Abstract
Newborn offspring of animals often exhibit fully functional innate antipredator behaviors, but they may also require learning or further development to acquire appropriate responses. Experience allows offspring to modify responses to specific threats and also leaves them vulnerable during the learning period. However, antipredator behaviors used at one stage of a predator encounter may compensate for deficiencies at another stage, a phenomenon that may reduce the overall risk of young that are vulnerable at one or more stages. Few studies have examined age differences in the effectiveness of antipredator behaviors across multiple stages of a predator encounter. In this study, we examined age differences in the antipredator behaviors of California ground squirrels (Otospermophilus beecheyi) during the detection, interaction, and attack stages of Pacific rattlesnake (Crotalus oreganus) encounters. Using free-ranging squirrels, we examined the ability to detect free-ranging rattlesnakes, snake-directed behaviors after discovery of a snake, and responses to simulated rattlesnake strikes. We found that age was the most important factor in snake detection, with adults being more likely to detect snakes than pups. We also found that adults performed more tail flagging (a predator-deterrent signal) toward snakes and were more likely to investigate a snake’s refuge when interacting with a hidden snake. In field experiments simulating snake strikes, adults exhibited faster reaction times than pups. Our results show that snake detection improves with age and that pups probably avoid rattlesnakes and minimize time spent in close proximity to them to compensate for their reduced reaction times to strikes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predators threaten the reproductive success of most animals. Encounters with predators typically progress through a series of stages that could each result in death (Lima and Dill 1990; Fig. 1); thus, adaptations to respond appropriately at each stage are important for survival. Animals decrease their likelihood of encountering predators through avoidance (Kotler et al. 2002; Abramsky et al. 2002), but they must also exhibit defenses after an encounter takes place (Hileman and Brodie 1994; Hopkins et al. 2011). Prey benefit from (1) detecting the predator quickly, (2) avoiding the predator before it attacks (or dissuading it from attacking), and (3) evading predators that do attack (Lima and Dill 1990). It is important to examine the effectiveness of antipredator behaviors across multiple stages of predatory encounters because a behavior used at one stage may compensate for predation risk at another stage (Lind and Cresswell 2005).
The five main stages of a predatory encounter during which prey may utilize different antipredator behaviors to avoid death. An encounter occurs when both parties are at a distance where they are both able to detect each other. An interaction occurs when the prey positively detects the predator and exhibits a behavioral response. Prey may try to deter an attack via pursuit-deterrent signaling (Hasson 1991) or active harassment. If a predator attacks and captures its prey, the prey may still escape after capture (not shown on this diagram). Flow chart adapted from Lima and Dill (1990)
Animals often express antipredator behaviors immediately after birth or hatching. Innate expression of fully functional antipredator behavior occurs when there is strong selection to express the defense, usually when predation risk is high, pervasive, and constant and when flexibility after birth adds little to no benefit (Nonacs and Blumstein 2010). Solitary and precocial species are likely to produce offspring with relatively functional antipredator defenses because they do not benefit as much from the social-learning opportunities and group defense as social and/or altricial species (Sordahl 1988; Kehmeier et al. 2011). The form of an innate antipredator behavior should not differ substantially across ages if all age classes experience similar predation risk. However, young can also express an innate functional response that differs from the adult form if they experience different risks than adults (Wiedenmayer 2009). When behavioral defenses by young are appropriate for the risks associated with their age, age differences in behavior represent age-specific adaptations (Alberts and Cramer 1988; Hawlena et al. 2006; Landová et al. 2013). For example, ground-nesting scrubwren (Sericornis frontali) chicks respond appropriately to alarm calls toward predators that prey on eggs and nestlings, and they develop adult-like responses to aerial predator calls just before fledging (Platzen and Magrath 2005).
When post-birth flexibility is adaptive, for instance under changing environmental conditions, young may be born with defenses inappropriate for the risks associated with their age (Wiedenmayer 2009). Young then express less effective defenses compared with adults, and these age differences stem from young requiring learning or further development to acquire appropriate responses. For example, both Belding’s ground squirrels, Urocitellus beldingi (Mateo 1996a) and meerkats, Suricata suricatta (Hollén and Manser 2006) require experience to correctly discriminate and respond to conspecific alarm calls. Young animals often enhance their antipredator responses by watching older experienced individuals (Griffin 2004).
Antipredator behaviors are a composite of many behaviors that an animal can adjust to reduce predation risk (Lind and Cresswell 2005). When young require experience or further development to complete the development of one antipredator response, they may exhibit other behaviors to compensate for this deficiency. Possessing such a defensive repertoire would enhance survival during the learning period, but few studies have examined age differences in the effectiveness of antipredator behaviors across multiple stages of a predator encounter. Here, we address this shortcoming by examining the ontogeny of several defenses in free-ranging California ground squirrels (Otospermophilus beecheyi) across multiple stages of encounters with Pacific rattlesnakes (Crotalus oreganus).
Our system is ideal for examining the ontogeny of antipredator behaviors because we can quantify actual attack rates on prey and also easily simulate attacks in the field. The behaviors of free-ranging predators are often unexamined due to logistical constraints (Lima 2002); however, some studies have incorporated live predators in natural settings, and these findings provide valuable insight into predator–prey relationships and community ecology (FitzGibbon and Fanshawe 1988; Cresswell 1994; FitzGibbon 1994; Abramsky et al. 1997; Abramsky et al. 2002; Whitfield 2003). In our system, the sit-and-wait hunting mode of rattlesnakes and their tolerability to human disturbance facilitate observations of their hunting behavior.
Ground squirrels live in social groups and young individuals (pups) likely learn from mothers who actively defend them during the close association that occurs for several weeks after emergence from the natal burrow (Poran and Coss 1990; Swaisgood et al. 2003; Owings and Coss 2008). The maturation of perceptual processes important for predator recognition and alarm call response behavior develops through learning in squirrels (Mateo 1996a, b). However, even though pups reap the defensive benefits of group living and parental care, they remain exceptionally vulnerable to predation by rattlesnakes, which have been estimated to consume about 34 % of the annual reproductive output in a typical squirrel population (Fitch 1949). Pups are highly susceptible to venom compared with adults because their venom-neutralizing enzymes are overwhelmed due to their small body volume (Poran et al. 1987; Poran and Coss 1990). This strong source of selection imposed on young squirrels by snakes should thus result in the early organization of antisnake behaviors that persist in the absence of learning opportunities (see Tromborg and Coss 2015).
Predation by rattlesnakes can be avoided as long as squirrels detect snakes before entering the strike range (approximately 50 cm) (Clark et al. 2012). To our knowledge, no study in any predator–prey system has yet to compare the ability of different age classes to detect free-ranging predators. Lab-born squirrel pups that had never experienced snakes previously responded fearfully to snake scale patterns and odors (Coss 1991), suggesting the recognition of snakes as dangerous predators is innate. However, their ability to detect hidden snakes in the natural habitat may be modified through experience and/or development. In addition, differences in microhabitat structure (Whittingham et al. 2004; Devereux 2005), and in snake scale-pattern visibility may affect detection of rattlesnakes, and vary based on the age of the squirrel.
After squirrels discover a rattlesnake, both pups and adults will approach the snake and tail flag (Owings and Coss 2008), moving a raised tail from side to side. Tail flagging deters snakes from striking by signaling the squirrel’s vigilance; it also causes snakes to abandon the area by increasing vigilance in nearby prey (Hasson 1991; Barbour and Clark 2012; Putman and Clark 2015). Even if a snake strikes, squirrels may still avoid injury or death because they have evolved the ability to rapidly leap away from an attack, and tail flagging also informs snakes that a squirrel is prepared to jump (Owings and Coss 2008; Putman and Clark 2015). In other systems, predator-directed signals similar to tail flagging are exhibited more by the young than by adults to compensate for their increased predation risk (Owings and Loughry 1985; Caro and FitzGibbon 1992; Hawlena 2009; Medill et al. 2011). However, previous studies have not examined whether increased signaling deters predation, because attack rates on free-ranging prey were not quantified.
We examined age differences in antisnake behaviors during the detection, interaction, and attack stages of a rattlesnake encounter. If age differences are absent, a behavior is “unlearned” and developmentally stable in the defensive repertoire. If age differences exist, we may generate hypotheses on their functional significance based on whether the behaviors are appropriate for age-related risks (Wiedenmayer 2009) and whether defenses exhibited at one stage of an encounter compensate for deficiencies at another stage. Our study consists of two parts: (1) natural observations of encounters between free-ranging ground squirrels and rattlesnakes to understand how age affects detection of and response to snakes; and (2) field experiments on free-ranging squirrels to compare the ability of pups and adults in evading simulated rattlesnake strikes.
Materials and methods
Natural squirrel–snake interactions
Study sites
We recorded natural squirrel–snake interactions at three sites: (1) the Blue Oak Ranch Reserve (BORR), Santa Clara County, California, (2) Camp Ohlone, Sunol Regional Wilderness, Alameda County, California, and (3) Frog Pond, Ohlone Regional Wilderness, Alameda County, California. The Sunol and Ohlone Regional Wildernesses are approximately 30 km north of BORR, and within them, Frog Pond is 3 km west of Camp Ohlone. BORR is at a higher elevation than the other two (800 versus 400 m, respectively), but all three possess habitat characterized by steep to moderate hills covered by mixed oak woodlands and grasslands. We collected data from May–July in 2009–2012. Squirrel pups emerged from natal burrows in late May/early June and snakes generally began hunting in squirrel colonies just before this time.
Snake collection and surgery
At each study site, we captured Pacific rattlesnakes (C. oreganus) and surgically implanted them with temperature sensitive radio transmitters (models A1-2T and SI-2T, Holohil Systems Ltd, ON, Canada; model G3, AVM Instrument Company Ltd, CA, USA) using the methods of Reinert and Cundall (1982). Transmitters weighed less than 5 % of snake body mass. After surgery, we kept snakes at a field station until they resumed normal behaviors, and then we released them at their place of capture. We captured 22 adults at Frog Pond and Ohlone and 23 adults at BORR. For this study, we only report data from snakes that actively hunted within squirrel colonies: 11 males and 4 females from Frog Pond and Ohlone and 11 males and 6 females from BORR.
Squirrel trapping and marking
We trapped squirrels continuously throughout the summers using Tomahawk traps baited with black oil sunflower seed. Once captured, squirrels were sedated with 40 mg/kg of ketamine through injection into the hind leg muscle. We marked squirrels with Nyanzol pelage dye for short-term visual identification and metal ear tags for long-term identification. We also recorded their mass (±5.0 g), snout-to-anus length (±0.5 cm), tail length (±0.5 cm), and hind foot length (±0.5 cm). We sexed squirrels by their ano-genital distance, of which males have longer distances than females. After processing, we kept squirrels captive until they regained normal movement, then released them back at the point of capture within the same day of capture.
Field videography
We radio tracked snakes at least once daily and recorded their positions using a global positioning unit (Garmin Geko, ±6 m accuracy). We positioned a battery-powered portable surveillance camera over a snake if it appeared to be actively hunting within a squirrel colony (as evident by a stereotyped ambush posture; Clark 2004; Reinert et al. 2011). At Frog Pond and Ohlone, we used fixed security cameras (Swann PNP-150 and SuperCircuit PC161IR-2) that recorded data onto mini-digital video recorders (SVAT CVP800 and Supercircuits MDVR14-3). At BORR, we used wireless network security cameras (Sony SNC-RZ25N) attached to network radios (Nanostation M2), which allowed cameras to communicate with a field wireless internet network. We used laptop computers in the field to monitor and record video feeds from these wireless cameras and control their pan/tilt/zoom mechanisms in real time. Because we were continually monitoring snakes with wireless video, whenever a snake gave up at an ambush site and moved away from the camera, we relocated it using radio telemetry and repositioned the camera. Additionally, if we did not visibly see a snake on camera for more than one hour (i.e., it remained within a burrow or log), we physically checked its position using radio telemetry. Thus, we remained confident of all snake locations even when the snake was not visible. For this study, we only examined videos that were recorded after pups had emerged from natal burrows (at least 45 days post-natal).
Video analysis
In total, we captured 214 squirrel–snake encounters on camera. Sample video recordings from this study can be publicly viewed at our YouTube channel (http://www.youtube.com/user/rulonclark). For this study, we defined an encounter as a situation when a squirrel and snake were within 1.5 m of each other, a range at which they could detect each other under most conditions. Individual squirrel identity sometimes could not be discerned from video recordings because: (1) the angle of the squirrel’s body to the camera often prevented us from viewing its dye markings, (2) the dye markings had faded and become unrecognizable, or (3) the squirrel was never marked. Consequently, we only analyzed encounters of the first pup and first adult squirrel at a single snake location, and we included more encounters only if squirrels could be positively identified as unique individuals. Ground squirrels usually occupy a small home range around their home burrows; the diameter of the core use area of adult females is approximately 9 m, while the diameter of the core use area of adult males is approximately 5 m (Boellstrorff and Owings 1995). We assumed encounters that occurred more than 9 m apart involved separate individual squirrels (see Barbour and Clark 2012). Snakes moved, on average, 34.6 m (range, 9.8–154.0 m) between hunting locations, so most recordings were well over 9 m apart. We also removed encounters for which we could not confidently identify the snake’s location. Thus, we analyzed a total of 107 distinct encounters (38 pups and 69 adults).
Snake detection
For each encounter, we determined the time in seconds the squirrel spent within 1.5 m of a rattlesnake (termed encounter duration). We assumed squirrels remained unaware of the presence of the snake when they did not exhibit tail flagging or the fixed orientation posture indicative of snake awareness, termed elongate investigative posture (EIP; Owings and Coss 1977). If squirrels remained unaware of snake presence, the end of an encounter occurred when the squirrel left the video camera’s field of view, was struck by the rattlesnake, or leaped away from a strike attempt. For squirrels that tail flagged toward snakes and exhibited EIPs, encounters ended when the squirrel either left the video camera’s field of view, was struck by a rattlesnake, leaped away from a strike attempt, or when the squirrel began foraging, grooming, or dust bathing (i.e., it no longer exhibited antisnake behaviors).
Within the encounter duration, we quantified the total time in seconds the squirrel directed attention toward the snake and the proportion of total tail-flagging bouts directed toward the snake. We considered squirrels to be directed toward the snake if the snake’s body fell within 45 degrees of the axis defined by the anteroposterior plane bisecting the head of the squirrel. Since squirrels are known to tail flag in the absence of snakes (Hersek and Owings 1993; Hersek and Owings 1994), we used the presence of EIP and high rates of snake-directed behavior to categorize positive snake detection. Specifically, positive detections were those encounters that included the presence of an EIP, >50 % time directed at the snake, and >70 % tail-flagging bouts directed at the snake. Squirrel behavior had to meet all three criteria to be quantified as positive detection.
We recorded each squirrel as either a pup or an adult. Because our study occurred just after pups emerged from their natal burrow (pups were approximately 50 days old), they were easily distinguishable from adults based on their small body size. Pups start to become indistinguishable from adults at around 100 days of age (Hanson and Coss 1997). We excluded any encounters for which we were unsure of the age of the squirrel. Finally, we categorized the microhabitat of the snake as grass (outside a refuge within or on top of vegetation), burrow, or shelter (inside fallen log or under rock) and noted whether or not the snake was exposed (scale pattern or head visible on camera). Although we captured three times more females on camera than males (which was likely due to mothers’ increase in antisnake behaviors after pup emergence; Hersek and Owings 1993), we did not include squirrel sex in this study because both sexes use similar behaviors to deter predation when in close proximity to hunting snakes.
Snake-interaction behaviors
An encounter became an interaction when the squirrel initiated a behavioral response indicative of positive snake detection. In total, we recorded 36 adults and 13 pups that detected snakes. Our inability to determine squirrel identity precluded the use of their body size as a reference distance in analyses on closeness of approach (i.e., distance between snake and squirrel). However, current studies in our lab have shown that application of snake scent near burrows elicits burrow investigation behavior in squirrels. Thus, we tested in this study whether the propensity to investigate a refuge differs between age classes after detection of a hidden snake (presumably through olfaction). For each encounter with an unexposed snake, we noted whether the squirrel (22 adults, 9 pups) investigated the snake’s refuge as defined by sticking any portion of its body starting from the tip of its nose into the refuge.
We also quantified the time spent investigating snakes, the number of tail-flagging bouts performed during an interaction, and the tail-flagging rate (number of bouts/time spent investigating). To directly compare signaling behaviors between adults and pups, we only used recordings that included a complete interaction from start to finish. Interactions started with an EIP followed by tail flagging and ended when the squirrel moved away from the snake. We recorded 24 complete interactions (16 adults, 8 pups) and used these to examine whether pups differed from adults in their signaling behaviors.
Simulated rattlesnake strikes
This experiment occurred solely at the Blue Oak Ranch Reserve from May–July in 2012 and 2013 following methods described in Putman and Clark (Putman and Clark 2015). All adults tested were marked individuals, but most pups were unmarked because they did not enter traps as often as adults. Hence, we identified home burrows of unmarked pup litters and only tested pups near those burrows once to control for repeated measures on the same individual. Squirrel pups do not roam far from their home burrows (Boellstorff and Owings 1995) so we are confident that all pup trials are independent. Near each focal squirrel’s burrow, we examined responses to simulated rattlesnake strikes under one of two experimental treatments that manipulated the presence of rattlesnakes: snake present and recent snake. For the snake present treatment, squirrels were tested with a tethered wild-caught rattlesnake present (see Putman and Clark 2015, for snake tethering methods). For the recent snake treatment, squirrels were presented with a tethered rattlesnake, and then we tested their responses less than one hour after the snake was removed from the area. After recently encountering a snake, squirrels exhibited enhanced vigilance at that site even when the snake was no longer present. Our previous study (Putman and Clark 2015) found that squirrels responded most strongly to simulated strikes under the recent snake treatment, but we included data from both treatments in this study to test whether pups and adults differed in their responses to strikes when they were actively confronting a snake, and when they were concerned about a potential attack from an undetected snake.
Snake strikes were simulated using a spring-loaded device that uncoiled at approximately the same velocity as a rattlesnake strike (see Putman and Clark 2015). We recorded squirrel responses to these simulated strikes using a Sony model DCR SR-200 video camera with high frame rate (120 frames per second), and we recorded their tail-flagging behaviors prior to the simulated strike using a Sony Handycam model DCR SR-85 video camera (30 frames per second). Each adult squirrel or pup litter was tested once to control for prior experience with the device.
Behavior quantification
We used the software Premiere Pro (Adobe Systems Inc., San Jose, CA, USA) to quantify from video both the reaction time and body displacement time of each squirrel in response to the simulated strike. Reaction time is the time from the first visible movement of the cork-topped spring out of the device to the first body movement made by the squirrel. It is important to note that our measurement of reaction time is based on the movement of the cork-topped spring in the recordings, and that several events occurred in rapid succession just prior to this movement that might prime an evasive response. This measure is best taken as a relative measure useful for comparison between individuals in different treatments and is not directly comparable with neurophysiological measures of reaction time.
Body displacement time is the time it took the squirrel to fully displace its body from the position it was in before the spring was launched. Reaction time and body displacement time were not positively correlated (N = 68, r = 0.133, P = 0.280). We quantified two distinct flee modalities that squirrels used to evade simulated strikes: evasive leap, a vertical or sideways jump in the air with all four feet off the ground while swinging the tail, or scramble, during which squirrels ran away without leaping into the air. Evasive leaps were qualitatively similar to the escape maneuvers squirrels use in response to free-ranging rattlesnake strikes (Hennessy and Owings 1988; Barbour and Clark 2012). For each trial, we also quantified tail-flagging rate by counting the number of tail-flagging bouts that occurred the minute before the release of the spring.
Statistical analyses
All statistical analyses were performed using SYSTAT 12.0 software (SPSS Inc., Chicago, IL, USA), and significance was assessed at α ≤ 0.05. We looked for significant interactions in all statistical models.
Snake detection
We ran a multiple logistic regression to test the effects of squirrel age, snake exposure, and snake microhabitat on squirrels’ ability to detect snakes (yes/no dependent variable). For the microhabitat independent variable, which had three categories, we used multiple Wald’s tests to examine all pairwise comparisons and included a Bonferroni correction to adjust the α level of these multiple comparisons.
Snake-interaction behaviors
We quantified both the length of time squirrels spent interacting with snakes and number of tail-flagging bouts, but since these two variables were highly correlated, we only used number of tail-flagging bouts in our analysis. We log transformed the data to meet the assumptions of normality and homoscedasticity and used a two-sample t test to examine age difference in tail-flagging bouts during an interaction with a rattlesnake. We also ran a two-sample t test to test whether the tail-flagging rate differed between age classes. We used a Fisher exact test to determine whether pups differed from adults in the frequency of investigating a snake’s refuge while interacting with an unexposed rattlesnake.
Free-ranging rattlesnake strikes
We examined whether any naturally occurring snake strikes were influenced by squirrel age and signaling during an interaction. We ran a multiple logistic regression using squirrel age, occurrence of tail flagging (yes/no), and number of tail-flagging bouts as independent variables and strike (yes/no) as the dependent variable. We also examined whether age classes differed in the likelihood of leaping away from a strike attempt (yes/no dependent variable) using a Fisher exact test.
Simulated rattlesnake strikes
We examined data from 47 adult squirrels and 22 squirrel pups. The reaction time of one adult was excluded from analysis because this individual appeared to be startled by ancillary movements before the simulated strike occurred (time = −116.67 ms). We implemented two separate general linear models (GLMs) that assumed multivariate Gaussian distribution of the errors to test the effects of squirrel age, treatment (recent snake and snake present), and tail-flag rate on responses to simulated strikes (reaction time and body displacement time). We square-root transformed body displacement and reaction times to meet the assumption of normality. We used a multiple logistic regression including the same independent variables to test their effect on flee modality (scramble vs. evasive leap). Because Putman and Clark (2015) found that snake size had a strong effect on squirrel reaction time, we only examined data associated with large snake presentations (>500 g, N = 7 snakes, 32 adults, 13 pups) in our model on reaction time. We included all data in our models on body displacement time and on flee modality because snake size did not affect these responses in this study (displacement time: F = 0.01, P = 0.936; flee modality: Z = −1.07, P = 0.286) or our previous study (Putman and Clark 2015).
Results
Snake detection
Most (64.5 %) snake encounters occurred with adult squirrels. We found that adult squirrels detected snakes reliably more often than squirrel pups (Z = −3.21, P = 0.001, Table 1), while snake exposure and microhabitat did not meet significance criteria to statistically predict squirrels’ ability to detect cryptic rattlesnakes. The odds of an adult detecting a snake were 4.9 times higher than pups (95 % confidence interval (CI) = 1.86 to 12.87). Pups consistently detected snakes at lower rates than adults regardless of snake microhabitat or exposure (Table 1).
Snake-interaction behaviors
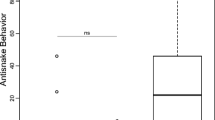
The total number of tail-flagging bouts for adults ranged from 1 to 215 during a complete interaction (X ± SE = 38.94 ± 12.78, N = 17) whereas the number for pups ranged from 1 to 18 bouts (X ± SE = 7.38 ± 2.24, N = 8). When interacting with a snake, adults performed more tail-flagging bouts (reflecting longer interactions) than pups (t test: t 23 = 2.29, P = 0.031, Fig. 2a). However, adults and pups did not differ in their tail-flagging rates (t test: t 23 = -0.182, P = 0.857). When snakes were unexposed, adults stuck their heads into the opening of the snake’s refuge more often than pups did (Fisher exact test: P = 0.044, Fig. 2b).
Ground squirrel age differences in antisnake behaviors. a Mean (±SE) tail-flagging bouts of 17 adults and 8 pups performed during rattlesnake interactions. b Proportions of 22 adults and 9 pups that investigated the snake’s refuge during an interaction with an unexposed snake. c Mean (±SE) reaction times of 46 adults and 22 pups in response to simulated rattlesnake strikes
Free-ranging rattlesnake strikes
Overall, 26.7 % of pups, while only 9.5 % of adult squirrels, were attacked by rattlesnakes. However, squirrel age did not affect rattlesnakes’ decision to strike in our logistic regression model (Z = 0.70, P = 0.484). Furthermore, pups were equally likely as adults to leap away from strike attempts (Fisher exact test: P = 0.768).
Strike initiation was negatively affected by the presence of tail flagging (Z = -2.50, P = 0.012), but not the number of tail-flagging bouts (Z = 0.05, P = 0.957). The odds of a snake striking were 7.0 times higher for a non-tail-flagging squirrel than a tail-flagging squirrel (95 % CI = 1.52 to 32.27).
Simulated rattlesnake strikes
Adult squirrels exhibited faster reaction times than squirrel pups (F 1, 36 = 5.65, P = 0.023, Fig. 2c). Squirrels also exhibited faster reaction times under the recent snake treatment (F 1, 36 = 5.08, P = 0.030). Tail-flagging rate did not predict reaction time significantly (F 1, 36 = 0.69, P = 0.413).
Body displacement time did not differ between pups and adults (F 1, 64 = 0.44, P = 0.509), and was not significantly affected by tail-flagging rate (F 1, 64 = 3.49, P = 0.066). Treatment was the only explanatory variable that significantly affected body displacement time: squirrels displaced their bodies more quickly under the recent snake treatment (F 1, 64 = 9.92, P = 0.002).
The type of flee modality used to evade simulated strikes did not differ between pups and adults (Z = −0.46, P = 0.647). All squirrels were more likely to use an evasive leap under the recent snake treatment (Z = 3.23, P = 0.001) and at higher tail-flagging rates (Z = 2.12, P = 0.034).
Discussion
Adult and juvenile ground squirrels differ in antisnake behaviors at the detection, interaction, and attack stages of a rattlesnake encounter. Pups are not as good as adults at detecting free-ranging snakes coiled in ambush, and also have slower reaction times to snake strikes. However, pups appear to compensate for these deficiencies by exhibiting different behaviors than adults at other stages of an encounter. Specifically, more so than adults, pups avoid rattlesnakes and minimize time spent in close proximity to them during anitsnake signaling interactions. Below, we discuss these findings in more detail.
Snake detection
Most of the squirrels that encountered rattlesnakes at our sites were adults, probably because adults are more active and have larger home ranges than pups (Boellstorff and Owings 1995). Alternatively, pups may have detected snakes beyond the 1.5 m radius of our camera’s field of view and avoided approaching a dangerous threat. Even though other studies have found the young of many species, including squirrels, to be less active, more vigilant, and more wary than adults (Dixon and Baker 1988; Hanson and Coss 2001; Hopkins et al. 2011; but see Arenz and Leger 2000), our results demonstrate that pups are still less capable of detecting a cryptic snake when in close proximity to it, a response that is inappropriate for the risks associated with their age. This suggests that pups are born with an incomplete form of the adult trait that becomes fully functional over time. In addition, since pups generally exhibit higher states of physiological arousal as they explore their environment (Hanson and Coss 2001), and because they need to be vigilant against a greater number of threats (e.g., infanticide; Trulio 1996), their attention might be more dispersed than adults, reducing the likelihood of snake detection. However, the reduced activity and increased wariness of pups (Hanson and Coss 2001) could compensate for their deficiencies in snake detection, such as a general preparation for evasive action. To our knowledge, this is the first study to quantify an animal’s ability to detect non-attacking cryptic predators in a completely natural setting.
Squirrels probably use sensory systems other than vision to detect snakes because they were able to detect unexposed snakes hiding within refuge. Hennessy and Owings (1977) found that olfaction plays an important role in modifying squirrels’ snake-interaction behaviors. Before eye-opening and prior to emergence from the natal burrow, pups respond fearfully to gopher snake (Pituophis catenifer) scent (Coss 1991). Their ability to detect rattlesnakes at this age is unknown but likely similar to that of gopher snakes since rattlesnakes have been observed to prey on pre-emergent pups in their burrows (BJP, personal observation). Because rattlesnakes are well hidden, scent is likely an important cue that informs squirrels a snake is nearby (Wasko et al. 2014). They probably then search for the scale-pattern regularity of snakes to locate the exact position of the hidden predator (as seen in the refuge-investigation behaviors, we recorded when unexposed snakes were detected). Ongoing studies by our research group are examining the relative importance of olfactory and visual cues in ground squirrel–snake detection and discrimination.
The pups in our study were also still undergoing developmental processes that could hinder their ability to visually detect snakes. Their ability to see color was likely limited because the squirrel retina usually does not reach adult-like dichromacy until 30 days post-emergence (McCourt and Jacobs 1983). Our finding that pups are less able than adults at detecting free-ranging snakes could result from the ongoing development of this sensory system, along with lack of experience. In the laboratory, pups alternate their attention from their mother to the snake while she interacts with a snake (Poran and Coss 1990). This same study found that all pups with their mother present detected snakes, while not all pups detected snakes when tested alone. This suggests that pups may use associative learning to distinguish cues linked with dangerous snakes (Heyes 1994). Likewise, young rhesus monkeys (Macaca mulutta) learn that snakes are dangerous through observations of experienced adults’ antipredator responses (Cook and Mineka 1987). Pups may also learn to detect snakes through trial-and-error because they often inspect snake-like sticks and rounded stones near their natal burrows and exhibit snake-directed behaviors toward them including long pauses with EIPs and tail flags (Hersek 1990; Coss 1991).
Variation in the microhabitat used by snakes did not exert a large effect on the ability of ground squirrels to detect snakes. Other studies have shown that microhabitat features indicative of high snake predation risk (e.g., bushy, occluded habitats) induces predator-avoidance behaviors in several rodent species (Kotler et al. 1993; Wasko et al. 2014). Although snakes in our study were most often found in burrows, they ambushed squirrels almost equally across microhabitat types. This suggests that no one of the microhabitat categories we tested is more dangerous than another, and so there would not be strong selection on squirrels to enhance vigilance in one microhabitat compared with another.
Snake-interaction behaviors
After detecting a rattlesnake, adult squirrels performed more tail-flagging bouts than pups. This finding interestingly contradicts the results of laboratory studies which have shown pups to mob snakes more intensely than adults (Owings and Coss 1977; Poran and Coss 1990). When tested alone compared with their mother present, pups engaged snakes for a longer time and exhibited more tail-flagging bouts (Poran and Coss 1990). Laboratory studies often test individuals in isolation, but the field environment and potential influence of nearby conspecifics in free-ranging animals may contribute to our contradictory results. However, our results also conflict with studies in other systems which have shown that both young prairie dogs, Cynomys cynomys (Owings and Loughry 1985) and skunks, Mephitis mephitis (Medill et al. 2011) display pursuit-deterrent signals more often than adults. The authors of these studies propose that the young exhibit a high propensity to signal because of their increased vulnerability to predators. However, they do not demonstrate whether increased signaling reduces predation.
Our findings show that squirrel pups should display tail flagging as much as adults because the occurrence of tail flagging was the most significant factor deterring rattlesnake strikes. Nonetheless, most pups failed to mount an appropriate response when in close proximity to a snake (i.e., they did not tail flag). The amount of tail flagging had no effect on rattlesnakes’ decision to strike; thus pups, which exhibited fewer bouts of tail flagging than adults, were not targeted because of reduced signal intensity. Age differences in signaling behavior are likely caused by other factors.
First, rattlesnakes do not abandon the hunting area after interactions with pups as they do with adults. Barbour and Clark (2012) found that the more time adult squirrels spent signaling toward a rattlesnake, the more likely the snake was to leave the area. In contrast, after interactions with squirrel pups, snakes were more likely to remain in the area and their likelihood of capturing a pup increased. When pups discover snakes, tail flagging apparently deters snakes from striking in that instance, but it does not cause snakes to abandon hunting sites. Thus, young squirrels do not benefit in the same ways as adults from exhibiting prolonged tail-flagging bouts during a rattlesnake interaction. The differential rattlesnake responses to squirrels of different age classes could either stem from or contribute to the reduced signaling we recorded in squirrel pups.
Pups may also not benefit from exhibiting prolonged tail-flagging bouts because adult squirrels do not readily respond to their displays. Hersek and Owings (1994) found that tail flagging by pups did not induce vigilance in nearby squirrels nor did it attract the attention of their mothers. This suggests that experienced adults may not view pups as reliable signalers, a similar phenomenon that occurs with the alarm calls of juvenile bonnet macaques (Macaca radiata) (Ramakrishnan and Coss 2000). In addition, prolonged bouts of tail flagging may be costly because they could draw the attention of other predators (Broom and Ruxton 2012). Thus, rattlesnake-induced tail flagging may facilitate attacks from other predatory species (e.g., Kotler et al. 1992; Embar et al. 2014), which pups should avoid due to their high vulnerability. Associative learning facilitated by conspecifics is likely the main process by which pups develop adult-like snake responses. Pups may learn appropriate responses by observing their mothers which defend them for several weeks after natal burrow emergence. In support of this, socially deprived squirrels that were kept isolated into adulthood retain a pup-like expectation of encountering snakes (Tromborg and Coss 2015).
We also tested whether pups would inspect snake refuges more closely than adults. Such risky behavior has been observed in juvenile nonhuman primates that have snake predators (Correa and Coutinho 1997; Ramakrishnan et al. 2005), but our results showed that adults were more likely than pups to enter an unexposed snake’s refuge. Hence, with age, squirrels become more willing to approach snakes closely, probably because they become more adept at evading strikes and because their physiological resistance to snake venom increases as a function of larger body size. By not inspecting snake refuges, pups are acting appropriately for the risks associated with their age.
Response to rattlesnake strikes
Even though we found that pups were just as likely as adults to leap away from free-ranging snake strikes, this ability improves with age because adult squirrels exhibited faster reaction times to simulated attacks than pups. The reaction of squirrels to strikes is likely a startle response that bypasses cerebral-cortex processing, producing extremely short response latencies (Putman and Clark 2015). Age differences in reaction times have been reported for meerkats; pups react slower to conspecific alarm calls than adults (Hollén and Manser 2006). Also, the latency to respond to startling acoustic stimuli decreases with age in laboratory rats (Sheets et al. 1988). Sheets et al. (1988) suggest that neuron axon development (increased myelination and axon diameter increasing conduction velocity) instead of motor development likely causes age differences in reaction times to startling stimuli. This same developmental process may place constraints on pup responses to snake strikes, but further research is needed to test this hypothesis. In addition, pups may require experience to enhance their reaction times to strikes, and the startle responses they exhibit toward inanimate objects support this idea (Coss 1991).
An alternative explanation is that adults react faster than pups because their large size impairs flight ability and faster reaction times compensate for this deficiency (Jones et al. 2009). However, we did not find age differences in body displacement time or type of flee modality used suggesting that locomotor ability (i.e., speed of movement) is similar between pups and adults. Selection appears to have fixed aerial leaping as an “unlearned” response early in ground squirrel ontogeny (Wiedenmayer 2009). Further research is needed to determine if evasive leaps are exhibited immediately after emergence from the natal burrow, and if specific biomechanical aspects of this behavior (agility, acceleration, maneuverability, etc.) differ between age classes (Carrier 1996). We also found in this study and in Putman and Clark (2015) that squirrels reacted faster under the recent snake treatment than the snake present treatment. When squirrels were uncertain of the location of a recently encountered snake, they appeared more wary and responded to simulated attacks more strongly, although pups were slower than adults to react under both these treatments. Overall, the slower reaction times of pups are likely not appropriate for risks associated with their age, making them vulnerable at the attack stage of rattlesnake encounters.
Concluding remarks
We found age differences in antisnake behaviors during three stages of a rattlesnake encounter. The age differences in squirrels’ ability to detect free-ranging rattlesnakes and their responses to strikes suggest that these behaviors could make pups more vulnerable than adults. However, antisnake behaviors expressed by pups at other stages of rattlesnake encounters appear to compensate for these risks: pups did not spend as much time as adults harassing snakes and were more reluctant to approach hidden snakes closely. Thus, the two age classes use distinct antisnake strategies: pups avoid snakes while adults harass snakes. This variability in antisnake defenses may place constraints on hunting rattlesnakes, which must distinguish between pup and adult behaviors.
Our study has important findings that may carry over to other aspects of squirrels’ fitness. For example, some rodents are known to alter their foraging behavior in response to vipers, while others remain unaffected (Kotler et al. 1993; Bouskila 1995; Wasko et al. 2014). Because squirrel pups should avoid risky habitats where rattlesnakes are found, they could have limited access to high-quality resources. As a result, they may not experience the same foraging benefits as their adult counterparts, but the benefit of increased safety could outweigh this cost. Once squirrels reach adulthood, they can explore new areas with decreased risk as they become more effective at detecting snakes and responding to strikes. Overall, our study demonstrates the value of examining defensive repertoires over multiple stages of predatory encounters to garner a more holistic understanding of the sources of natural selection shaping the development of antipredator behavior.
References
Abramsky Z, Rosenzweig ML, Subach A (1997) Gerbils under threat of owl predation: isoclines and isodars. Oikos 78:81–90
Abramsky Z, Rosenzweig ML, Subach A (2002) The costs of apprehensive foraging. Ecology 83:1330–1340
Alberts JR, Cramer CP (1988) Ecology and experience: sources of means and meaning of developmental change. In: Blass EM (ed) Developmental psychobiology and behavioral ecology. Plenum Press, New York, pp 1–39
Arenz C, Leger D (2000) Antipredator vigilance of juvenile and adult thirteen-lined ground squirrels and the role of nutritional need. Anim Behav 59:535–541
Barbour MA, Clark RW (2012) Ground squirrel tail-flag displays alter both predatory strike and ambush site selection behaviours of rattlesnakes. Proc R Soc Lond B 279:3827–3833
Boellstorff DE, Owings DH (1995) Home range, population structure, and spatial organization of California ground squirrels. J Mammal 76:551–561
Bouskila A (1995) Interactions between predation risk and competition: a field study of kangaroo rats and snakes. Ecology 76:165–178
Broom M, Ruxton GD (2012) Perceptual advertisement by the prey of stalking or ambushing predators. J Theor Biol 315:9–16
Caro TM, FitzGibbon CD (1992) Large carnivores and their prey: die quick and die dead. In: Crawtey MJ (ed) Natural enemies: the population biology of predators. Parasites and diseases. Blackwell Scientific, Oxford, pp 117–142
Carrier R (1996) Ontogenetic limits on locomotor performance. Physiol Zool 69:467–488
Clark RW (2004) Timber rattlesnakes (Crotalus horridus) use chemical cues to select ambush sites. J Chem Ecol 30:607–617
Clark RW, Tangco S, Barbour MA (2012) Field video recordings reveal factors influencing predatory strike success of free-ranging rattlesnakes (Crotalus spp.). Anim Behav 84:183–190
Cook M, Mineka S (1987) Second-order conditioning and overshadowing in the observational conditioning of fear in monkeys. Behav Res Ther 25:349–364
Correa HKM, Coutinho PEG (1997) Fatal attack of a pit viper, Bothrops jararaca, on an infant buffy-tufted ear marmoset (Callithrix aurita). Primates 38:215–217
Coss RG (1991) Context and animal behavior III: the relationship between early development and evolutionary persistence of ground squirrel antisnake behavior. Ecol Psychol 3:277–315
Cresswell W (1994) Song as a pursuit-deterrent signal, and its occurrence relative to other anti-predation behaviors of skylark (Alauda arvensis) on attack by merlins (Falco columbarius). Behav Ecol Sociobiol 34:217–223
Devereux CL (2005) Predator detection and avoidance by starlings under differing scenarios of predation risk. Behav Ecol 17:303–309
Dixon SM, Baker RL (1988) Effects of size on predation risk, behavioural response to fish, and cost of reduced feeding in larval Ischnura verticalis (Coenagrionidae: Odonata). Oecologia 76:200–205
Embar K, Raveh A, Hoffmann I, Kotler BP (2014) Predator facilitation or interference: a game of vipers and owls. Oecologia 174:1301–1309
Fitch HS (1949) Study of snake populations in central California. Am Midl Nat 41:513–579
FitzGibbon CD (1994) The costs and benefits of predator inspection behaviour in Thomson’s gazelles. Anim Behav 34:139–148
FitzGibbon CD, Fanshawe JH (1988) Stotting in Thomson’s gazelles: an honest signal of condition. Behav Ecol Sociobiol 23:69–74
Griffin AS (2004) Social learning about predators: a review and prospectus. Anim Learn Behav 32:131–140
Hanson MT, Coss RG (1997) Age differences in the response of California ground squirrels (Spermophilus beecheyi) to avian and mammalian predators. J Comp Psychol 111:174–84
Hanson MT, Coss RG (2001) Age differences in arousal and vigilance in California ground squirrels (Spermophilus beecheyi). Dev Psychobiol 39:199–206
Hasson O (1991) Pursuit-deterrent signals: communication between prey and predator. Trends Ecol Evol 6:325–329
Hawlena D (2009) Colorful tails fade when lizards adopt less risky behaviors. Behav Ecol Sociobiol 64:205–213
Hawlena D, Boochnik R, Abramsky Z, Bouskila A (2006) Blue tail and striped body: why do lizards change their infant costume when growing up? Behav Ecol 17:889–896
Hennessy DF, Owings DH (1977) Snake species discrimination and the role of olfactory cues in the snake-directed behavior of the California ground squirrel. Behaviour 65:115–123
Hennessy F, Owings H (1988) Rattlesnakes create a context for localizing their search for potential prey. Ethology 77:317–329
Hersek MJ (1990) Behavior of predator and prey in a highly coevolved system: northern Pacific rattlesnakes and California ground squirrels. Thesis, University of California, Davis
Hersek MJ, Owings DH (1993) Tail flagging by adult California ground squirrels: a tonic signal that serves different functions for males and females. Anim Behav 46:129–138
Hersek M, Owings DH (1994) Tail flagging by young California ground squirrels, Spermophilus beecheyi: age-specific participation in a tonic communicative system. Anim Behav 48:803–811
Heyes CM (1994) Social learning in animals: categories and mechanisms. Biol Rev 69:207–231
Hileman KS, Brodie ED Jr (1994) Survival strategies of the salamander Desmognathus ochrophaeus: interaction of predator-avoidance and anti-predator mechanisms. Anim Behav 47:1–6
Hollén LI, Manser MB (2006) Ontogeny of alarm call responses in meerkats, Suricata suricatta: the roles of age, sex and nearby conspecifics. Anim Behav 72:1345–1353
Hopkins GR, Gall BG, Brodie ED Jr (2011) Ontogenetic shift in efficacy of antipredator mechanisms in a top aquatic predator, Anax junius (Odonata: Aeshnidae). Ethology 117:1093–1100
Jones KA, Krebs JR, Whittingham MJ (2009) Heavier birds react faster to predators: individual differences in the detection of stalking and ambush predators. Behav Ecol Sociobiol 63:1319–1329
Kehmeier S, Schloegl C, Scheiber IBR, Weiß BM (2011) Early development of gaze following into distant space in juvenile Greylag geese (Anser anser). Anim Cogn 14:477–485
Kotler BP, Blaustein L, Brown JS (1992) Predator facilitation: the combined effect of snakes and owls on the foraging behavior of gerbils. Ann Zool Fenn 29:199–206
Kotler BP, Brown JS, Dall SRX, Gresser S, Ganey D, Bouskila A (2002) Foraging games between gerbils and their predators: temporal dynamics of resource depletion and apprehension in gerbils. Evol Ecol Res 4:495–518
Kotler BP, Brown JS, Slotow RH, Goodfriend WL, Strauss M (1993) The influence of snakes on the foraging behavior of gerbils. Oikos 67:309–316
Landová E, Jančúchová-Lásková J, Musilová V, Kadochová S, Frynta D (2013) Ontogenetic switch between alternative antipredatory strategies in the leopard gecko (Eublepharis macularius): defensive threat versus escape. Behav Ecol Sociobiol 67:1113–1122
Lima SL (2002) Putting predators back into behavioral predator–prey interactions. Trends Ecol Evol 17:70–75
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Lind J, Cresswell W (2005) Determining the fitness consequences of antipredation behavior. Behav Ecol 16:945–956
Mateo JM (1996a) Early auditory experience and the ontogeny of alarm-call discrimination in Belding’s ground squirrels (Spermophilus beldingi). J Comp Psychol 110:115–124
Mateo JM (1996b) The development of alarm-call response behaviour in free-living juvenile Belding’s ground squirrels. Anim Behav 52:489–505
McCourt ME, Jacobs GH (1983) Effects of photic environment on the development of spectral response properties of optic nerve fibers in the ground squirrel. Exp Brain Res 49:443–452
Medill SA, Renard A, Larivière S (2011) Ontogeny of antipredator behaviour in striped skunks (Mephitis mephitis). Ethol Ecol Evol 23:41–48
Nonacs P, Blumstein D (2010) Predation risk and behavioral life history. In: Westneat DF, Fox CW (eds) Evolutionary behavioral ecology. Oxford University Press, New York, pp 207–221
Owings DH, Coss RG (1977) Snake mobbing by California ground squirrels: adaptive variation and ontogeny. Behaviour 62:50–69
Owings DH, Coss RG (2008) Hunting California ground squirrels: constraints and opportunities for northern Pacific rattlesnakes. In: Hayes WK, Beaman KR, Cardwell MD, Bush SP (eds) Biology of the rattlesnakes. Loma Linda University Press, Loma Linda, pp 155–167
Owings DH, Loughry WJ (1985) Variation in snake-elicited jump-yipping by black-tailed prairie dogs: ontogeny and snake-specificity. Z Tierpsychol 70:177–200
Platzen D, Magrath RD (2005) Adaptive differences in response to two types of parental alarm call in altricial nestlings. Proc R Soc Lond B 272:1101–1106
Poran NS, Coss RG (1990) Development of antisnake defenses in California ground squirrels (Spermopilus beecheyi): I. Behavioral and immunological relationships. Behaviour 112:222–224
Poran NS, Coss RG, Benjamini E (1987) Resistance of California ground squirrels (Spermophilus beecheyi) to the venom of the northern Pacific rattlesnake (Crotalus viridis oreganus): a study of adaptive variation. Toxicon 25:767–777
Putman BJ, Clark RW (2015) The fear of unseen predators: ground squirrel tail flagging in the absence of snakes signals vigilance. Behav Ecol 26:185–193
Ramakrishnan U, Coss RG, Schank J, Dharawat A, Kim S (2005) Snake species discrimination by wild bonnet macaques (Macaca radiata). Ethology 111:337–356
Ramakrishnan U, Coss RG (2000) Age differences in the responses to adult and juvenile alarm calls by bonnet macaques (Macaca radiata). Ethology 106:131–144
Reinert HK, Cundall D (1982) An improved surgical implantation method for radio-tracking snakes. Copeia 1982:702–705
Reinert HK, MacGregor GA, Esch M, Bushar LM, Zappalorti RT (2011) Foraging ecology of timber rattlesnakes, Crotalus horridus. Copeia 2011:430–442
Sheets LP, Dean KF, Reiter LW (1988) Ontogeny of the acoustic startle response and sensitization to background noise in the rat. Behav Neurosci 102:706–713
Sordahl TA (1988) The American avocet (Recurvirostra americana) as a paradigm for adult automimicry. Evol Ecol 2:189–196
Swaisgood RR, Rowe MP, Owings DH (2003) Antipredator responses of California ground squirrels to rattlesnakes and rattling sounds: the roles of sex, reproductive parity, and offspring age in assessment and decision-making rules. Behav Ecol Sociobiol 55:22–31
Tromborg CT, Coss RG (2015) Isolation rearing reveals latent antisnake behavior in California ground squirrels (Otospermophilus becheeyi) searching for predatory threats. Anim Cogn. doi:10.1007/s10071-015-0853-5
Trulio LA (1996) The functional significance of infanticide in a population of California ground squirrels (Spermophilus beecheyi). Behav Ecol Sociobiol 38:97–103
Wasko DK, Bonilla F, Sasa M (2014) Behavioral responses to snake cues by three species of neotropical rodents. J Zool 292:142–150
Whitfield DP (2003) Redshank Tringa totanus flocking behaviour, distance from cover and vulnerability to sparrowhawk Accipiter nisus predation. J Avian Biol 34:163–169
Whittingham MJ, Butler SJ, Quinn JL, Cresswell W (2004) The effect of limited visibility on vigilance behaviour and speed of predator detection: implications for the conservation of granivorous passerines. Oikos 106:377–385
Wiedenmayer CP (2009) Plasticity of defensive behavior and fear in early development. Neurosci Biobehav Rev 33:432–441
Acknowledgments
Funding for this research was provided by San Diego State University, the National Geographic Society Waitts Grant (W17-08 to RWC), the National Science Foundation (DBI-0951010 to RWC), the Mildred E. Mathias Graduate Student Research Grant (to B JP), and the Animal Behavior Society Student Research Grant (to BJP). For assistance with fieldwork, we thank Matthew Barbour, Caesar Rahman, Emily Mastrelli, Jessica Fort, Zachary Cava, Michelle Goh, Elinor Israel, Emily Stulik, Hossein Ayazi, Eric Schroder, Cleo Grieve, Sean Tangco, Kenneth Huang, Curt Barnes, Darren Fraser, Rey Ayon, Annie Maguire, Matthew Strimas-Mackey, Gretchen Anderson, Tara Easter, Jessica Tingle, Erynn Rebol, Mark Herr, Trevor Darragh, Lauren Kong, Susan Anthony, Mike Hogan, Joseph Chase, and Jenny Schefski. For assistance with reviewing and quantifying video recordings, we thank Omar Singleton, Sky Jacobson, Ashley Benson, Armando Magana, Ivana Bui, Rebecca Ehrlich, Sarfaraz Serang, Jenny Schefski, Jaime Lane McKenzie, and Alexis Leon. We thank Michael Hamilton for general field support and for assistance with erecting the wireless internet network at the BORR field site, and members of the 2013 Clark Lab and two anonymous reviewers for comments improving the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All methods were approved by the San Diego State University Institutional Animal Care and Use Committee (APF 10-09-025C).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. B. Banks
Rights and permissions
About this article
Cite this article
Putman, B.J., Coss, R.G. & Clark, R.W. The ontogeny of antipredator behavior: age differences in California ground squirrels (Otospermophilus beecheyi) at multiple stages of rattlesnake encounters. Behav Ecol Sociobiol 69, 1447–1457 (2015). https://doi.org/10.1007/s00265-015-1957-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-1957-2