Abstract
Purpose
To define and analyze the learning curve of percutaneous endoscopic transforaminal decompression (PETD) for lumbar spinal stenosis (LSS).
Methods
From July 2015 to September 2016, 78 patients underwent PETD; one of whom was converted to open surgery, two were lost, and 75 were included in this study. Clinical results were assessed by using the Oswestry Disability Index (ODI) and visual analog scale (VAS). The learning curve was assessed by a logarithmic curve-fitting regression analysis. Of these 75 patients, 35 were defined as the “early” group, and 40 were defined as the “late” group for comparison.
Results
The mean follow-up was 25.37 ± 4.71 months. The median operative time gradually decreased from 95 (interquartile range, IQR, 85–110) minutes for the early group to 70 (IQR, 60–80) minutes for the late group (P < .000), and an asymptote was reached after approximately 35 cases. After surgery, the VAS for leg pain (LP) and ODI decreased significantly and remained constant during the follow-up. However, the VAS of low back pain (LBP) increased mildly. The total complication rate was 6.6%. ODI, VAS of LP and of LBP, and complication rate did not significantly differ between two groups. Early ambulation and short hospital stay after surgery were achieved.
Conclusion
The learning curve of PETD for LSS was assessed and good clinical results were achieved. The surgeon’s experience with this technique correlated with reduced operation time. Proper patient selection, familiarity with pathological anatomy, and manipulation under endoscopic view may shorten the learning curve and decrease complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lumbar spinal stenosis (LSS) can cause chronic low back pain (LBP), leg pain (LP), and intermittent neurological claudication, which may severely restrict function, walking ability, and quality of life [1]. When conservative management is unsuccessful, surgical treatment is considered [2]. Currently, LSS is the most common indication for spinal surgery [3, 4].
The surgical options for LSS are decompression with fusion or decompression alone (non-fusion). Since minimally invasive spinal surgery (MISS) was introduced, a variety of MISS lumbar interbody fusion (LIF) procedures have been applied to treat LSS [5,6,7,8]. Despite reported good clinical results, these techniques have some inherent disadvantages, including loss of motion unit, risk of non-fusion, internal implant-related complications, demand for general anaesthesia, and high cost [2, 9,10,11,12].
Non-fusion MISS techniques may overcome those disadvantages in selected patients such as patients without intervertebral segmental instability or patients with complex general diseases and elderly patients. Micro-endoscopic discectomy (MED) can successfully treat LSS via a unilateral approach to achieve bilateral decompression [13, 14]. Recently, with the development of endoscopic equipment and surgical skills, full-endoscopic decompression surgery (FEDS) was used to treat LSS [15,16,17] and was considered to be less invasive than MED. The proper indications for percutaneous endoscopic transforaminal decompression (PETD) and percutaneous endoscopic interlaminal decompression (PEID) for LSS were proposed [18]. For the anatomical restriction in the upper lumbar spine, and different demands of anaesthesia, PETD has a wider application than PEID in the lumbar spine.
Because of the complicated pathological changes in LSS, using PETD for treating LSS is still a technical challenge for most surgeons. Therefore, understanding the learning curve of PETD is essential to avoid severe complications when beginning to use this technique. To our knowledge, no study has evaluated operative time, complications, or outcomes associated with gaining experience with PETD for LSS. The aim of this study was to assess the learning curve of PETD for LSS.
Materials and methods
Before using PETD for LSS, the author observed ten cases treated with this surgery at a spinal endoscopy centre and practiced on cadavers five times. The author has experience in percutaneous endoscopic lumbar discectomy (PELD) of more than 100 cases, in which transforaminal approach was used for nearly three-fifths. Between July 2015 and September 2016, 91 patients with LSS were treated by FEDS. Thirteen patients were treated with PEID, and 78 were treated with PETD. One of the 78 patients was converted to open surgery and 77 completed PETD. Two of the 77 patients were lost during the follow-up for the change of phone number and address. The remaining 75 patients were included in this study and their demographic details are presented in Table 1.
The inclusion criteria were as follows: (1) patients with LSS, including lateral recess, foraminal stenosis, and central stenosis (Schizas grade B and C) [19, 20], as confirmed by imaging characteristics and clinical symptoms; (2) with single level pathology and unilateral symptoms; (3) with > three months of failed conservative treatment; (4) with sufficient follow-up data. The exclusion criteria were the following: (1) patients with extreme LSS [20]; (2) with segmental instability; (3) with visual analog scale (VAS) score of LBP > 3; (4) with abnormal nerve root in the neuroforamen on magnetic resonance imaging (MRI); (5) with pathologies such as infection or tumor. This retrospective study was approved by the ethics committee of our hospital.
Operation steps
All operations were performed under basic sedation and local anaesthesia. A prone position with flexed hip and knee on a radiolucent table was used for all patients. The skin entry point of the spinal needle was basically located 8–13 cm lateral from the midline according to the patient’s body size. The initial target point of the needle was the middle part of the superior articular process (SAP) of the inferior vertebrae. After proper initial needle placement and local anesthesia with 0.5% lidocaine, a guide rod was placed at the middle-inferior part of the foramen on anterior-posterior fluoroscopy and at the ventral surface of the middle part of the SAP on the lateral view (Fig. 1a). The rod was gently tapped to fix it on the SAP. A series of dilators were inserted consecutively, and a trephine protection tube was placed finally. After the protection tube was firmly fixed within the foraminal portion, a trephine (diameter, 8.5 mm) with a deep-control device was used to undercut the bone from the ventral part of the SAP under fluoroscopic inspection (Fig. 1b). While removing the cutting bone and introducing the endoscope, the hole in the SAP created by the trephine was used as a good landmark to guide our endoscopic decompression. An endo-Kerrison was used to further bony decompression (Fig. 1c). At this point, the ligamentum flavum, foraminal ligaments, shoulder osteophyte, and disc surface were observed. The repeated dissection-and-cutting maneuvers decompressed the lateral recess and released the dural sac and traversing nerve root. Ventral pathologies, such as osteophytes and bulged intervertebral discs, could be decompressed. A bipolar radiofrequency coagulator was used to ablate the ligamentous structures and for bleeding control. The end point of the procedure was free mobilization and release of the dural sac and the affected nerve roots (Fig. 1d). If a patient has intervertebral foramen stenosis and the corresponding symptoms, enlargement of the whole foramen for decompressing the exiting root is crucial. After confirming the full-scale lateral recess and foramen decompression and haemostasis, the endoscope and working tube were withdrawn. A single-point subcutaneous suture was applied. Thereafter, the patients were monitored for post-operative problems and discharged mostly within 48 hours after finishing the remaining post-operative examinations. A representative case is shown in Fig. 2.
Surgical steps. a A guide rod is placed at the target point. b A trephine with a deep-control device (white arrow) is used in the foraminoplasty. c The hole within the SAP is created by the trephine as a landmark to guide decompression. An endo-Kerrison punch was used to decompress the remaining hard structures. d A bipolar radiofrequency coagulator is used to ablate the ligamentous structures and the nerve root is released. The yellow arrow indicates the ligamentum flavum, the green arrow indicates osteophytes and the bulged disc, and the red arrow indicates the nerve root
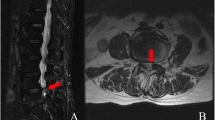
A 55-year-old man with L4/5 spinal stenosis. a Pre-operative images showing L4/5 left lateral recess stenosis with disc herniation (red arrow). b The lateral recess and neuroforamen have been enlarged, and the ventral part of the superior facet and the herniated disc had been removed after surgery (yellow arrow)
Outcome evaluation and statistical analysis
Operative time (the time from making skin landmark to ending the surgery), intra-operative fluoroscopy exposure, and intra-operative and post-operative complications were analyzed. Post-operative X-ray and three-dimensional reconstruction computed tomography imaging were performed for all patients. Post-operative MRI was indicated only for patients with deteriorated post-operative neurological symptoms. Clinical results were assessed by the VAS score and the Oswestry Disability Index (ODI) by two independent observers at day one, at months three, six and 12, and yearly after surgery.
Statistical analysis was conducted with SPSS 19.0 (SPSS Inc., Chicago, IL, USA). The normality of data was tested using Shapiro–Wilk test. Continuous data were presented as mean and standard deviation or interquartile range (IQR), if available. The learning curve for PETD was assessed by logarithmic curve-fitting regression analysis. Continuous data were compared with paired t test for parametric data and Wilcoxon symbolic rank test for non-parametric data. The repeated-measures data were compared using Friedman test followed by a pairwise comparison. The χ2 test or Fisher’s exact test was used to compare the frequencies of categorical data. A P value < 0.05 was considered significant.
Results
Clinical results of the patients (n = 75)
The mean follow-up period for the patients was 25.37 ± 4.71 (range, 12–36) months. Nine (12%) patients were lost in different time points during follow-up. Therefore, the data of the last follow-up for all patients was chosen to be the end point for a statistical analysis. The median operative time of all patients was 80 (IQR, 65–105) minutes. The median intra-operative fluoroscopy exposure was 16 (IQR, 12–19) times. Intra-operative blood loss was unmeasurable. The post-operative median time to ambulation was 20 (IQR, 18–24) hours. The median hospital stay after surgery was 30 (IQR, 24–39) hours (Table 1). The VAS score of LP decreased from 6 (IQR, 5–6) pre-operatively to 1 (IQR, 1–2) post-operatively and to 1 (IQR, 1–2) at the last follow-up. The VAS score of LBP changed from 1 (IQR, 1–2) preoperatively to 1 (IQR, 1–2) postoperatively and to 2 (IQR, 1–2) at the last follow-up. The ODI values decreased from 34 (IQR, 32–36) pre-operatively to 9 (IQR, 7–10) post-operatively and to 9 (IQR, 7–10) at the last follow-up (Table 2).
Learning curve of PETD (n = 75)
The operative time decreased significantly with accumulation of the number of cases, as demonstrated by the equation from the logarithmic curve-fitting regression analysis (P < .000), y = − 46.94 ln(x) + 153 with a coefficient of determination R2 of 0.567 (case number, x; operative time, y (min)) (Fig. 3). A steady state, which was defined as the asymptote of the learning curve, was assumed to have been achieved after approximately 35 cases.
Comparison of outcome measures between the early group (n = 35) and late group (n = 40)
On the basis of the learning curve findings, the 75 patients were divided into two groups: the early group (35 patients) and the late group (40 patients). The pre-operative baseline data were equal between the two groups (Tables 3 and 4). Operative time decreased significantly from 95 (IQR, 85–110) minutes in the early group to 70 (IQR, 60–80) minutes in the late group (P < .000) (Table 3). Intra-operative fluoroscopy exposure decreased from 19 (IQR, 18–22) times in the early group to 12 (IQR, 12–16) times in the late group (P < .000). The post-operative ODI and VAS scores of LP in both groups significantly decreased from the pre-operative values, and these results were steadily maintained during the follow-up (Table 2). However, the VAS score of LBP in the total 75 patients showed a significant increase from 1 (IQR, 1–2) post-operatively to 2 (IQR, 1–2) at the last follow-up (P = .050). There were no significant differences in the ODI and VAS scores of LP and LBP at the same time points between the groups (Table 4) or in the time to ambulation and hospital stay after surgery (Table 3).
Complications and converted operation (n = 76)
In one patient, PETD had to be converted to open surgery, and this operation was considered to be a type of complication of PETD. Therefore, 76 cases were finally analyzed with respect to complications of this surgery. The converted patient (case 29th) was allocated to the early group. The total complication rate was 6.6% (5/76). The rates in the early group and late group were 8.6% (3/36) and 5% (2/40), respectively. The number of complications and rate were higher in the early group, which indicated a higher risk of complications in the early learning stage of this new technique. However, the complication incidences were not significantly different between the 2 groups (P = .663), this may have been related to the relatively small sample size. On the other hand, this result indicated the need to exercise great care during the operation even if the surgeon is familiar with this technique. Detailed information is presented in Table 5.
Discussion
Pathological changes are more complicated in LSS than in LDH [1]; therefore, more specialized tools, skills, and experience are demanded in LSS than in LDH if an endoscope is used [18]. The learning curve of PELD cannot represent the learning course of PETD for LSS, although the two techniques have similarities. Actually, PETD should be considered to be a new and complex technique and should be “pushed” by a few selected experienced surgeons to explore the learning course of this technique [21]. Following this strategy, a senior doctor assessed the learning curve of PETD for LSS in the present study. Additionally, the strict inclusion criteria were adopted to minimize the potential selection bias to facilitate the further comparison (Table 3), because the difference in pathological severity and location of LSS can significantly affect the clinical results.
In general, when comparing a new technique with an existing “gold standard” technique, identification of the asymptote or steady state of the learning curve can facilitate an accurate comparison [22, 23]. Operative time is the best indicator of familiarity with and/or mastering of a technique. The operative time should decrease during the beginning stage and then reach an asymptote as a surgeon accumulates cases, and the form of the curve should be logarithmic. Therefore, we mathematically derived a logarithmic function and showed that the operative time gradually decreased until approximately the 35th case. Therefore, accumulation of 35 cases was near the beginning of the learning curve asymptote. The learning stage of 35 cases of PETD is longer than that of other techniques which ranged from around 20 to 30 cases [22, 24, 25]. Despite difference in techniques and diseases, this longer learning stage may indicate the complexity of this new technique.
The operative time in the total 75 patients was 80 (IQR, 65–105) minutes. In the early group, the operative time was 95 (IQR, 85–110) minutes, which was longer than the time of previous studies using PETD for LSS from 54 to 89.7 minutes [16, 17]. With the increase in knowledge and skills acquired with increased experience, in the late group, the time was decreased to 70 (IQR, 60–80) minutes, which was similar with the time of previous similar studies [16, 17]. Differences in understanding for pathological anatomy under endoscopic view, technique familiarity, and the surgeon’s skill may account for this longer operative time in the early group than that for previous studies. Combined with minimal injury and earlier recovery, we achieved decreased intra-operative fluoroscopy exposure during PETD in the late group (P < .000), which may benefit both patients and surgeons. This benefit is also a function of increased experience with accumulation of cases.
We observed a significant improvement in the ODI and VAS score of LP in all 75 patients after PETD. These results were steadily maintained throughout the follow-up, which suggested that this technique effectively improved the functional ability of the patients. There were no significant differences in the ODI and VAS scores of LP at the same time point between the two groups, which indicated that similar results could be achieved even with a difference in the operation time. These results were comparable with the results of previous similar studies [16, 17] and fusion surgeries [6, 7, 9, 11]. The shorter time to ambulation and hospital stay after operation offers an obvious advantage over MIS-fusion surgeries [7, 9, 24]. A distinctive finding was that the VAS scores of LBP for all patients increased significantly from 1 (IQR, 1–2) post-operatively and to 2 (IQR, 1–2) at the last follow-up. This result was different from previous findings [15, 16]. No patient needed subsequent surgery for this increased LBP, and we did not find radiographic evidence of segmental instability during the follow-up. Two reasons may account for this finding. First, this is a non-fusion surgery. With progressive degeneration of the lumbar spine, LBP may increase [26, 27]. Second, foraminoplasty may cause injury to the facet joint and increase the opportunity for lumbar segmental instability to cause LBP [28]. Therefore, care is needed to minimize injury to the facet joint in the following surgery.
A major concern during the initial stage of performing PETD is complications. We did not encounter serious complications; the total complication rate was 6.6% (5/76) and comparable with that for PELD (3% to 6.6%) [29,30,31] and that for FEDS (0% to 10.4%) [15, 16, 32]. The complication rate (8.6%) in the early group was higher than that for PELD which indicated that PETD presented a greater technical challenge reflected by the differences in pathologies and required skills. One converted surgery in this study meant that insufficient understanding of the pathological anatomy for LSS caused improper patient selection and significantly increased the complication rate. In the late group, we thought that the complication rate of 5.0% was acceptable. In this study, there was no significant difference in the complication rates between the two groups (P = .663), which suggests that the surgeon was making a continual effort to prevent complications at the cost of increased operative time. To reduce the complication rate of PETD, we agree with the recommendation that surgeons should start with simple cases in the initial learning stage and work under the supervision of an experienced surgeon to gain adequate experience [33, 34].
Two technique issues may reduce the complications in the learning stage of PETD: First, the trephine should not cut through the medial cortex of the SAP completely, and then the cannula should be gently rotated clockwise to cause a fracture of the bony cortex, with use of endo-Kerrisons to carefully remove the bone fragments. This temporarily reserved cortex might act as a barrier to decrease the risk of cutting the nerve. Second, the most compressive region was the junction area between the posterior and inferior margin of the intervertebral disc and superior part of the inferior pedicle. Hence, removal of the medial and superior parts of the pedicle was sometimes needed to achieve complete decompression.
The retrospective design was a major limitation of this study. Other limitations included the different ending time points during the follow-up which may somewhat affect the interpretation of the results, and the non-quantified distance of intermittent neurological claudication. Further, PETD has difficulties in treating patients at the L5-S1 level with a high iliac crest.
References
Katz JN, Harris MB (2008) Clinical practice. Lumbar spinal stenosis. N Engl J Med 358:818–825. https://doi.org/10.1056/NEJMcp0708097
Phillips FM, Slosar PJ, Youssef JA et al (2013) Lumbar spine fusion for chronic low back pain due to degenerative disc disease: a systematic review. Spine 38:E409–E422. https://doi.org/10.1097/BRS.0b013e3182877f11
Deyo RA, Mirza SK, Martin BI et al (2010) Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 303:1259–1265. https://doi.org/10.1001/jama.2010.338
Du Bois M, Szpalski M, Donceel P (2012) A decade’s experience in lumbar spine surgery in Belgium: sickness fund beneficiaries, 2000–2009. Eur Spine J 21:2693–2703. https://doi.org/10.1007/s00586-012-2381-1
Foley KT, Holly LT, Schwender JD (2003) Minimally invasive lumbar fusion. Spine 28:S26–S35
Ozgur BM, Aryan HE, Pimenta Let al (2006) Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 6:435–443
Mayer HM (1997) A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine 22:691–699 discussion 700
Silvestre C, Mac-Thiong JM, Hilmi R et al (2012) Complications and morbidities of mini-open anterior retroperitoneal lumbar Interbody fusion: oblique lumbar interbody fusion in 179 patients. Asian Spine J 6:89–97. https://doi.org/10.4184/asj.2012.6.2.89
Seng C, Siddiqui MA, Wong KP et al (2013) Five-year outcomes of minimally invasive versus open transforaminal lumbar interbody fusion: a matched-pair comparison study. Spine 38:2049–2055. https://doi.org/10.1097/BRS.0b013e3182a8212d
Deyo RA, Ciol MA, Cherkin DC et al (1993) Lumbar spinal fusion-a cohort study of complications, reoperations, and resource use in the Medicare population. Spine 18:1463–1470
Khan NR, Clark AJ, Lee SL et al (2015) Surgical outcomes for minimally invasive vs open transforaminal lumbar interbody fusion: an updated systematic review and meta-analysis. Neurosurgery 77:847–874. https://doi.org/10.1227/NEU.0000000000000913
Goldstein CL, Phillips FM, Rampersaud YR (2016) Comparative effectiveness and economic evaluations of open versus minimally invasive posterior or transforaminal lumbar interbody fusion: a systematic review. Spine 41:S74–S89. https://doi.org/10.1097/BRS.0000000000001462
Xu BS, Tan QS, Xia Q et al (2010) Bilateral decompression via unilateral fenestration using mobile microendoscopic discectomy technique for lumbar spinal stenosis. Orthop Surg 2:106–110. https://doi.org/10.1111/j.1757-7861.2010.00072.x
Perez-Cruet MJ, Foley KT, Isaacs RE et al (2002) Microendoscopic lumbar discectomy: technical note. Neurosurgery 51:S129–S136
Kim HS, Paudel B, Jang JS et al (2017) Percutaneous full endoscopic bilateral lumbar decompression of spinal stenosis through uniportal-contralateral approach: techniques and preliminary results. World Neurosurg 103:201–209. https://doi.org/10.1016/j.wneu.2017.03.130
Xiong C, Li T, Kang H et al (2019) Early outcomes of 270-degree spinal canal decompression by using TESSYS-ISEE technique in patients with lumbar spinal stenosis combined with disk herniation. Eur Spine J 28:78–86. https://doi.org/10.1007/s00586-018-5655-4
Shin SH, Bae JS, Lee SH et al (2018) Transforaminal endoscopic decompression for lumbar spinal stenosis: a novel surgical technique and clinical outcomes. World Neurosurg 114:e873–ee82. https://doi.org/10.1016/j.wneu.2018.03.107
Ahn Y (2014) Percutaneous endoscopic decompression for lumbar spinal stenosis. Expert Rev Med Devices 11:605–616. https://doi.org/10.1586/17434440.2014.940314
Wang Y, Dou Q, Yang J et al (2018) Percutaneous endoscopic lumbar decompression for lumbar lateral spinal canal stenosis: classification of lateral region of lumbar spinal canal and surgical approaches. World Neurosurg 119:e276–e283. https://doi.org/10.1016/j.wneu.2018.07.133
Schizas C, Theumann N, Burn A et al (2010) Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine 35:1919–1924. https://doi.org/10.1097/BRS.0b013e3181d359bd
Benzel EC, Orr RD (2011) A steep learning curve is a good thing! Spine J 11:131–132. https://doi.org/10.1016/j.spinee.2010.12.012
Nowitzke AM (2005) Assessment of the learning curve for lumbar microendoscopic discectomy. Neurosurgery 56:755–762
Ramsay CR, Grant AM, Wallace SA et al (2000) Assessment of the learning curve in health technologies. A systematic review. Int J Technol Assess Health Care 16:1095–1108
Lee JC, Jang HD, Shin BJ (2012) Learning curve and clinical outcomes of minimally invasive transforaminal lumbar interbody fusion: our experience in 86 consecutive cases. Spine 37:1548–1557. https://doi.org/10.1097/BRS.0b013e318252d44b
Hua W, Zhang Y, Wu X et al (2019) Full-endoscopic visualized foraminoplasty and discectomy under general anesthesia in the treatment of L4-L5 and L5-S1 disc herniation. Spine 44:E984–E991. https://doi.org/10.1097/BRS.0000000000003014
Anderson DG, Tannoury C (2005) Molecular pathogenic factors in symptomatic disc degeneration. Spine J 5:260S–266S
Zhang YG, Guo TM, Guo X et al (2009) Clinical diagnosis for discogenic low back pain. Int J Biol Sci 5:647–658
Haher TR, O’Brien M, Dryer JW et al (1994) The role of the lumbar facet joints in spinal stability. Identification of alternative paths of loading. Spine 19:2667–2670 discussion 2671
Yeung AT, Tsou PM (2002) Posterolateral endoscopic excision for lumbar disc herniation-surgical technique, outcome, and complications in 307 consecutive cases. Spine 27:722–731
Ruetten S, Komp M, Merk H et al (2008) Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine 33:931–939. https://doi.org/10.1097/BRS.0b013e31816c8af7
Wang B, Lu G, Patel AA et al (2011) An evaluation of the learning curve for a complex surgical technique: the full endoscopic interlaminar approach for lumbar disc herniations. Spine J 11:122–130. https://doi.org/10.1016/j.spinee.2010.12.006
Nellensteijn J, Ostelo R, Bartels R et al (2010) Transforaminal endoscopic surgery for lumbar stenosis: a systematic review. Eur Spine J 19:879–886. https://doi.org/10.1007/s00586-009-1272-6
Ruetten S, Komp M, Merk H et al (2007) Use of newly developed instruments and endoscopes: full-endoscopic resection of lumbar disc herniations via the interlaminar and lateral transforaminal approach. J Neurosurg Spine 6:521–530
Choi G, Lee SH, Raiturker PP et al (2006) Percutaneous endoscopic interlaminar discectomy for intracanalicular disc herniations at L5-S1 using a rigid working channel endoscope. Neurosurgery 58:59–68
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The device(s) is/are FDA approved or approved by corresponding national agency for this indication. This retrospective study was approved by the West China Hospital Ethics Committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jin Yang and Chuan Guo are co-first authors
Rights and permissions
About this article
Cite this article
Yang, J., Guo, C., Kong, Q. et al. Learning curve and clinical outcomes of percutaneous endoscopic transforaminal decompression for lumbar spinal stenosis. International Orthopaedics (SICOT) 44, 309–317 (2020). https://doi.org/10.1007/s00264-019-04448-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-019-04448-1