term memory are in line with behaviour-based modelling studies that suggested that swing movements are subject to a position feedback system (Schumm and Cruse 2006) that is capable of generating the cyclic foot trajectories as observed during searching movements (Dürr 2001). Together, the evidence from behavioural and modelling experiments supports the view that at least two types of mechanically uncoupled leg movements—swing and searching—may be subject to the same feedback controller and rely on the same afferent information. According to this view, the difference between swing and searching is that a swing movement is interrupted by touch-down, whereas searching is not. With touch-down, the leg becomes mechanically coupled with other limbs and load/force feedback becomes relevant as the function of the movement changes (for all we know, load/force sensors are not active during mechanically uncoupled leg movements in which muscle forces are not resisted; see Zill et al. 2013). In summary, the separation of mechanically uncoupled and position-controlled swing and searching movements on the one hand, and mechanically coupled and load/force-controlled stance movements on the other hand may be described as the “two-control mode” hypothesis. According to this hypothesis, a step cycle is a concatenation of distinct movement episodes that serve distinct behavioural functions and are dominated by distinct control mechanisms that differ in the afferent feedback used (for a schematic, see Fig. 4a, right).
Two alternative interpretations of behavioural and neurophysiological results on distinct types of rhythmic leg movements. a Schematic side view of foot trajectories during stepping and searching, and during swing/search and stance. Left: Stepping (red) and searching (blue) movements are two rhythmical movement sequences that strongly differ in kinematics. This difference could indicate that they are driven by distinct motor programs. Right: During swing and searching (red), a leg is completely unloaded and mechanically uncoupled from the other legs (at least largely). Movement is controlled so as to reach or traverse a certain posture sub-space. Both movements are strongly affected by posture-encoding hair plates, whereas strain-encoding campaniform sensilla are not stimulated. During stance (blue), a leg is mechanically coupled to all other legs in ground contact and controlled so as to contribute to the desired propulsion, heading and support. During stance, interaction forces between the leg and substrate stimulate campaniform sensilla. b Left: Activation time courses of the thoracic pre-motor interneuron I4 that is rhythmically active during both stepping (red, top) and searching (blue, bottom). Time courses are idealised but drawn to scale after Berg et al. (2015), with the dashed line indicating the resting membrane potential (rest). Grey shading highlights episodes of flexion of the femur-tibia joint. During stepping, this flexion episode largely coincides with ground contact. According to the “two motor patterns” hypothesis, this neuron is a prime example of how distinct neuronal activation patterns (red vs blue) may correlate with distinct leg movement types in A. Injection of depolarising/hyperpolarising current can trigger/abort searching (Berg et al. 2015, not shown). Right: According to the “two control modes” hypothesis, the neuron’s activity is affected by proprioceptive afferent activity: blue and red episodes differ in self-induced proprioceptive feedback. During stepping, afferent activity alternates between strong (blue) and absent (red) load feedback from campaniform sensilla. During searching, only postural proprioceptors are excited, as during swing
An alternative hypothesis may be called the “two motor patterns” hypothesis (Fig. 4a, left). It suggests that a step cycle should be considered a cyclic movement pattern that is distinct from cyclic searching movements. This hypothesis is supported by at least two observations: First, Berg et al. (2015) found that they could reliably trigger/abort searching movements of a stick insect’s middle leg by injecting depolarising/hyperpolarising current into a certain local non-spiking interneuron (I4) of the mesothoracic ganglion. Second, the inter-joint coordination of a searching movement cycle strongly differs from that of a walking step cycle. The membrane potential of interneuron I4 fluctuates rhythmically during both movement patterns (Berg et al. 2015) and differs mainly during flexion of the femur-tibia joint (which, in the stepping condition of these experiments largely corresponds to stance). According to the “two motor patterns” hypothesis, interneuron I4 acts very much like a command neuron that may switch between two mutually exclusive states (stepping and searching). In this case, the states correspond to two cyclic movement patterns. Concerning the neurophysiological evidence on interneuron I4, the “two control modes” hypothesis would predict that interneuron I4 should show a similar time course of its membrane potential during a swing movement as during a swing-like “extension part” of a searching cycle (red lines in right panel of Fig. 4b). Indeed, the corresponding depolarisation maxima are similar and the time courses of depolarisation leading to these maxima are similar, too. In contrast, the time courses strongly differ during the “flexion part” which, during stepping, is dominated by ground contact and corresponding mechanical interaction of foot and substrate. The lack of ground contact during searching allows this flexion part to follow a totally different trajectory.
In summary, both the “two motor patterns” and the “two control modes” hypotheses contrasted in Fig. 4 have arguments supporting them. Essentially, they differ in claiming the existence of a command neuron that switches between motor patterns (stepping or searching), as opposed to claiming that the sudden occurrence of load/force feedback initiates a transition between distinct movement episodes (search is equivalent swing with subsequent loops). Potentially, the matter could be resolved by measuring the effect of sensory afferents that encode loading or unloading of a leg on interneuron I4 and other premotor interneurons. According to the “two motor patterns” hypothesis, loading should cause a switch from searching to stepping, for example by inhibiting interneuron I4 and exciting at least one other command neuron that can drive the entire stepping pattern. Moreover, unloading during stepping should not be sufficient to trigger searching movements with several loops. According to the “two control modes” hypothesis, loading should persistently inhibit interneuron I4 until unloading induces dis-inhibition, followed by triggering a swing and subsequent search. As it stands, the discrepancies between the two hypotheses may be largely attributed to the methodological limitations of the studies that support them (electrophysiology on reduced preparations on the one hand, behavioural movement analyses and computational modelling on the other hand). At the same time, this underscores the complementary potential and necessity of these different approaches.
Behaviour-based modelling of flexible locomotion
In computational modelling of motor behaviour, the use of sensory feedback has two major functional consequences: First, it allows closed-loop control for appropriate counteraction against disturbances. Second, it allows to include information about the current situation of the agent. This may comprise cues arising from immediate physical interaction (e.g., slipperiness or softness), near-range information relevant to the execution of limb movements (e.g., obstacles within reach) and far-range information relevant to steering, navigation (e.g., landmarks) and choices among competing goals (e.g., visual scene cues indicating potential food sources, presence of conspecifics, or danger). Since all of these aspects are of immediate behavioural relevance to animals, behavioural flexibility requires appropriate weighting of various sensory cues according to context and behavioural goal. Moreover, since behaviours as complex as locomotion may require multiple aspects to be taken care of (e.g., propulsion, heading and balance), differential weighting of sensory cues is likely to be beneficial even during plain walking. This idea is central to Walknet, a behaviour-based model of adaptive multi-legged locomotion. Walknet was originally proposed more than 20 years ago (Cruse et al. 1995) and may be considered a benchmark in modelling behavioural flexibility in insect locomotion. The model rests on two main concepts: The first concept is the differential significance of sensory cues during swing and stance, and the strict separation of two single-leg control modes, one for each phase of the step cycle (as in Fig. 4, right). The second concept is that “communication” among legs governs inter-leg coordination by determining the transitions from one control mode to the other (see Fig. 1b, c). Since the original version of this model was of purely kinematic nature, only ground contact and postural cues were used as sensory cues: In the single-leg control concept, the stance mode relies on feedback about leg retraction velocity, whereas the swing mode is governed by an integral controller of joint angle velocities that receives position feedback from three leg joints (Cruse et al. 1998). Ground contact determines which of these control modes is used. In the inter-leg coordination concept, the current posture of a leg is used to calculate the distance of the foot to a default lift-off position (the Posterior Extreme Position, PEP, in forward walking), and to set a desired “target posture” of the next posterior leg’s touch-down (the Anterior Extreme Position, AEP, in forward walking). A set of three “coordination rules” specifies how each leg affects the lift-off position of its anterior, posterior and contralateral neighbouring legs. Two of these are shown in Fig. 1c. The rationale behind this model is that inter-leg coordination subscribes to the goals of maintaining stability and coherence of stepping, based on the current interaction with the substrate (ground contact) and on the distributed proprioceptive feedback about whole-body posture (Dürr et al. 2004). Owing to the strict functional separation of swing and stance control, the model can accommodate variants of control modules that allow for a seamless transition from a targeted swing movement to a cyclic searching movement (Dürr 2001), or for swing movements of different height and touch-down location (e.g., Schumm and Cruse 2006). Owing to the inclusion of both ground contact and postural cues into inter-leg coordination, the model can change gaits, walk curves, and climb stairs of approximately half the body clearance (Kindermann 2002). Later versions of this model have augmented (e.g., Bläsing 2006) or re-arranged the computational structure (Schilling et al. 2013a) so as to improve its flexibility in climbing (Bläsing 2006) or to introduce motion planning and navigation skills (Schilling et al. 2013b). As yet, the concepts of strict functional separation of swing and stance control, and of sensory-based inter-leg coordination, have remained essential hallmarks in all variants of Walknet.
More recent models of insect locomotion strive for more realistic implementation of physiological facts, such as the combination of known reflex pathways (Ekeberg et al. 2004), the rigorous formulation through neural networks (e.g., Twickel et al. 2011), the inclusion of central oscillators for each joint (Toth and Daun 2017) and/or the use of multiple ion currents per computational element (e.g., Hodgkin-Huxley-type neuron models; Szczecinski et al. 2014). Arguably, each of these models comprises more details about physiological mechanisms than Walknet. However, all of them clearly fall short of the behavioural flexibility exhibited by the “synthetic neuroethology” of Walknet (Kindermann 2002). Three typical criticisms about lacking neurobiological detail in Walknet concern:
-
(i)
The lack of endogenous rhythmic activity as found in the central nervous system in insects,
-
(ii)
the lack of neurophysiological evidence as yet of separate neuronal controllers for swing and stance movements, and
-
(iii)
the kinematic nature of the model and the use of a binary ground contact signal instead of cues on dynamics, such as load or force.
Regarding the first of these criticisms, there are both conceptual (see Hoinville et al. 2015) and physiological reasons why endogenous rhythmic activity is probably not very helpful for our understanding of behavioural flexibility. Many physiological studies on insects have shown that pilocarpine, an agonist of metabotropic acetylcholine receptors, can induce rhythmic activity in the central nervous system of arthropods. In stick insects, this leads to a rhythmic drive to pre-motor interneurons (Büschges 1995). However, pilocarpine-induced rhythms have periods that are eight to ten times longer than typical step cycles, show little or no persistence in de-afferented ganglia (Büschges et al. 1995), and cannot cause inter-segmental coordination through central rhythms alone (Ludwar et al. 2005). In a brain-antennae preparation in which proprioceptive feedback remains intact, pilocarpine can induce persistent rhythmic movements of the antennae (Krause et al. 2013), albeit much slower than in spontaneous movements. A strong case for the functional significance of centrally generated activity rhythms in locomotion comes from recent genetic ablation studies in Drosophila (Isakov et al. 2016; Fujiwara et al. 2017). These studies showed that animals can still walk when lacking parts of their mechanoreceptive infrastructure, albeit with considerable differences in overall performance, e.g., straightness of walking path (Fujiwara et al. 2017), or the inability to compensate for effects caused by the loss of a limb, (Isakov et al. 2016). More generally speaking, Drosophila deficient of certain proprioceptors shows reduced behavioural flexibility. This is paralleled by the fact that until today, the inclusion of central oscillators into computational models of insect locomotion has not led to any advantage in behavioural performance.
Regarding the second criticism, studies in which individual thoracic interneurons were recorded during the execution of different types of cyclic leg movements agree in that all neurons that show rhythmic excitation or inhibition during one type of movement (e.g., searching), also show rhythmic changes during the other type of movement (e.g., stepping; Tryba and Ritzmann 2000; Berg et al. 2015). In stick insects, a particularly intriguing interneuron is I4 (as discussed in the context of Fig. 4), which is thought to be part of the central pattern generator in the thoracic ganglion (Büschges 1995). Its properties are reminiscent of a command neuron that governs the execution of a more or less fixed motor program (e.g., Hedwig and Heinrich 1997) in that experimental depolarisation of interneuron I4 can cause searching, but not stepping. As yet, depending on whether or not stepping is considered as “one motor pattern” or rather an alternation of “two control modes”, its properties are also consistent with the idea that searching is equivalent to a swing movement that is not stopped by physical contact with the ground or another obstacle (Fig. 4). Generally, the fact that Walknet strictly separates swing and stance controllers does not mean that the neural networks in both controllers have to be disjunct. Rather, single neurons and even parts of the neural networks may be identical, provided that their interconnectivity and synaptic weights are modulated in a way that is consistent with two controllers. In other words, the separation into two controllers (as done in Walknet) is foremost based on a functional distinction, not on a physical separation of networks.
Regarding the third criticism, it is very likely that the replacement of a binary ground contact signal by local load feedback from campaniform sensilla could only improve model performance. A binary ground contact signal is equivalent to a thresholded load provided that the threshold is very low. However, phasic-tonic encoding of load as done by campaniform sensilla provides more information that may be used, for example, to estimate how much a leg is contributing to the support of body weight. A technical demonstration of the potential of distributed load sensing for controlling inter-leg coordination was provided by a biorobotics study using one stance-swing oscillator per leg and no direct, “neural” coupling among them (Owaki et al. 2013). Instead, the oscillators were affected as soon as legs became mechanically coupled through the substrate. Whenever local load feedback rose beyond a certain threshold, this caused a transition into a non-oscillatory mode until load feedback decreased and legs got mechanically uncoupled again. In a four-legged robot, this proved to be sufficient for generating a coordinated stepping pattern, despite the lack of “neural” communication among legs. This was attributed to mechanical load transfer among the legs with ground contact, inducing a local, load-dependent switch between oscillatory and non-oscillatory modes. To some degree, this approach even allows a velocity-dependent transition between stepping patterns (Owaki and Ishiguro 2017). Further work will be required to assess the robustness of this control scheme, and to test its applicability to six-legged locomotion, where load transfer appears less straight-forward because of multi-leg interactions.
In the motor physiology of animals, local load-dependent effects on the step cycle of a walking leg have been analysed in mammals, where—other than in insects—distributed load sensing is done by Golgi tendon organs (GTO) that encode tendon strain (for review, see Duysens et al. 2000). For example, group 1b afferents from GTO in the cat hind leg are considered important for the maintenance of stance (Donelan and Pearson 2004) as well as for timing of hind leg lift-off (e.g., Whelan et al. 1995). The interpretation is that the GTO afferents monitor the rhythmic loading/unloading of a leg, thus signalling the current contribution of this leg to propulsion and support of the body weight. If the load is high, the leg remains in stance until the leg is unloaded, for example via load transfer upon touch-down of the contralateral leg. This interpretation has received support from a neuromechanical model of hind-leg stepping in cats, where the load feedback entrains rhythmic coordination of contralateral stepping through mechanical load transfer alone (Ekeberg and Pearson 2005). In this model, the step cycle of each leg is determined by sensory-induced transitions of muscle activation (without central oscillators) and inter-leg coordination is accomplished through sensory encoding of load changes that, in turn, arise through mechanical coupling. In insects, an analogous mechanism has been proposed, based on the observation that strain-encoding campaniform sensilla in the cuticle can encode unloading of the leg (cockroach: Keller et al. 2007; stick insect: Zill et al. 2011). In walking cockroaches, campaniform sensilla afferents of the middle leg fire when the neighbouring hind leg touches down (Zill et al. 2009), as if signalling load transfer from the middle leg to the hind leg. Although this is a plausible assumption, so far the unloading event was implied from a kinematic event (touch-down). More compelling evidence in favour of this mechanism would require the direct measurement of unloading in a walking insect. Recently, this was achieved by Dallmann et al. (2017). In their study on freely walking stick insects, they showed that campaniform sensilla can reliably encode the unloading caused by load-transfer to another leg, and that unloading can reliably trigger a lift-off in the middle leg by activating the appropriate muscle. This suggests the presence of a mechanism equivalent to an “indirect” implementation of Cruse’s inter-leg coordination rule No 2 (see Fig. 1b, c) through local encoding of loading/unloading events caused by mechanical coupling of neighbouring legs.
Interaction of locomotion and near-range exploration
As load- and posture-encoding proprioceptors are embedded virtually everywhere in the musculoskeletal system of insects (as in other animals, too), their afferents will always and everywhere supply information about the current movement of the body and its interaction with its environment. Even if some afferent inputs may be gated out during active movement (e.g., Staudacher and Schildberger 1999; Poulet and Hedwig 2002), a large amount of afferent information is likely to be integrated into thoracic motor networks all the time. The sections above provided examples of how the integration of rich, multi-faceted and distributed proprioceptive feedback favours flexible adjustments of single-leg movements and inter-leg coordination in unrestrained locomotion. An intriguing special case of single-leg movements concerns searching. Irrespective of which hypothesis about its control is correct (see Fig. 4), the cyclic foot trajectories are generally thought to serve near-range exploration for suitable foothold. If this is true, then animals can show exploration behaviour intermittently with locomotion behaviour. This is accompanied by an important part of the otherwise proprioceptive input taking on additional exteroceptive quality during searching: The same mechanoreceptors that—during walking—are part of control loops regulating foot position, leg posture or interaction forces, during searching provide additional and concurrent feed-forward information about the presence of a foothold or obstacle. Thus, since near-range exploration through leg searching movements occurs in service of locomotion (by finding new foothold), and searching is always preceded by locomotor activity in the form of at least one swing movement (irrespective of which hypothesis in Fig. 4 will turn out to be correct), locomotion and near-range exploration are mutually interdependent.
Another variant of this mutual interdependency exists in limbs that no longer serve the generation of interaction forces in locomotion, but have become dedicated sensory appendages for continuous exploration of the near-range environment. In insects, this is the case in antennae. Antennae are serial homologues of the thoracic limbs. They share a lot of morphological, sensory and neural characters with walking legs (for review, see Krishnan and Sane 2015) but have evolved to serve as dedicated sensory appendages of the head. Many insect groups actively move their antennae during walking and climbing (e.g., cockroaches: Okada and Toh 2004; Harley et al. 2009; crickets: Horseman et al. 1997; stick insects: Dürr et al. 2001; Krause and Dürr 2012; beetles: Pelletier and McLeod 1994; Zurek and Gilbert 2014). It is clear that active antennal movements serve near-range exploration. In some insects, antennae are of similar length as, or longer than, the walking-legs. As a consequence, active movement of the antennae increases the likelihood to touch objects within reach of the front legs, and in time to react with appropriate adjustment of body inclination (e.g., Pelletier and McLeod 1994), walking height (e.g., Harley et al. 2009), and/or the execution of aimed limb movements, as in reach-to-grasp movements (Schütz and Dürr 2011; Fig. 5e).
Near-range exploration in service of adaptive locomotion. Active tactile exploration in stick insects depends on locomotor activity (a, b), but may also strongly affect locomotion (d, e). a Trajectories of the head (blue), left (red) and right (green) antennal tips of a stick insect climbing a stair. Top: In the intact animal (see Supplementary Video 3), both antennae are continuously swept through the near-range environment, so as to search for obstacles. Bottom: After de-cerebration, the same animal can still walk but no longer moves its antennae (see Supplementary Video 4). The animal fails to climb, and antennal tips are passively deflected downwards when contacting the stair. Inserts show the distributions of antennal pointing directions on a sphere centred on the head. Axes: x, forward/rostral (red); y, leftward/lateral (green); z, upward/dorsal (blue). b During visually induced transition from straight walking to curve walking, both antennae shift their beating field into the direction of turning. Time courses of the head (blue, H), outer (red, O) and inner (green, I) antenna during a clockwise turn. Same paradigm as used for Fig. 2a. Grey shading marks the duration of the visual motion stimulus. c Behavioural paradigms in which the stick insect walks towards a rectangular block (d) or a horizontal rod (e). In both paradigms, intact animals first touch and then climb the obstacle. d) Animals with intact antennae (top) touch the obstacle earlier, begin to raise the body earlier and maintain a larger distance during climbing than animals with shortened antennae (bottom). For samples of head trajectories similar to the blue line in a, symbols show the mean head locations at the time of (i) first contact, (ii) nearest point to the lower corner, and (iii) elevation above 25 mm. Red symbols show sighted animals, blue show the same animals after blindfolding. The light blue line in the lower panel copies the blue line of the top panel. e Antennal contact with a horizontal rod induces fast re-retargeting of the on-going swing movement towards the rod. Red trajectory shows the movement of the left foot in a trial illustrated by the snapshots above (white numbers indicate time in s relative to first antennal contact). Blue asterisks mark foot position in the instants of four antennal contact events. Bottom panel shows the corresponding joint angle time courses. Soon after the first antennal contact with the rod, the leg is levated (blue) before being protracted (red) and extended (green). Thick line segments indicate episodes of leg contact with the rod. [a: Data from Krause et al. 2013; b: adapted from Dürr and Ebeling 2005; d: adapted from Dürr et al. 2003]
The entire neuromuscular infrastructure underlying antennal movements is located in the head (for review, see Staudacher et al. 2005), whereas the corresponding neuromuscular infrastructure of the legs is located in the thorax. As a consequence, four segments of the brain and ventral nerve cord separate the motor and sensory integration centre of antennae (the deutocerebrum) and legs (the thoracic ganglia). Therefore, it would not be surprising if antennal movements were largely independent of leg movements. Instead, antennal movements have been reported to be very different in standing and walking cockroaches (Okada and Toh 2004). In stick insects, it is very difficult to elicit rhythmic antennal movements in animals that do not walk, and stick insects never walk without moving their antennae (see Supplementary Video 3). Moreover, de-cerebrate stick insects can still walk but no longer move their antennae (Fig. 5a; see Supplementary Video 4). Nevertheless, it is possible to trigger coordinated rhythmic antennal movements in these de-cerebrated animals by application of pilocarpine (Krause et al. 2013); similar to pilocarpine-induced motor nerve activity in the cockroach brain (Okada et al. 2009). This suggests that antennal motor networks are still functional in de-cerebrate animals, but lack an activating input. In turning stick insects, the antennal beating field shifts towards the turning direction (Fig. 5b; Dürr and Ebeling 2005) and loss of foothold by a front leg not only induces searching movements of the legs, but also affects the concurrent movement patterns of the antennae (Dürr 2001). All of this shows that locomotor activity of the legs strongly affects the pattern of active antennal movements. By doing so, locomotion behaviour goes in-hand with an adjustment of active tactile exploration of the near-range environment.
Once antennal movement leads to contact with an external object, it can induce short-latency effects in ongoing leg movements, but also in the antennal movement pattern itself. As a result, active near-range exploration by the antennae improves the efficiency of locomotion in an unpredictable environment (Fig. 5c, d). In cockroaches, antennal contact induces a turning movement towards the contacted side (Okada and Toh 2001) and a sustained contact is relevant to tactile orientation behaviour (Camhi and Johnson 1999; Mongeau et al. 2013). In stick insects, the front-leg that is ipsilateral to the contacting antenna often shows an aimed reach-to-grasp movement to the newly detected object (Schütz and Dürr 2011), while the antennae change their pattern of inter-joint coupling, both on the ipsilateral (Schütz and Dürr 2011) and on the contralateral side (Krause and Dürr 2012). As illustrated in Fig. 5e, front legs may respond to an antennal contact even during an ongoing swing movement. This response is not a fixed reflex action but adapts at least to the height of the antennal contact (Schütz and Dürr 2011; in Fig. 5e, this is reflected by adjustment of femoral levation soon after the first antennal contact). Such rapid motor responses of the legs to antennal contact events show that antennal near-range exploration efficiently detects novel objects and induces appropriate changes in locomotion. Thus, antennal exploration clearly is in the service of locomotion. Together with the observation that antennal near-range exploration depends on locomotion and may even require an initiating ascending input to the brain (Fig. 5; Krause et al. 2013), the integration of antennal tactile cues in motor control of the legs underscores the mutual interdependency of locomotion and near-range exploration.
In summary, there is an intimate link between locomotion and near-range exploration in both antennal and leg movements. Given the relevance of sensory feedback for flexible locomotion (“sensing for acting”) and the relevance of active exploratory movements for the acquisition of suitable sensory input (“acting for sensing”), the distinction between locomotion and near-range exploration becomes a gradual one, at least within the action volume spanned by the possible movements of all limbs of the animal.
Summary and conclusions
In taking a dedicated behavioural perspective, this review covered five aspects of distributed and multifaceted sensory feedback in the flexible control of insect motor behaviour. The first of these concerned the behavioural relevance of the experimental paradigm and the diverse functions of the so-called walking legs of insects. We argue that, because walking legs do not serve locomotion only but may be recruited for many different behaviours, the goal of the behaviour should determine which kinds of sensory feedback are integrated into the control of leg movements, and in which way. By loosely grouping the various mechanoreceptors into those that monitor interaction forces between the body and the environment, and those that monitor body posture, we propose to categorise movements into (i) those that are mechanically uncoupled from other legs and from the ground (e.g., the swing episode of the step cycle, searching and reaching in stick insects, grooming in locusts) and (ii) those that are mechanically coupled to other legs via the ground (e.g., the stance episode of a step cycle). Whereas the former are likely to be dominated by postural feedback, the latter are likely to be dominated by load/force feedback. We further argue that, depending on the goal of the current behaviour, or on the experimental paradigm used, the relevant types and working-ranges of sensory feedback channels may differ. Therefore, the behavioural relevance of the experimental paradigm and, in particular, the appropriate involvement of sensory feedback, must be considered when interpreting experimental results with regard to neural mechanisms at work.
The second aspect concerns the flexibility of coordination among legs, the quasi-rhythmicity of unrestrained locomotion in particular. We argue that much of the observed experimental variability is likely to be more than just a sign of noisy or imprecise neural computations. Instead, it should be considered a sign of variable sensory feedback. For example, when walking or climbing through an unpredictable environment, sensory feedback is continuously integrated into the control of single-leg movements and inter-leg coordination. Variation of limb kinematics and surface structure gives rise to considerable variation in the sensory experience of the interaction between body and environment. We claim that this variation is functionally relevant because it depends on the current context and therefore contributes to flexibility of sensorimotor control. Although we focus on the gait continuum in insect locomotion (Fig. 1), the quasi-rhythmicity of inter-leg coordination for a given behavioural output (Fig. 2a) and the inadequacy of considering one degree of freedom alone to account for behavioural flexibility of inter-leg coordination (Fig. 2b), we could have chosen examples of variability of single-leg movements as well. Future research will need to address the question how much of the variability—intra-trial, inter-trial, inter-individual, and inter-species—is caused by the interaction of the animal with its environment, by internal state, individual predisposition, or simply by noise. Potentially, this may also reveal how differences between species relate to their behavioural repertoire.
The third part of the review focuses on recent evidence for the significance of sensory feedback in locomotor flexibility. In particular, we discuss the occurrence of intermittent short steps (Fig. 3b), spatial coordination among legs (Fig. 3c), and evidence regarding the functional distinction of searching and stepping (behavioural, Fig. 3d; neurophysiological, Fig. 4b). Sensory feedback about interaction forces affects the likelihood of short steps and, therefore, a class of steps that may serve the finding of appropriate grip during climbing. On the other hand, postural feedback from a proprioceptive hair field affects the control of swing height (Fig. 3d, right), the spatial coordination of touch-down (Fig. 3c) and the likelihood of intermittent searching movements (Fig. 3d, left and middle), all of which concern the choice of appropriate foothold location (of two legs, the one carrying the proprioceptor, and its posterior neighbour). This led us to the discussion of the possibility that stepping (and, therefore, walking) should be considered an alternating sequence of posture-controlled swing and interaction-force-controlled stance movements, rather than considering a step cycle as one functional unit of locomotion. By contrasting two alternative hypotheses concerning the functional dissociation of cyclic searching and stepping movements (Fig. 4), we emphasise the need for further experimental evidence on the questions as to (i) how pre-programmed certain movement types really are, and (i) how variable sensory feedback is involved in the transition from one movement type to the next.
This issue was then taken up by the fourth section, discussing evidence from computational studies that demonstrate how the alternation of posture- and force-dependent control may greatly improve the behavioural flexibility of six-legged locomotion models. One aspect of this was that postural control of a single leg allows rapid modification of a standard swing movement into a targeted reach as well as into a cyclic search. Moreover, it allows spatial coordination among legs that may be of particular relevance in behaviours such as turning and climbing. Another aspect concerns the fact that force-dependent control of stance simplifies the context-dependent adaptation of interaction forces so as to propel the centre of mass into a desired direction at a desired speed. Future research will have to show how much afferent feedback about load/force may also contribute to inter-leg coordination in insect locomotion.
Finally, we discuss the intimate interplay of locomotion and dedicated near-range exploratory movements by antennae and legs. With reference to the effects of locomotor activity on exploration and the continuous integration of afferent information in the adjustment of ongoing movements, we argue that the distinction of locomotion and near-range exploration is rather artificial. This is true for the action volume of the limbs: a volume analogous to what is called the peri-personal space of humans. Because of the confinement to this near-range volume, near-range exploration may differ from other exploration behaviours. An example of this is navigation behaviour where a spatial location (e.g., of the nest) has to be inferred from comparing current experience with cues stored in memory. Therefore, exploration behaviour in the context of navigation requires some internal representation. To date, it is unclear whether near-range exploration of antennae and legs leads to the formation of any kind of spatial representation in insects. In cats, it has been shown that a spatial memory is formed as the animal steps across an obstacle (McVea and Pearson 2006). Potentially, the tactually induced short-term memory component in the searching movements of stick insects (Berg et al. 2013) may point at a related spatial memory in insects (see also Horridge 1962). However, so far, there is little more evidence than transient changes in limb kinematics.
We conclude that future research on motor flexibility should consider five aspects in order to improve our understanding of how functionally distinct movements may be triggered, controlled and coordinated according to the current context and goal of the animal:
-
(i)
Behavioural relevance of the experimental paradigm, in particular with regard to the natural input range of all mechanoreceptors potentially involved.
-
(ii)
Analysis of reasonably large data sets (both in terms of trial numbers and degrees of freedom of movement recorded) on unrestrained moving insects, allowing the functional distinction of movement types per leg, and of consistent changes in coordination among several degrees of freedom.
-
(iii)
Separate analyses of mechanically coupled and mechanically uncoupled leg movements, accounting for flexible control of interaction forces on the one hand, and the flexible control of foot position and velocity on the other.
-
(iv)
Inclusion of top-down aspects in computational modelling of motor flexibility, emphasising distinct behavioural functions as opposed to distinct neurons and networks.
-
(v)
Consideration of all limb movements as an act of active near-range exploration, either of body-environment interaction, or of ambient space.
References
Ahn AN, Full RJ (2002) A motor and a brake: two leg extensor muscles acting at the same joint manage energy differently in a running insect. J Exp Biol 205(Pt 3):379–389
Alexander RM (2003) Principles of animal locomotion. Princeton University Press, Princeton. https://doi.org/10.1515/9781400849512
Bässler U (1983) Neural basis of elementary behavior in stick insects. Springer, Berlin. https://doi.org/10.1007/978-3-642-68813-3
Bässler U, Büschges A (1998) Pattern generation for stick insect walking movements—multisensory control of a locomotor program. Brain Res Rev 27(1):65–88. https://doi.org/10.1016/S0165-0173(98)00006-X
Berendes V, Zill SN, Büschges A, Bockemühl T (2016) Speed-dependent interplay between local pattern-generating activity and sensory signals during walking in Drosophila. J Exp Biol 219(23):3781–3793. https://doi.org/10.1242/jeb.146720
Berg E, Büschges A, Schmidt J (2013) Single perturbations cause sustained changes in searching behavior in stick insects. J Exp Biol 216(6):1064–1074. https://doi.org/10.1242/jeb.076406
Berg EM, Hooper SL, Schmidt J, Büschges A (2015) A leg-local neural mechanism mediates the decision to search in stick insects. Curr Biol 25(15):2012–2017. https://doi.org/10.1016/j.cub.2015.06.017
Berkowitz A, Laurent G (1996) Local control of leg movements and motor patterns during grooming in locusts. J Neurosci 16(24):8067–8078
Bläsing B (2006) Crossing large gaps: a simulation study of stick insect behavior. Adapt Behav 14(3):265–285. https://doi.org/10.1177/105971230601400307
Bläsing B, Cruse H (2004) Stick insect locomotion in a complex environment: climbing over large gaps. J Exp Biol 207(8):1273–1286. https://doi.org/10.1242/jeb.00888
Blickhan R, Full RJ (1993) Similarity in multilegged locomotion: bouncing like a monopode. J Comp Physiol A 173:509–517
Borgmann A, Hooper SL, Büschges A (2009) Sensory feedback induced by front-leg stepping entrains the activity of central pattern generators in caudal segments of the stick insect walking system. J Neurosci 29(9):2972–2983. https://doi.org/10.1523/JNEUROSCI.3155-08.2009
Brunn DE, Dean J (1994) Intersegmental and local interneurons in the metathorax of the stick insect Carausius morosus that monitor middle leg position. J Neurophysiol 72(3):1208–1219
Büschges A (1995) Role of local nonspiking interneurons in the generation of rhythmic motor activity in the stick insect. J Neurobiol 27(4):488–512. https://doi.org/10.1002/neu.480270405
Büschges A (2012) Lessons for circuit function from large insects: towards understanding the neural basis of motor flexibility. Curr Opin Neurobiol 22(4):602–608. https://doi.org/10.1016/j.conb.2012.02.003
Büschges A, Gruhn M (2007) Mechanosensory feedback in walking: from joint control to locomotor patterns. Adv Insect Physiol 34:193–230. https://doi.org/10.1016/S0065-2806(07)34004-6
Büschges A, Schmitz J, Bässler U (1995) Rhythmic patterns in the thoracic nerve cord of the stick insect induced by pilocarpine. J Exp Biol 198:435–456
Camhi JM, Johnson EN (1999) High-frequency steering maneuvers mediated by tactile cues: antennal wall-following in the cockroach. J Exp Biol 202(Pt 5):631–643
Cruse H (1976) The function of the legs in the free walking stick insect Carausius morosus. J Comp Physiol 112(2):235–262. https://doi.org/10.1007/BF00606541
Cruse H (1979) The control of the anterior extreme position of the hindleg of a walking insect, Carausius morosus. Physiol Entomol 4(2):121–124. https://doi.org/10.1111/j.1365-3032.1979.tb00186.x
Cruse H (1990) What mechanisms coordinate leg movement in walking arthropods? Trends Neurosci 13(1):15–21. https://doi.org/10.1016/0166-2236(90)90057-H
Cruse H, Dean J, Suilmann M (1984) The contributions of diverse sense organs in the control of leg movement by a walking insect. J Comp Physiol 154(5):695–705. https://doi.org/10.1007/BF01350223
Cruse H, Bartling C, Dreifert M, Schmitz J, Brunn DE, Dean J, Kindermann T (1995) Walking: a complex behaviour controlled by simple networks. Adapt Behav 3(4):385–418. https://doi.org/10.1177/105971239500300403
Cruse H, Kindermann T, Schumm M, Dean J, Schmitz J (1998) Walknet - a biologically inspired network to control six-legged walking. Neural Netw 11(7-8):1435–1447. https://doi.org/10.1016/S0893-6080(98)00067-7
Cruse H, Dürr V, Schilling M, Schmitz J (2009) Principles of insect locomotion. In: Arena P, Patanè L (eds) Spatial temporal patterns for action-oriented perception in roving robots. Springer, Berlin, pp 43–96. https://doi.org/10.1007/978-3-540-88464-4_2
Dallmann CJ, Dürr V, Schmitz J (2016) Joint torques in a freely walking insect reveal distinct functions of leg joints in propulsion and posture control. Proc R Soc B 283(1823):20151708. https://doi.org/10.1098/rspb.2015.1708
Dallmann CJ, Hoinville T, Dürr V, Schmitz J (2017) A load-based mechanism for inter-leg coordination in insects. Proc R Soc B 284(1868):20171755. https://doi.org/10.1098/rspb.2017.1755
Dean J (1990) Coding proprioceptive information to control movement to a target: simulation with a simple neural network. Biol Cybern 63(2):115–120. https://doi.org/10.1007/BF00203033
Dean J, Schmitz J (1992) The two groups of sensilla in the ventral coxal hairplate of Carausius morosus have different roles during walking. Physiol Entomol 17(4):331–341. https://doi.org/10.1111/j.1365-3032.1992.tb01031.x
Dean J, Wendler G (1983) Stick insect locomotion on a walking wheel: interleg coordination of leg position. J Exp Biol 103:75–94
Delcomyn F (1980) Neural basis of rhythmic behaviour in animals. Science 210(4469):492–498. https://doi.org/10.1126/science.7423199
Dickinson MH, Farley CT, Full RJ, Koehl MAR, Kram R, Lehman S (2000) How animals move: an integrative view. Science 288(5463):100–106. https://doi.org/10.1126/science.288.5463.100
Donelan JM, Pearson KG (2004) Contribution of force feedback to ankle extensor activity in decerebrate walking cats. J Neurophysiol 92(4):2093–2104. https://doi.org/10.1152/jn.00325.2004
Duch C, Pflüger HJ (1995) Motor patterns for horizontal and upside-down walking and vertical climbing in the locust. J Exp Biol 198(Pt 9):1963–1976
Dürr V (2001) Stereotypic leg searching-movements in the stick insect: kinematic analysis, behavioural context and simulation. J Exp Biol 204(Pt 9):1589–1604
Dürr V (2005) Context-dependent changes in strength and efficacy of leg coordination mechanisms. J Exp Biol 208(12):2253–2267. https://doi.org/10.1242/jeb.01638
Dürr V, Ebeling W (2005) The behavioural transition from straight to curve walking: kinetics of leg movement parameters and the initiation of turning. J Exp Biol 208(12):2237–2252. https://doi.org/10.1242/jeb.01637
Dürr V, Matheson T (2003) Graded limb targeting in an insect is caused by the shift of a single movement pattern. J Neurophysiol 90(3):1754–1765. https://doi.org/10.1152/jn.00416.2003
Dürr V, König Y, Kittmann R (2001) The antennal motor system of the stick insect Carausius morosus: anatomy and antennal movement pattern during walking. J Comp Physiol A 187(2):131–144
Dürr V, Krause AF, Schmitz J, Cruse H (2003) Neuroethological concepts and their transfer to walking machines. Int J Robot Res 22(3-4):151–167. https://doi.org/10.1177/0278364903022003002
Dürr V, Schmitz J, Cruse H (2004) Behaviour-based modelling of hexapod locomotion: linking biology and technical application. Arthropod Struct Dev 33(3):237–250. https://doi.org/10.1016/j.asd.2004.05.004
Duysens J, Clarac C, Cruse H (2000) Load regulation mechanisms in gait and posture: comparative aspects. Physiol Rev 80(1):83–133
Ebeling W, Dürr V (2006) Perturbation of leg protraction causes context-dependent modulation of inter-leg coordination, but not of avoidance reflexes. J Exp Biol 209(11):2199–2214. https://doi.org/10.1242/jeb.02251
Ekeberg Ö, Pearson KG (2005) Computer simulation of stepping in the hind legs of the cat: an examination of mechanisms regulating the stance-to-swing transition. J Neurophysiol 94(6):4256–4268. https://doi.org/10.1152/jn.00065.2005
Ekeberg Ö, Blümel M, Büschges A (2004) Dynamic simulation of insect walking. Arthropod Struct Dev 33(3):287–300. https://doi.org/10.1016/j.asd.2004.05.002
Elias DO, Maddison WP, Peckmezian C, Girard MB, Mason AC (2012) Orchestrating the score: complex multimodal courtship in the Habronattus coecatus group of Habronattus jumping spiders (Araneae: Salticidae). Biol J Linn Soc 105(3):522–547. https://doi.org/10.1111/j.1095-8312.2011.01817.x
Field LH, Matheson T (1998) Chordotonal organs of insects. Adv Insect Physiol 27:1–288. https://doi.org/10.1016/S0065-2806(08)60013-2
Fujiwara T, Cruz TL, Bohnslav JP, Chiappe ME (2017) A faithful internal representation of walking movements in the Drosophila visual system. Nat Neurosci 20(1):72–81. https://doi.org/10.1038/nn.4435
Full RJ, Blickhan R, Ting LH (1991) Leg design in hexapedal runners. J Exp Biol 158:369–390
Goldman DI, Chen TS, Dudek DM, Full RJ (2006) Dynamics of rapid vertical climbing in a cockroach reveals a template. J Exp Biol 209(15):2990–3000. https://doi.org/10.1242/jeb.02322
Grabowska M, Godlewska E, Schmidt J, Daun-Gruhn S (2012) Quadrupedal gaits in hexapod animals - inter-leg coordination in free-walking adult stick insects. J Exp Biol 215(24):4255–4266. https://doi.org/10.1242/jeb.073643
Harley CM, English BA, Ritzmann RE (2009) Characterization of obstacle negotiation behaviors in the cockroach, Blaberus discoidalis. J Exp Biol 212(10):1463–1476. https://doi.org/10.1242/jeb.028381
Hedwig B, Heinrich R (1997) Identified descending brain neurons control different stridulatory motor patterns in an acridid grasshopper. J Comp Physiol A 180(3):285–294. https://doi.org/10.1007/s003590050048
Hoinville T, Schilling M, Cruse H (2015) Control of rhythmic behavior: central and peripheral influences to pattern generation. In: Proceedings of ICRA workshop on “pros and cons of central pattern generators” IEEE, Seattle
Horridge GA (1962) Learning of leg position by the ventral nerve cord in headless insects. Proc R Soc Lond B 157(966):33–52. https://doi.org/10.1098/rspb.1962.0061
Horseman BG, Gebhardt MJ, Honegger H-W (1997) Involvement of the suboesophageal and thoracic ganglia in the control of antennal movements in crickets. J Comp Physiol A 181:195–204
Hughes GM (1952) The co-ordination of insect movements: I. The walking movements of insects. J Exp Biol 29:267–285
Ikeda K, Wiersma CAG (1964) Autogenic rhythmicity in the abdominal ganglia of the crayfish: the control of swimmeret movements. Comp Biochem Physiol 12:107–115
Isakov A, Buchanan SM, Sullivan B, Ramachandran A, Chapman JKS, ES L, Mahadevan L, de Bivort BL (2016) Recovery of locomotion after injury in Drosophila depends on proprioception. J Exp Biol 219(11):1760–1771. https://doi.org/10.1242/jeb.133652
Keller BR, Duke ER, Aymer AS, Zill SN (2007) Tuning posture to body load: decreases in load produce discrete sensory signals in the legs of freely standing cockroaches. J Comp Physiol A 193(8):881–891. https://doi.org/10.1007/s00359-007-0241-y
Kindermann T (2002) Behavior and adaptability of a six-legged walking system with highly distributed control. Adapt Behav 9:16–41
Krause AF, Dürr V (2012) Active tactile sampling by an insect in a step-climbing paradigm. Front Behav Neurosci 6:1–17
Krause AF, Winkler A, Dürr V (2013) Central drive and proprioceptive control of antennal movements in the walking stick insect. J Physiol Paris 107(1-2):116–129. https://doi.org/10.1016/j.jphysparis.2012.06.001
Krishnan A, Sane SP (2015) Antennal mechanosensors and their evolutionary antecedents. Adv Insect Physiol 49:59–99. https://doi.org/10.1016/bs.aiip.2015.06.003
Ludwar BC, Goeritz ML, Schmidt J (2005) Intersegmental coordination of walking movements in stick insects. J Neurophysiol 93(3):1255–1265. https://doi.org/10.1152/jn.00727.2004
Markl H (1962) Borstenfelder an den Gelenken als Schweresinnesorgane bei Ameisen und anderen Hymenopteren. Z Vergl Physiol 45(5):475–569. https://doi.org/10.1007/BF00342998
McGhee RB, Iswandhi GI (1979) Adaptive locomotion of a multilegged robot over rough terrain. IEEE T Syst Man Cyb 9(4):176–182. https://doi.org/10.1109/TSMC.1979.4310180
McVea DA, Pearson KG (2006) Long-lasting memories of obstacles guide leg movements in the walking cat. J Neurosci 26(4):1175–1178. https://doi.org/10.1523/JNEUROSCI.4458-05.2006
Moll K, Roces F, Federle W (2013) How load-carrying ants avoid falling over: mechanical stability during foraging in Atta vollenweideri grass-cutting ants. PLoS One 8(1):e52816. https://doi.org/10.1371/journal.pone.0052816
Mongeau JM, Demir A, Lee J, Cowan NJ, Full RJ (2013) Locomotion- and mechanics-mediated tactile sensing: antenna reconfiguration simplifies control during high-speed navigation in cockroaches. J Exp Biol 216(24):4530–4541. https://doi.org/10.1242/jeb.083477
Müller-Wilm U, Dean J, Cruse H, Weidemann HJ, Eltze J, Pfeiffer F (1992) Kinematic model of stick insect as an example of a 6-legged walking system. Adapt Behav 1(2):155–169. https://doi.org/10.1177/105971239200100202
Okada J, Toh Y (2001) Peripheral representation of antennal orientation by the scapal hair plate of the cockroach Periplaneta americana. J Exp Biol 204(Pt 24):4301–4309
Okada J, Toh Y (2004) Spatio-temporal patterns of antennal movements in the searching cockroach. J Exp Biol 207(21):3693–3706. https://doi.org/10.1242/jeb.01201
Okada J, Morimoto Y, Toh Y (2009) Antennal motor activity induced by pilocarpine in the American cockroach. J Comp Physiol A 195(4):351–363. https://doi.org/10.1007/s00359-008-0411-6
Owaki D, Ishiguro A (2017) A quadruped robot exhibiting spontaneous gait transitions from walking to trotting to galloping. Sci Rep 7(1):277. https://doi.org/10.1038/s41598-017-00348-9
Owaki D, Kano T, Nagasawa K, Atsushi T, Ishiguro A (2013) Simple robot suggests physical interlimb communication is essential for quadruped walking. J R Soc Interface 10(78):20120669. https://doi.org/10.1098/rsif.2012.0669
Page KL, Matheson T (2009) Functional recovery of aimed scratching movements following a graded proprioceptive manipulation. J Neurosci 29(12):3897–3907. https://doi.org/10.1523/JNEUROSCI.0089-09.2009
Pal PK, Jayarajan K (1990) A free gait for generalized motion. IEEE T Robot Autom 6(5):597–600. https://doi.org/10.1109/70.62049
Pelletier Y, McLeod CD (1994) Obstacle perception by insect antennae during terrestrial locomotion. Physiol Entomol 19(4):360–362. https://doi.org/10.1111/j.1365-3032.1994.tb01063.x
Pfeffer SE, Wittlinger M (2016) Optic flow odometry operates independently of stride integration in carried ants. Science 353(6304):1155–1157. https://doi.org/10.1126/science.aaf9754
Pfeffer SE, Wahl VL, Wittlinger M (2016) How to find home backwards? Locomotion and inter-leg coordination during rearward walking of Cataglyphis fortis desert ants. J Exp Biol 219(14):2110–2118. https://doi.org/10.1242/jeb.137778
Pick S, Strauss R (2005) Goal-driven behavioral adaptations in gap-climbing Drosophila. Curr Biol 15(16):1473–1478. https://doi.org/10.1016/j.cub.2005.07.022
Poulet JFA, Hedwig B (2002) A corollary discharge maintains auditory sensitivity during sound production. Nature 418(6900):872–876. https://doi.org/10.1038/nature00919
Reinhardt L, Weihmann T, Blickhan R (2009) Dynamics and kinematics of ant locomotion: do wood ants climb on level surfaces? J Exp Biol 212(15):2426–2435. https://doi.org/10.1242/jeb.026880
Riley JR, Greggers U, Smith AD, Reynolds DR, Menzel R (2005) The flight paths of honeybees recruited by the waggle dance. Nature 435(7039):205–207. https://doi.org/10.1038/nature03526
Schilling M, Hoinville T, Schmitz J, Cruse H (2013a) Walknet, a bio-inspired controller for hexapod walking. Biol Cybern 107(4):397–419. https://doi.org/10.1007/s00422-013-0563-5
Schilling M, Paskarbeit J, Hoinville T, Hüffmeier A, Schneider A, Schmitz J, Cruse H (2013b) A hexapod walker using a heterarchical architecture for action selection. Front Comput Neurosci 7:126. https://doi.org/10.3389/fncom.2013.00126
Schumm M, Cruse H (2006) Control of swing movement: influences of differently shaped substrate. J Comp Physiol A 192(10):1147–1164. https://doi.org/10.1007/s00359-006-0147-0
Schütz C, Dürr V (2011) Active tactile exploration for adaptive locomotion in the stick insect. Philos Trans R Soc B 366(1581):2996–3005. https://doi.org/10.1098/rstb.2011.0126
Seeds AM, Ravbar P, Chung P, Hampel S, Midgley FM, Mensh BD, Simpson JH (2014) A suppression hierarchy among competing motor programs drives sequential grooming in Drosophila. elife 3:e02951. https://doi.org/10.7554/eLife.02951
Staudacher E, Schildberger K (1999) A newly described neuropile in the deuterocerebrum of the cricket: antennal afferents and descending interneurons. Zool Anal Complex Syst 102:212–226
Staudacher E, Gebhardt MJ, Dürr V (2005) Antennal movements and mechanoreception: neurobiology of active tactile sensors. Adv Insect Physiol 32:49–205. https://doi.org/10.1016/S0065-2806(05)32002-9
Strauss R, Heisenberg M (1990) Coordination of legs during straight walking and turning in Drosophila melanogaster. J Comp Physiol A 167(3):403–412
Szczecinski NS, Brown AE, Bender JA, Quinn RD, Ritzmann RE (2014) A neuromechanical simulation of insect walking and transition to turning of the cockroach Blaberus Discoidalis. Biol Cybern 108(1):1–21. https://doi.org/10.1007/s00422-013-0573-3
Theunissen LM, Dürr V (2013) Insects use two distinct classes of steps during unrestrained locomotion. PLoS One 8(12):e85321. https://doi.org/10.1371/journal.pone.0085321
Theunissen LM, Vikram S, Dürr V (2014) Spatial co-ordination of foot contacts in unrestrained climbing insects. J Exp Biol 217(18):3242–3253. https://doi.org/10.1242/jeb.108167
Theunissen LM, Bekemeier HH, Dürr V (2015) Comparative whole-body kinematics of closely related insect species with different body morphology. J Exp Biol 218(3):340–352. https://doi.org/10.1242/jeb.114173
Ting LH, Blickhan R, Full RJ (1994) Dynamic and static stability in hexapedal runners. J Exp Biol 197:251–269
Toth TI, Daun S (2017) Effects of functional decoupling of a leg in a model of stick insect walking incorporating three ipsilateral legs. Physiol Rep 5(4):e13154. 10.14814/phy2.13154
Tryba AK, Ritzmann RE (2000) Multi-joint coordination during walking and foothold searching in the Blaberus cockroach. II. Extensor motor neuron pattern. J Neurophysiol 83(6):3337–3350
Twickel AV, Büschges A, Pasemann F (2011) Deriving neural network controllers from neuro-biological data: implementation of a single-leg stick insect controller. Biol Cybern 104:95–119
von Buddenbrock W (1920) Der Rhythmus der Schreitbewegungen der Stabheuschrecke Dyxippus. Biol Zentralbl 41:41–48
von Holst E (1943) Über relative Koordination bei Arthropoden. Pflügers Arch 246(6):847–865. https://doi.org/10.1007/BF01751829
Wahl V, Pfeffer SE, Wittlinger M (2015) Walking and running in the desert ant Cataglyphis fortis. J Comp Physiol A 201(6):645–656. https://doi.org/10.1007/s00359-015-0999-2
Wehner R (2003) Desert ant navigation: how miniature brains solve complex tasks. J Comp Physiol A 189(8):579–588. https://doi.org/10.1007/s00359-003-0431-1
Weihmann T, Blickhan R (2009) Comparing inclined locomotion in a ground-living and a climbing ant species: sagittal plane kinematics. J Comp Physiol A 195(11):1011–1020. https://doi.org/10.1007/s00359-009-0475-y
Wendler G (1964) Laufen und Stehen der Stabheuschrecke: Sinnesborsten in den Beingelenken als Glieder von Regelkreisen. Z Vergl Physiol 48(2):198–250. https://doi.org/10.1007/BF00297860
Whelan PJ, Hiebert GW, Pearson KG (1995) Stimulation of the group I extensor afferents prolongs the stance phase in walking cats. Exp Brain Res 103(1):20–30
Wilson DM (1966) Insect walking. Annu Rev Entomol 11(1):103–122. https://doi.org/10.1146/annurev.en.11.010166.000535
Wittlinger M, Wehner R, Wolf H (2006) The ant odometer: stepping on stilts and stumps. Science 312(5782):1965–1967. https://doi.org/10.1126/science.1126912
Wöhrl T, Reinhardt L, Blickhan R (2017) Propulsion in hexapod locomotion: how do desert ants traverse slopes? J Exp Biol 220(9):1618–1625. https://doi.org/10.1242/jeb.137505
Wosnitza A, Bockemühl T, Dübbert M, Scholz H, Büschges A (2013) Inter-leg coordination in the control of walking speed in Drosophila. J Exp Biol 216(3):480–491. https://doi.org/10.1242/jeb.078139
Zill SN, Schmitz J, Büschges A (2004) Load sensing and control of posture and locomotion. Arthropod Struct Dev 33(3):273–286. https://doi.org/10.1016/j.asd.2004.05.005
Zill SN, Keller BR, Duke ER (2009) Sensory signals of unloading in one leg follow stance onset in another leg: transfer of load and emergent coordination in cockroach walking. J Neurophysiol 101(5):2297–2304. https://doi.org/10.1152/jn.00056.2009
Zill SN, Keller BR, Chaudhry S, Duke ER, Neff D, Quinn R, Flannigan C (2010) Detecting substrate engagement: responses of tarsal campaniform sensilla in cockroaches. J Comp Physiol A 196(6):407–420. https://doi.org/10.1007/s00359-010-0526-4
Zill SN, Büschges A, Schmitz J (2011) Encoding of force increases and decreases by tibial campaniform sensilla in the stick insect, Carausius morosus. J Comp Physiol A 197(8):851–867. https://doi.org/10.1007/s00359-011-0647-4
Zill SN, Schmitz J, Chaudhry S, Büschges A (2012) Force encoding in stick insect legs delineates a reference frame for motor control. J Neurophysiol 108(5):1453–1472. https://doi.org/10.1152/jn.00274.2012
Zill SN, Chaudhry S, Büschges A, Schmitz J (2013) Directional specificity and encoding of muscle forces and loads by stick insect tibial campaniform sensilla, including receptors with round cuticular caps. Arthropod Struct Dev 42(6):455–462. https://doi.org/10.1016/j.asd.2013.10.001
Zill SN, Chaudhry S, Exter A, Büschges A, Schmitz J (2014) Positive force feedback in development of substrate grip in the stick insect tarsus. Arthropod Struct Dev 43(5):441–455. https://doi.org/10.1016/j.asd.2014.06.002
Zill SN, Chaudhry S, Büschges A, Schmitz J (2015) Force feedback reinforces muscle synergies in insect legs. Arthropod Struct Dev 44(6):541–553. https://doi.org/10.1016/j.asd.2015.07.001
Zollikofer CPE (1994) Stepping patterns in ants. I. Influence of speed and curvature. J Exp Biol 192(1):95–106
Zurek DB, Gilbert C (2014) Static antennae act as locomotory guides that compensate for visual motion blur in a diurnal, keen-eyed predator. Proc R Soc B 281(1779):20133072. https://doi.org/10.1098/rspb.2013.3072
Acknowledgements
The authors thank the students of the “control of behaviour” master course 2016 and Bianca Jaske for collecting data on free walking insects. Part of these data was used for Fig. 1a, and Fig. 2b. Moreover, we thank two anonymous reviewers for their helpful comments and suggestions, and Yannick Günzel for his assistance in data management and analysis. This work was supported by the cluster of excellence 277, CITEC, funded by of the German Research Council, DFG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by H. Bleckmann
This manuscript is a contribution to the Topical Collection From Sensory Perception to Behavior — Guest Editors: Theo C. M. Bakker, Horst Bleckmann, Joachim Mogdans, Vera Schlüssel
Rights and permissions
About this article
Cite this article
Dürr, V., Theunissen, L.M., Dallmann, C.J. et al. Motor flexibility in insects: adaptive coordination of limbs in locomotion and near-range exploration. Behav Ecol Sociobiol 72, 15 (2018). https://doi.org/10.1007/s00265-017-2412-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2412-3