Abstract
Differences in gender and age and the balance between aggressive behavior and the ability to escape are fundamental in predator–prey interactions, as well as for survival, foraging, and mating success. We investigated the defensive behavior of the scorpion Tityus pusillus and assessed possible differences in their behavior responses associated with sex, age, and diel period, by simulating a predation threat. Predator attacks were simulated by pressing the telsons with forceps, dropping the animals from a height of 25 cm on a plastic tray, restraining the pincers using large rubber-tipped tweezers, or restricting the prosoma. Tityus pusillus (Buthidae) showed five defensive behaviors: thanatosis, fleeing, stinging, standing still, and tail wagging. The scorpions responded with thanatosis or fleeing when their telsons were restricted. The frequency of these responses varied with sex and diel period. Stinging was the primary behavior response to prosoma restriction in both adults and juveniles while standing still was the most frequently observed behavior response to restraining pincers. These results indicate that the plasticity of defensive behavior in T. pusillus in response to predation is influenced by sex, age, diel period, and the body part targeted by the predator.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation is one of the most important selective forces in evolution, shaping several morphological characteristics and animal behavior (Lima and Dill 1990; Sansom et al. 2009; Preisser and Orrock 2012). Failing to avoid predation may result in death for the animal; thus, the ability to perceive and respond to a threat is under high selective pressure throughout evolutionary history (Lima and Dill 1990; Kats and Dill 1998).
Arthropods possess several defense mechanisms to avoid predation including crypsis, mimicry, thanatosis, autotomization of appendices, and fleeing, and aggressive behaviors like stinging (Tullberg et al. 2005; Fleming et al. 2007; Gnaspini and Hara 2007; Pomini et al. 2010; Coelho et al. 2017). Sexual dimorphism and the developmental stage are multi-integrated components affecting the defensive actions (Williams et al. 2001; Uma and Weiss 2012; Miller et al. 2016). For example, Centruroides vittatus (Say, 1821) males and females differ in morphology and behavior. Males sprint faster than females, while females with their larger, heavier bodies move slower and are more likely to sting and deliver repetitive stings more rapidly when threatened (Carlson and Rowe 2009; Carlson et al. 2014).
Many arthropods also exhibit ontogeny-related defensive behavior (Breed et al. 1990; Jeanne et al. 1992; Judd 2000; Haight 2008; Ramirez et al. 2010). For example, in the wasp Polybia occidentalis Olivier, 1791, the probability of a young worker engaging in defensive behavior is low but increases with age (Jeanne et al. 1992). Dangles et al. (2007) found that juveniles of the cricket Nemobius sylvestris (Bosc, 1792) exhibit a higher incidence of escape performances than older instars, suggesting that these variations may be related to the lower predatory risk perceived by large adults than by juveniles.
Modulation of antipredatory behavior according to diel rhythms has also been described in many arthropod species (McIntosh and Townsend 1994; Jones et al. 2011; Effertz and von Elert 2014; Watts et al. 2014). Watts et al. (2014) found that the orb-weaving spider Cyclosa turbinate (Walckenaer, 1841) exhibits thanatosis more frequently and for more extended periods during daytime when confronted with a predator stimulus in an experimental setup. Similarly, the orb-weaving spider Larinioides cornutus (Clerck, 1757) holds a huddled posture longer in the light than in the dark (Jones et al. 2011).
Being both a predator and prey, scorpions offer a robust opportunity to examine trade-offs associated with defensive behavior. To avoid predation, scorpions have evolved different defense mechanisms such as retreating to a burrow or other hiding place (Polis 1990), using pincers (chelae), venomous telsons (stingers), or both (Warburg 1998; van der Meijden et al. 2013). Morphology is also a key feature influencing scorpion defensive behavior. Many species (e.g., Tityus sp., Centruroides sp., and Isometrus sp.) from the family of Buthidae C. L. Koch, 1837 that possesses less robust pedipalp pincers react more often with stinging compared to species with more robust pedipalp pincers such as Scorpionidae Latreille, 1802, Diplocentridae Karsch, 1880, Liochelidae Fet and Bechly, 2001, or Iuridae Thorell, 1876 (Polis 1990; Warburg 1998; van der Meijden et al. 2013). Additionally, van der Meijden et al. (2013) found that species with strong pincers used these more often as a defense mechanism compared to species with weak pincers that rather used stinging.
In this study, we analyzed the defensive behavior of Tityus pusillus Pocock, 1893 by simulating a risk predation situation and demonstrated possible differences in the behavior response associated with sex, age, and diel period. Tityus pusillus, the litter-dwelling scorpion, is a sexually dimorphic species with males having more robust pincers and enlarged metasomas compared to females (Lira et al. 2018a). We addressed the following questions: (1) Do differences in the age of T. pusillus influence defensive behavior? (2) Do morphological differences, such as more robust pincers in males, caused by sexual dimorphism, influence the defensive performance in this species? (3) Does T. pusillus exhibit alternative defensive behavior according to the diel period, such as being more responsive during the nighttime?
Materials and methods
Studied species
Tityus pusillus is a sedentary, small-sized scorpion (30-35 mm) that occurs in the Atlantic Forest and Caatinga in Northeastern Brazil (Porto et al. 2010; Lira et al. 2018a). The species is found primarily in leaf litter layers (Lira et al. 2013, 2018b; Santos et al. 2018) and is sensitive to microhabitat structure changes (Lira et al. 2015; Dionisio-da-Silva et al. 2018). Tityus pusillus is a protandric species that reach adulthood after 4–5 molts, with females being able to give birth to 8 to 12 live offspring in 85 ± 12 days (Albuquerque and Lira 2016; Lira et al. 2018a).
In this study, 248 T. pusillus individuals (68 males, 90 females, and 90 juveniles in the second instar phase), collected in a remnant Atlantic forest in the Moreno municipality, Pernambuco state, northeast Brazil, were used. All animals were collected during the dry season in February 2014; during this season, T. pusillus exhibit an increase in foraging activity (Dionisio-da-Silva et al. 2018). In the laboratory, the animals were placed in individual plastic terraria (14 cm × 10 cm × 8 cm) and supplied with cardboard as a shelter and wetted cotton wool as a water source. They were left in observation for 30 days before the start of the experiments. Laboratory conditions were maintained at 24 °C ± 2 °C and 80% ± 5% relative humidity, and a 12:12 h light:dark photoperiod. The scorpions were fed weekly with cockroach nymphs of the species Nauphoeta cinerea (Oliver, 1789). The test animals were subjected to a fast 15 days before the experiments, and no gravid females (with embryos visible through the ventral mesosoma) were used.
Simulated predator experiments
Two sets of experiments using a simulated predator stimulus were performed to verify T. pusillus anti-predator response and to assess differences in behavior caused by age (adult/juvenile), sex (male/female), and diel period (night/day). All the procedures were performed by two researchers (AFAL and FMFA).
In the first trial based on Pomini et al. (2010), 123 scorpions (33 males, 45 females, and 45 juveniles) were used and predator attacks were simulated by holding the telson with forceps for 10 s and by dropping the animals from a height of 25 cm on a plastic tray (47 × 32 × 9 cm) containing 3 mm of litter layer. Between the trials, the litter layer was changed and the plastic tray was washed with 70% ethanol. In situations where thanatosis (freezing posture) was observed, the time individuals took to start moving again was recorded for up to 15 min. Behavioral assessments were performed at night (19:00–21:00) under red light (Machan 1968) and repeated after 15 days during the day between 13:00 and 14:00 to verify if diel period influenced the intensity of response to predation. All scorpions were fed before the second round of experiments.
In the second trial, a different group of scorpions (35 males, 45 females, and 45 juveniles in the second instar phase) was transferred to individual 1000 ml plastic terrariums (9 cm high and 14 cm in diameter) and allowed to acclimate for 15 min. Each scorpion had a separate terrarium in which the trials were carried out. In the second trial, each of the pincers was first restrained laterally in a random order for 5 s (per pincer) using large rubber-tipped tweezers, followed by a similar restriction (dorso-ventrally) of the prosoma (van der Meijden et al. 2013). Behavioral assessments were performed at night and during the day as described for the first trial. Scorpions were arbitrarily assigned to the day or the night group to ensure the randomness of the dataset. After 15 days, the groups were inverted. Voucher specimens were deposited in the Arachnological Collection of Universidade Federal de Pernambuco.
Statistical analyses
Age and sex differences in frequencies on defensive behavior (thanatosis or fleeing) were assessed using a Chi square test for the first trial. For the second trial, the frequencies of responses from the pincer and prosoma restrictions were assessed through a Chi square for independence. Differences in thanatosis time of adults vs. juveniles, males vs. females, and daytime vs. nighttime were assessed by the Mann–Whitney pairwise test with p values being adjusted using the Bonferroni’s correction. Before these analyses, the normality and variance of the data were assessed by the Shapiro–Wilk and the Levene tests, respectively. All statistical analyses were performed with the PAST 3.22 software (Hammer et al. 2001).
Results
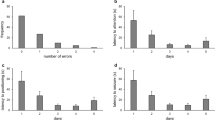
In response to a simulated predator attack, T. pusillus exhibited five defensive behaviors (Table 1). Scorpions responded with thanatosis or fleeing when their telsons were pressed and released (Fig. 1) and with stinging, standing still, fleeing, or tail wagging when their pincers or prosomas were immobilized (Tables 2, 3). However, the frequency of these behaviors was influenced by sex, age (adult/juvenile stage), and diel period (Tables 2, 3).
After pressing the telsons with forceps and dropping the animals from a height in the first set of experiments, both juvenile and adult T. pusillus exhibited defensive behavior characterized by fleeing or thanatosis. However, a clear differential response related to age, sex, and diel period was observed. Juveniles and females exhibited a preference for fleeing. The flight response significantly increased among juveniles (χ2 = 4.52, df = 1, p = 0.03) if the simulated attack occurred during the nocturnal trials, while thanatosis was the main response of males independent of the diel period (Fig. 1a). Juveniles displayed significantly more fleeing behavior than adults in both daytime (χ2 = 3.59, df = 1, p = 0.05) and nighttime (χ2 = 13.9, df = 1, p < 0.01). Thanatosis was performed by 30% of the juveniles, while a fleeing response was performed by 60% of the juveniles during the diurnal trial. In nocturnal trials, thanatosis was performed seven times less by the juveniles compared to fleeing (Fig. 1a). Adult individuals showed no differences (χ2 = 0.01, df = 1, p = 0.73) in the frequency of thanatosis and fleeing, independent of the period (Fig. 1a).
The time spent in thanatosis (Table S1) (mean ± standard deviation) by adults during the day (758 ± 263 s) was not significantly different (U = 349, adjusted p = 0.14) from the time spent in thanatosis during the night (484.71 ± 412 s) (Fig. 2). Similarly, juveniles did not show a diel period effect in terms of the time spent in thanatosis (U = 13.5, adjusted p = 1) (Fig. 2). Compared to adults, juveniles spent significantly less time in thanatosis in daytime (U = 56.0, adjusted p < 0.01), but not in nighttime (U = 34.0, adjusted p = 1) (Fig. 2).
During diurnal trials, males showed a higher preference for thanatosis (χ2 = 7.65, df = 1, p = 0.05) compared to females (Fig. 1b). However, the duration of the thanatosis response of males and females was similar (males: 723.63 ± 291.91 s; females: 822.25 ± 194.97 s; U = 105, adjusted p = 1). Likewise, no significant difference between males and females was recorded for time spent in thanatosis during the nocturnal trials, although the time spent in that state decreased for both sexes (males: 379.78 ± 412.44 s; females: 638.07 ± 374.75 s; U = 87.5, adjusted p = 1).
In the second trial, four types of defense strategies were registered: standing still, fleeing, tail wagging, and stinging. The type of strategy used was mostly influenced by which body part was restricted in both adults and juveniles. When the prosomas were pinched, the primary defense mechanism was to sting while when the pincers were immobilized, the individuals chose to either show no reactions or to flee (Table 2). Stinging behavior was not influenced by diel period (daytime χ2(3,2) = 1.85, df = 2, p = 0.39; nighttime χ2(3,2) = 0.67, df = 2, p = 0.71) in both adults and juveniles. However, in response to pincer restriction, standing still was more frequently observed than stinging. This behavior was predominantly performed by adults during both diel periods (daytime χ2(4,2) = 15.4, df = 3, p < 0.01; nighttime χ2(4,2) = 16.6, df = 3, p < 0.01).
Tail wagging was only performed by adults and was independent of body part restriction (prosoma or pincers) and diel period (Table 2). Differences in the response of pincer restriction were observed between sexes, but only in diurnal trials (χ2(4,2) = 54.4, df = 3, p < 0.01) and not in nocturnal trials (χ2(4,2) = 4.96, df = 3, p = 0.17). In diurnal trials, males performed primarily two reactions: fleeing or stinging, while females exhibited no reaction or tail wagging (Table 3). Contrarily, males and females performed mainly stinging in response to prosoma restriction, with no differences between daytime (χ2(3,2) = 4.84, df = 2, p = 0.08) or nighttime (χ2(3,2) = 1.08, df = 2, p = 0.58) (Table 3).
Discussion
This study described the defensive mechanisms used by the litter-dwelling scorpion Tityus pusillus to simulate predation situations. Our results indicate that this species exhibits behavioral plasticity in defensive strategies, with stinging, thanatosis, and fleeing as the main defensive behavioral reactions. Nevertheless, these responses can be modulated according to factors such as the body structure immobilized by a predator, sex, diel period, and developmental stage.
In response to threats, juveniles of T. pusillus would rather flee than to engage in combat, while adults are more actively defensive. Similar results were described in other arachnids such as the cobweb spider Parasteatoda tepidariorum C. L. Koch, 1841 (Uma and Weiss 2012), in which juvenile spiders fled in response to attacks by the predator, the mud-dauber wasp Chalybion californicum (Saussure, 1867), while adult spiders fought in response to the wasp attacks (Uma and Weiss 2012). In the black widow spider Latrodectus mactans (Fabricius, 1775), an ontogenetic shift in female aggressiveness has also been positively associated with developmental stage, with adults being more aggressive than juveniles (Troupe 2009). A possible explanation for the differential response to predation between juveniles and adults is the relative body size that often determines how an animal interacts with its surroundings (Werner and Gilliam 1984; Polis and McCormick 1987; Polis et al. 1989). Young animals and small-sized individuals are more easily preyed upon by other larger animals. Therefore, it is plausible that these animals flee rather than face the predators. Although scorpions possess a venomous stinger as a defensive weapon at all stages of the life cycle, they do not deviate from this trend (Polis 1990). Known as generalist predators capturing a wide diversity of preys (Polis 1990), juvenile and smaller sized scorpions are also subject to predation, resulting in a high mortality rate (e.g., Polis and McCormick 1987; Moreno-González and Hazzi 2012; Lira et al. 2016, 2017a, b).
Tityus pusillus also shows sex-related differences in defensive behavior with males performing thanatosis and stinging more often and females performing fleeing, tail wagging, and stinging more often. Morphological differences between T. pusillus sexes such as males possessing larger metasomal segments and more robust pedipalp pincers and females possessing larger prosomas and mesosomas (Lira et al. 2018a) may explain the differences in defensive behavior. Similarly, differences in defensive behavior of the scorpion species Centruroides vittatus may be associated with sexual dimorphism (Carlson et al. 2014). In this species, females are more aggressive than males, possibly because the females have larger bodies compared to males. This observation suggests that in this species the females are the combative sex to compensate for their locomotive restriction for reproduction while males possess longer legs for sprinting to evade predators and to find mates (Carlson et al. 2014). Another explanation for the differences between male and female T. pusillus could be related to the life strategies of each sex, as researched by Coelho et al. (2017). Additionally, only T. pusillus adults exhibit unusual ‘tail wagging’ behavior, which is commonly considered as a reproductive dance (e.g., Melville et al. 2003; Gaffin and Brownell 2010; Taylor et al. 2012) and not as a defensive behavior. Tail wagging consists of metasomal movements that occur before stinging and are more common in Tityus pusillus females than males. In a non-reproductive context, this behavior has been described for juveniles of Tityus uruguayensis Borelli, 1901 when sharing the same prey (Toscano-Gadea and Costa 2006). Moving the metasomal before stinging may be considered as a warning of the aggressiveness level of the scorpion against potential predators or competitors.
Differences in behavioral strategies in response to the observed diel period in T. pusillus adults suggest that this scorpion species shows behavioral plasticity according to environmental pressure. Diurnal predators (e.g., lizards and snakes) typically hunt for prey through visual stimulus (Vitt and Cooper Jr. 1986; Husak et al. 2006); thus, the variegated coloration related to thanatosis behavior exhibited by T. pusillus makes these scorpions difficult to find in leaf litter. In addition, nocturnal predators (e.g., spider and scorpions) can use other mechanisms for prey detection such as chemical cues and substrate vibration (Brownell and Farley 1979; Persons and Rypstra 2000; Mineo and Del Claro 2006). For these types of predators, prolonged thanatosis is not advantageous, while fleeing to lower leaf litter layers or stinging, as exhibited by T. pusillus, is an effective mechanism to avoid predation. Contrarily, there were no differences in the fleeing response for T. pusillus juveniles in the different diel periods. The lack of differential fleeing behavior could be explained by their smaller size, allowing them to hide in places difficult to access by predators.
Our findings show that the litter-dwelling scorpion, Tityus pusillus, exhibit defensive behavioral plasticity influenced by developmental stage, sex, and diel period. However, differences found in our study between adults and juveniles should be interpreted with caution because of the effect of the perceived size of the stimulus compared to the scorpion body size. Finally, this is the first study revealing the ontogenic differences in defensive mechanisms of scorpions. Also, we observed that males and females perform different behavioral reactions, possibly because of sexual dimorphism or different life strategies.
References
Albuquerque CMR, Lira AFA (2016) Insights into reproductive strategies of Tityus (Archaeotityus) pusillus Pocock, 1893 (Scorpiones, Buthidae). C R Biol 339:179–184. https://doi.org/10.1016/j.crvi.2016.03.003
Breed MD, Robinson GE, Page RE Jr (1990) Division of labor during honey bee colony defense. Behav Ecol Sociobiol 27:395–401. https://doi.org/10.1007/BF00164065
Brownell P, Farley RD (1979) Prey-localizing behaviour of the nocturnal desert scorpion, Paruroctonus mesaensis: orientation to substrate vibrations. Anim Behav 27:185–193. https://doi.org/10.1016/0003-3472(79)90138-6
Carlson BE, Rowe MP (2009) Temperature and desiccation effects on the antipredator behavior of Centruroides vittatus (Scorpiones: Buthidae). J Arachnol 37:321–330. https://doi.org/10.1636/Hi09-06.1
Carlson BE, McGinley S, Rowe MP (2014) Meek males and fighting females: sexually-dimorphic antipredator behavior and locomotor performance is explained by morphology in bark scorpions (Centruroides vittatus). PLoS One 9:e97648. https://doi.org/10.1371/journal.pone.0097648
Coelho P, Kaliontzopoulou A, Rasko M, van der Meijden A (2017) A ‘striking’ relationship: scorpion defensive behaviour and its relation to morphology and performance. Funct Ecol 31:1390–1404. https://doi.org/10.1111/1365-2435.12855
Dangles O, Pierre D, Christides JP, Casas J (2007) Escape performance decreases during ontogeny in wild crickets. J Exp Biol 210:3165–3170. https://doi.org/10.1242/jeb.004648
Dionisio-da-Silva W, Lira AFA, Albuquerque CMR (2018) Distinct edge effects and reproductive periods of sympatric litter-dwelling scorpions (Arachnida: Scorpiones) in a Brazilian Atlantic forest. Zoology 129:17–24. https://doi.org/10.1016/j.zool.2018.06.001
Effertz C, von Elert E (2014) Light intensity controls anti-predator defenses in Daphnia: the suppression of life-history changes. Proc R Soc Lond 281:20133250. https://doi.org/10.1098/rspb.2013.3250
Fleming PA, Muller D, Bateman PW (2007) Leave it all behind: a taxonomic perspective of autotomy in invertebrates. Biol Rev 82:481–510. https://doi.org/10.1111/j.1469-185X.2007.00020.x
Gaffin DD, Brownell PH (2010) Evidence of chemical signaling in the sand scorpion, Paruroctonus mesaensis (Scorpionida: Vaejovida). Ethology 91:59–69. https://doi.org/10.1111/j.1439-0310.1992.tb00850.x
Gnaspini P, Hara MR (2007) Defense mechanisms. In: Pinto-da-Rocha R, Machado G, Giribet G (eds) Harvestmen: the biology of opiliones. Harvard University Press, Harvard, pp 374–399
Haight KL (2008) Ontogeny of the defensive stinging behavior of the fire ant, Solenopsis invicta. J Insect Behav 21:147–152. https://doi.org/10.1007/s10905-007-9114-z
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica
Husak JF, Macedonia JM, Fox SF, Sauceda RS (2006) Predation cost of conspicuous male coloration in collared lizards (Crotaphytus collaris): an experimental test using clay-covered model lizards. Ethology 112:572–580. https://doi.org/10.1111/j.1439-0310.2005.01189.x
Jeanne RL, Williams NM, Yandell BS (1992) Age polyethism and defense in a tropical social wasp (Hymenoptera: Vespidae). J Insect Behav 5:211–227. https://doi.org/10.1007/BF01049290
Jones TC, Akoury TS, Hauser CK, Moore D (2011) Evidence of circadian rhythm in antipredator behaviour in the orb-weaving spider Larinioides cornutus. Anim Behav 82:549–555. https://doi.org/10.1016/j.anbehav.2011.06.009
Judd TM (2000) Division of labor in colony defense against vertebrate predators by the social wasp Polistes fuscatus. Anim Behav 60:55–61. https://doi.org/10.1006/anbe.2000.1449
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394. https://doi.org/10.1080/11956860.1998.11682468
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640. https://doi.org/10.1139/z90-092
Lira AFA, Souza AM, Silva Filho AAC, Albuquerque CMR (2013) Spatio-temporal microhabitat use by two co-occurring species of scorpions in Atlantic rainforest in Brazil. Zoology 116:182–185. https://doi.org/10.1016/j.zool.2013.01.002
Lira AFA, Rego FNAA, Albuquerque CMR (2015) How important are environmental factors for the population structure of co-occurring scorpion species in a tropical forest? Can J Zool 93:15–19. https://doi.org/10.1139/cjz-2014-0238
Lira AFA, Araújo VFN, Albuquerque CMR (2016) Predation of a scorpion (Scorpiones: Buthidae) by an assassin bug (Heteroptera: Reduviidae) in the Brazilian Atlantic Forest. Turk J Zool 40:294–296. https://doi.org/10.3906/zoo-1504-27
Lira AFA, Pordeus LM, Albuquerque CMR (2017a) A new species of Ananteris (Scorpiones: Buthidae) from Caatinga biome, Brazil. Acta Arachnol 66:9–15. https://doi.org/10.2476/asjaa.66.9
Lira AFA, DeSouza AM, Albuquerque CMR (2017b) Report of intraguild predation and cannibalism in scorpions (Scorpiones: Buthidae) in the Brazilian Atlantic forest. North-West J Zool 13:356–358
Lira AFA, DeSouza AM, Albuquerque CMR (2018a) Environmental variation and seasonal changes as determinants of the spatial distribution of scorpions (Arachnida: Scorpiones) in Neotropical forests. Can J Zool 96:963–972. https://doi.org/10.1139/cjz-2017-0251
Lira AFA, Pordeus LM, Rego FNAA, Iannuzzi K, Albuquerque CMR (2018b) Sexual dimorphism and reproductive behavior in the Brazilian scorpion Tityus pusillus (Scorpiones, Buthidae). Invert Biol 137:221–230. https://doi.org/10.1111/ivb.12221
Machan L (1968) Spectral sensitivity of scorpion eyes as possible roles of shielding pigment effect. J Exp Biol 49:95–105
McIntosh AR, Townsend CR (1994) Interpopulation variation in mayfly antipredator tactics: differential effects of contrasting predatory fish. Ecology 75:2078–2090. https://doi.org/10.2307/1941612
Melville JM, Tallarovic SK, Brownell PH (2003) Evidence of mate trailing in the giant hairy desert scorpion, Hadrurus arizonensis (Scorpionida, Iuridae). J Insect Behav 16:97–115. https://doi.org/10.1023/A:1022853413104
Miller DW, Jones AD, Goldston JS, Rowe MP, Rowe AH (2016) Sex differences in defensive behavior and venom of the striped bark scorpion Centruroides vittatus (Scorpiones: Buthidae). Integr Comp Biol 56:1022–1031. https://doi.org/10.1093/icb/icw098
Mineo MF, Del Claro K (2006) Mechanoreceptive function of pectines in the Brazilian yellow scorpion Tityus serrulatus: perception of substrate-borne vibrations and prey detection. Acta Ethol 9:79–85. https://doi.org/10.1007/s10211-006-0021-7
Moreno-González JA, Hazzi NA (2012) Intraguild predation case: Tityus forcipula Gervais, 1843 (Scorpiones, Buthidae) feeding on Chactas vanbenedeni Gervais, 1843 (Scorpiones, Chactidae) in Colombia. Rev Ibér Aracnol 20:117–120
Persons MH, Rypstra AL (2000) Preference for chemical cues associated with recent prey in the wolf spider Hogna helluo (Araneae: Lycosidae). Ethology 106:27–35. https://doi.org/10.1046/j.1439-0310.2000.00496.x
Polis GA (1990) The biology of scorpions. University Press, Stanford
Polis GA, McCormick SJ (1987) Intraguild predation and competition among desert scorpions. Ecology 68:332–343. https://doi.org/10.2307/1939264
Polis GA, Myers CA, Holt RD (1989) The ecology and evolution of intraguild predation. Ann Rev Ecol Evol Syst 20:297–330. https://doi.org/10.1146/annurev.es.20.110189.001501
Pomini AM, Machado G, Pinto-da-Rocha R, Macías-Ordóñez R, Marsaioli AJ (2010) Lines of defense in the harvestman Hoplobunus mexicanus (Arachnida: Opiliones): Aposematism, stridulation, thanatosis, and irritant chemicals. Biochem Syst Ecol 38:300–308. https://doi.org/10.1016/j.bse.2010.03.003
Porto TJ, Brazil TK, Lira-da-Silva RM (2010) Scorpions, state of Bahia, northeastern Brazil. Check List 6:292–297
Preisser EL, Orrock JL (2012) The allometry of fear: interspecific relationships between body size and response to predation risk. Ecosphere 3:1–27. https://doi.org/10.1890/ES12-00084.1
Ramirez RA, Crowder DW, Snyder GB, Strand MR, Snyder WE (2010) Antipredator behavior of Colorado potato beetle larvae differs by instar and attacking predator. Biol Control 53:230–237. https://doi.org/10.1016/j.biocontrol.2010.01.004
Sansom A, Lind J, Cresswell W (2009) Individual behavior and survival: the roles of predator avoidance, foraging success, and vigilance. Behav Ecol 20:1168–1174. https://doi.org/10.1093/beheco/arp110
Santos GCSG, Dionisio-da-Silva W, Souza-Alves JP, Albuquerque CMR, Lira AFA (2018) Random or clumped: how litter dwelling scorpions are distributed in a fragment of Brazilian Atlantic forest. Eur J Entomol 115:445–449
Taylor MS, Cosper CR, Gaffin DD (2012) Behavioral evidence of pheromonal signaling in desert grassland scorpions Paruroctonus utahensis. J Arachnol 40:240–244
Toscano-Gadea CA, Costa FG (2006) Is Tityus uruguayensis (Buthidae) an araneophagic scorpion? An experimental analysis of its predatory behaviour on spiders and insects. Bull Brit Arachnol Soc 13:256–264
Troupe JE (2009) Ontogenetic shift in agonistic behavior of the southern black widow spider, Latrodectus mactans (Araneae: Theridiidae). Thesis, University of Tennessee, Knoxville
Tullberg BS, Merilaita S, Wiklund C (2005) Aposematism and crypsis combined as a result of distance dependence: functional versatility of the colour pattern in the swallowtail butterfly larva. Proc Biol Sci R Soc 272:1315–1321. https://doi.org/10.1098/rspb.2005.3079
Uma DB, Weiss MR (2012) Flee or fight: ontogenetic changes in the behavior of cobweb spiders in encounters with spider-hunting wasps. Environ Entomol 41:1474–1480. https://doi.org/10.1603/EN12126
van der Meijden A, Coelho PL, Sousa P, Herrel A (2013) Choose your weapon: defensive behavior is associated with morphology and performance in scorpions. PLoS One 8:e78955. https://doi.org/10.1371/journal.pone.0078955
Vitt LJ, Cooper WE Jr (1986) Foraging and diet of a diurnal predator (Eumeces laticeps) feeding on hidden prey. J Herpetol. https://doi.org/10.2307/1564503
Warburg M (1998) Qualitative and quantitative analysis of intra-and interspecific behavioural patterns among scorpions. J Ethol 16:115–121. https://doi.org/10.1007/BF02769290
Watts JC, Herrig A, Allen WD, Jones TC (2014) Diel patterns of foraging aggression and antipredator behaviour in the trashline orb-weaving spider, Cyclosa turbinata. Anim Behav 94:79–86. https://doi.org/10.1016/j.anbehav.2014.05.020
Werner E, Gilliam J (1984) The ontogenetic niche and species interactions in size-structured populations. Annu Rev Ecol Evol Syst 15:393–426. https://doi.org/10.1146/annurev.es.15.110184.002141
Williams JL, Snyder WE, Wise DH (2001) Sex-based differences in antipredator behavior in the spotted cucumber beetle (Coleoptera: Chrysomelidae). Environ Entomol 30:327–332. https://doi.org/10.1603/0046-225X-30.2.327
Acknowledgements
We are grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for granting a PhD scholarship to A.F.A. Lira and to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Fellowship #307759/2015-6 for research productivity) for a financial support for C.M.R. Albuquerque. We also grateful to Fundação de Amparo a Ciência e Tecnologia de Pernambuco (FACEPE) for financial support (APQ-0437-2.04/15).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Experiments using invertebrate animals conducted in Brazil do not require approval by the Ethics Committees, as established by the Brazilian Council for the Control of Animal Experimentation (CONCEA) (Law 11.794/08, § 3). In addition, the authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Lira, A.F.A., Almeida, F.M.F. & Albuquerque, C.M.R. Reaction under the risk of predation: effects of age and sexual plasticity on defensive behavior in scorpion Tityus pusillus (Scorpiones: Buthidae). J Ethol 38, 13–19 (2020). https://doi.org/10.1007/s10164-019-00615-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-019-00615-4