Abstract
Background
It is a general belief among orthopedists that the muscle damage of the hip abductors after total hip arthroplasty (THA) is theoretically minimal via posterior approach. However, there is little data scientifically supporting the purported advantage. The purpose of this study was to quantifiably assess the injury to the gluteus medius (GMED) after THA via the minimally invasive (MIS) posterior and the modified direct lateral (mDL) approaches.
Methods
Sixty-four consecutive patients enrolled prospectively were randomly assigned to the MIS posterior and the mDL approach groups. Three-dimensional MRI reconstruction of bilateral GMED, abductor strength measurement as well as post-operative pain assessment were included in the analysis. Data were collected pre-operatively, six, 12, and 52 weeks post-operatively.
Results
Interestingly, in terms of the morphological changes of GMED, the MIS posterior approach showed a more significant degeneration caused by the surgical trauma compared with the mDL approach in both muscle volume atrophy and fatty infiltration from six to 52 weeks post-operatively. However, the improvement of abductor muscle strength on surgical side and VAS pain score were comparable between the two groups during the entire follow-up.
Conclusion
The injury of hip abductors after THA via posterior approach cannot be neglected. And, the planned detachment of partial GMED tendon combined with the reconstruction in situ could also achieve the satisfactory muscle recovery. Moreover, the post-operative rehabilitation of abductor strength was the aggregated results of a battery of factors, especially the pain, not just determined by the muscular morphological changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the so-called protection of hip abductors, there is substantial interest localized on the minimally invasive (MIS) approaches of total hip arthroplasty (THA) [1,2,3]. It is generally accepted that, for the posterior approach, hardly any surgical dissection of the hip abductors should result in the slightest muscle injury. On the contrary, the modified direct lateral (mDL) approach from original Hardinge’s approach has been considered to be one of the most violent options for the abductor muscles [4,5,6].
Even so, there are still a handful of literatures refuting the plausible viewpoints above through the cadaveric experiments or electromyography (EMG) measurements [7,8,9,10]. Interestingly, these are studies that directly measure the damage of the hip abductors after THA. However, whatever the results, the previous researches related to the injury of the hip abductors are relatively old and the methods have certain limitations. Therefore, the related conclusions are not entirely convincing. More importantly, no matter what surgical approach was used, poor hip abductor strength and gait abnormalities after THA were widely reported, which had a great impact on the life quality of the patients [11,12,13].
Based on the above, this study aimed to address the following two questions quantificationally by using three-dimensional MRI reconstruction technique: (1) whether the damage to the gluteus medius (GMED) could be ignored through the MIS posterior approach compared with the mDL approach; (2) whether the rehabilitation of abductor strength was solely determined by the morphological changes of abductor muscles.
Methods
Patients
This prospective study has been approved by Ethics Committee of the First Affiliated Hospital of Chongqing Medical University and each patient has signed an informed consent. The methods were carried out in accordance with the relevant guidelines and regulations. Meanwhile, the study has been registered in Chinese Clinical Trial Registry (ChiCTR), the Clinical Trial Registry Number is ChiCTR-IOR-17013007. Four hundred and sixty-nine consecutive patients prepared for THA were enrolled from March 2016 to March 2017 including 278 males and 191 females with an average age of 55.39 years (range, 19–80 years). Each patient has signed the informed consent. Inclusion criteria comprise admission diagnosis of the primary hip osteoarthritis. Exclusion criteria consist of contralateral hip disease or surgical history, joint ankylosis or stiffness, suppurative coxarthritis, femoral or acetabular osteotomy, ipsilateral surgical history, severe systemic infection or tumor diseases, severe medical diseases, muscle weakness, muscle dystrophy- or muscle atrophy-related diseases, physical disability, or mental illness. At last, 64 patients have met the criteria (Fig. 1). Patients were randomly assigned to the MIS posterior approach group (32 patients) or the mDL approach group (32 patients) using the opaque envelope method. During the follow-up, ten patients (6 of the MIS posterior group, 4 of the mDL group) withdrew or lost from the study for a variety of reasons. In the end, 54 patients in this study were eligible to have completed the entire assessments throughout the entire follow-up (26 patients in the MIS posterior approach group, 28 in the mDL approach group). Demographic data of all patients involved were registered (Table 1).
Surgical procedure
For the MIS posterior approach, the minimally invasive form of that popularized by Gibson [14] was utilized: the tendon insertion of the short external rotators including piriformis, internal obturator muscle, superior gemellus, and inferior gemellus were cut off; the posterior joint capsule was cut through with a flap-shaped incision. Only the tendon of piriformis in combination with the posterior joint capsule was non in-situ reattached through a suture hole on the posterior part of femoral great trochanter using the non-absorbable suture (Ethibond) (Fig. 2).
The mDL approach was a modification of the approach which was initially described by Hardinge [15] with the detachment and subsequent in situ repair of the anterior forth to third of the tendons of GMED and gluteus minimus. Notably, the GMED was incised to a maximum length of 3 cm to protect the superior gluteal nerve (SGN) and the incision prolonging into the vastus lateralis was strictly avoided (Fig. 3).
All the MIS posterior THA were performed by one senior surgeon with rich experience and all the mDL THA were completed by another veteran senior surgeon. No complications were found in either group during the entire follow-up. Patient-controlled intravenous analgesia as well as oral non-steroidal anti-inflammatory drugs (NSAIDs) were routinely administrated as post-operative pain control protocol. All the patients followed the same post-operative rehabilitation protocol under instructions of physical therapists: passive and active leg-raising training from the first day, partial weight bearing walking from the third day, going up and down stairs from seventh to tenth day. Surgical performers were blinded to the randomization of the participants.
Three-dimensional MRI reconstruction technique
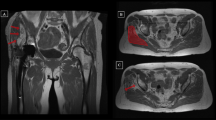
MRI was performed on a 1.5-T scanner (Signa HDxt, GE MEDICAL SYSTEMS, USA) according to the standard protocol pre-operatively, six, 12, and 52 weeks post-operatively in our hospital for all 54 patients and no data was missing. Images were acquired in 4-mm slices using a flexible phased-array coil and clinically established MRI sequences at our radiology department. T1-weighted fast spin-echo (FSE) images were obtained using configured scan parameters (TE, Min Full; TR, 760 ms; matrix size, 512 × 512; bandwidth, 31.25 kHz; field of view, 480 mm; slice thickness, 4 mm; spacing, 0.5 mm). T2-weighted fast recovery with fast spin-echo (FRFSE) sequence was also acquired (Auto TR, 2820 ms; Echo Train Length(18 mm); matrix size, 512 × 512; bandwidth, 31.25 kHz; field of view, 480 mm; Freq, 228 kHz; slice thickness, 4 mm; spacing, 0.5 mm). The use of coronal sections established the first cut from the level of anterior superior iliac spine to the middle of femoral shaft in each patient. The Digital Imaging and Communications in Medicine (DICOM) data of all the 54 bilateral hip joint MRI images were saved as DVD and loaded into Mimics 17.0 (Materialise, Belgium). Two orthopaedic radiologists independently identified the contours of the bilateral GMED using the LiveWare tool. If controversy existed over the identification between the two researchers, a third professional veteran musculoskeletal radiologist with decades of experience would make the final determination. The range of the reconstruction included all sections of the GMED that can be clearly recognized on the coronal sections. Due to the influence of muscle atrophy, the number of the bilateral sections can vary. The adipose threshold was determined based on the built-in “predefined thresholds sets” of Mimics software and was consistent for each patient in both groups. The Cronbach’s alpha and intraclass correlation coefficient for consistency between the two radiologists on each thresholding value selection for all included MRI images were 0.993 and 0.984 (95% CI = 0.972–0.990, F = 121.543, p < 0.01), respectively. After selection of the adipose threshold, the “negative value” operation was applied in the course of the adipose tissue reconstruction in order to ensure that the range of the two masks was exactly the same. The adipose tissue was separated from the muscle through the Boolean operation. The volume of muscle, adipose tissue and the corresponding fat-muscle ratio were calculated from the polygonal volume and surfaces on the basis of the three-dimensional reconstruction technique (Fig. 4).
Clinical assessment
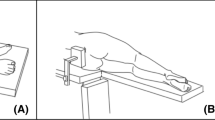
Measurement of the bilateral abductor strength and VAS pain score was conducted on each patient before operation, six, 12, as well as 52 weeks post-operatively in our department and no data was missing. The portable dynamometer (Lafayette Manual Muscle Test System model 01163; Lafayette Instrument Company, USA) was used for the abductor strength measurement in a lateral decubitus position (Fig. 5).
Statistical analysis
Data analysis was performed by an independent statistician using SPSS (Version 22; SPSS Inc., Chicago, IL). Univariate analysis of continuous variables was compared with a parametric t test or a nonparametric Mann-Whitney U test. Power and Precision software was used for the calculation of a sample size. A p value less than 0.05 was deemed to be statistic significant.
Results
GMED morphology analysis by three-dimensional MRI reconstruction
GMED volume
There was not a significant difference regarding the muscle volume of the bilateral GMED preoperatively in both groups (MIS posterior, p = 0.130; mDL, p = 0.099). At six weeks post-operatively, compared with the contralateral side, the GMED volume on the operated side decreased by 16.89% in MIS posterior group (p < 0.01) and by 7.27% in mDL group (p < 0.01), which were both statistically significant. The remarkable change was seen at 12 weeks; no obvious recovery of the GMED volume was observed via MIS posterior approach which still decreased by 14.05% (p < 0.01), while only 1.91% via mDL approach without significant difference (p = 0.098). After 52 weeks of rehabilitation, the GMED muscle volume in MIS posterior group also returned to normal that only decreased by 1.97% (p = 0.241) and 0.15% for mDL group (p = 0.877) (Table 2). There was no significant change in the GMED muscle volume on the contralateral side during the entire follow-up (p > 0.05).
GMED fat-muscle ratio
Both MIS posterior and mDL groups were homogeneous for pre-operative bilateral fat-muscle ratio (MIS posterior, p = 0.115; mDL, p = 0.139). At six weeks post-operatively, compared with the contralateral side, the GMED fat-muscle ratio on the operated side went up notably in both groups: 5.22% higher in MIS posterior approach (p < 0.01) as well as 7.26% higher in mDL approach (p < 0.01). Neither MIS posterior nor mDL group showed prominent improvement in this ratio at 12 weeks with 7.21% augmented via the MIS posterior (p < 0.01) and 5.01% increased through the mDL (p < 0.05) approach. At 52 weeks, the GMED fat-muscle ratio barely recovered to the normal level in both groups without statistical difference (MIS posterior, 0.34% increased, p = 0.411; mDL, 0.03% increased, p = 0.915) (Fig. 6). There was no dramatic change in the fat-muscle ratio on the contralateral side during the entire follow-up (p > 0.05).
Mean ± standard deviation of GMED fat-muscle ratio on the operated side in comparison with the contralateral side for both groups from pre-operation to 52 weeks post-operatively. a The GMED fat-muscle ratio in MIS posterior group increased by 0.29% (p = 0.115) pre-operatively, 5.22% (p < 0.01) at 6 weeks, 7.21% (p < 0.01) at 12 weeks, and 0.22% (p = 0.411) at 52 weeks. b The GMED fat-muscle ratio in mDL group increased by 0.34% (p = 0.139) pre-operatively, 7.26% (p < 0.01) at 6 weeks, 5.01% (p < 0.01) at 12 weeks, and 0.03% (p = 0.915) at 52 weeks
Clinical assessment
Isometric abductor muscle strength
Preoperatively, due to pain and disuse, the abductor muscle strength of the affected side was significantly lower than that of the contralateral side in both groups (MIS posterior, 35.53% reduced; mDL, 35.62% reduced; p < 0.01). At six weeks post-operatively, neither MIS posterior group nor mDL group presented an obvious amelioration of the muscle strength (MIS posterior, 30.06% decreased; mDL, 39.60% decreased; p < 0.01). The abductor strength increased rapidly from six to 12 weeks, but there was still a statistical significance compared to the contralateral side (MIS posterior, 14.31% decreased; mDL, 17.47% decreased; p < 0.01). Eventually, at the 52-week follow-up visit, the abductor strength on the operated side of both approaches reached the normal level (MIS posterior, 0.86% decreased, p = 0.221; mDL, 0.66% decreased; p = 0.127) (Fig. 7). There was no remarkable difference in the isometric abductor strength on the contralateral side during the entire follow-up (p > 0.05).
Mean ± standard deviation of isometric abductor muscle strength on the operated side in comparison with the contralateral side for both groups from pre-operation to 52 weeks post-operatively. a The isometric abductor muscle strength in MIS posterior group decreased by 35.53% (p < 0.01) pre-operatively, 30.06% (p < 0.01) at six weeks, 14.31% (p < 0.01) at 12 weeks, and 0.86% (p = 0.221) at 52 weeks. b The isometric abductor muscle strength in mDL group decreased by 35.62% (p < 0.01) pre-operatively, 39.60% (p < 0.01) at six weeks, 17.47% (p < 0.01) at 12 weeks, and 0.66% (p = 0.127) at 52 weeks
VAS pain score
There were no statistic differences between the two surgical approaches in the post-operative pain from six to 52 weeks (MIS posterior: 7.15, 5.62, 1.77, 0.69; mDL: 7.36, 5.71, 1.79, 0.68; Min p = 0.092).
Discussion
There is a considerable interest of minimally invasive THA among orthopaedic surgeons for the potential enhanced recovery due to the hip abductors release. Theoretically, no measurable damage to the hip abductors should be seen via MIS posterior approach because it possesses the attributes of “abductor sparing.” However, our results suggested that the GMED also suffered the injury in the MIS posterior approach, which was reflected in the muscular morphology, especially the volume atrophy, and it cannot be negligible compared to that of the direct lateral approach.
Muscle atrophy is the most visual morphological indicator of muscle damage by inspection or imaging diagnosis. Nevertheless, muscle atrophy cannot entirely account for losses in muscle strength or dynamic function, suggesting that the fatty infiltration is another important determinant [16,17,18]. Among few studies that directly evaluated the morphology of the abductor muscles through either cadaver experiment or MRI analysis, the majority of the conclusions were consistent with this study. A cadaveric study conducted by R. Michael Meneghini et al. reported the mean amount of damage reached up to 18% of gluteus minimus muscular area, 2.85% of GMED, and 22.81% of minimus tendon surface area in the MIS posterior approach group which were all higher than the Smith-Peterson approach group [19]. In another cadaver research, the evidence of injury in 4.73% of GMED muscular area, 8.62% of gluteus minimus, and 5.6% of medius tendon surface area via MIS posterior THA has been found [20]. In addition, Christoph A. Agten et al. completed a semiquantitative assessment analyzing periarticular muscles changes after four THA approaches by two-dimensional MRI in combination with Goutallier classification system, presenting that posterior THA did induce a comparable injury to GMED and gluteus minimus compared with the others [10]. Moreover, A. Rasch et al. indicated that fat infiltration of the muscles was slower to recover than size, which strongly supported our findings [21].
Muscle injury is a result of multifactorial synergy, occurring under various circumstances. It has been generally accepted that the direct incision or detachment is likely to cause the GMED damage. Nevertheless, inadvertent lesions of SGN or blunt surgical injury by retractor could represent some other problems, and eventually lead to a continued deficiency of GMED [22, 23]. There is a common risk of the SGN damage regardless of the THA approach used, including retraction, direct dissection, compression due to haematoma or cicatricial tissue, and thermal injury from methyl methacrylate [13]. In practice, however, SGN damage is indeed frequently seen after MIS posterior THA [12, 22]. J. J. Abitbol et al. reported abnormal EMG findings on 77% of hip abductors due to the blunt surgical trauma of SGN at six weeks and 35% at 52 weeks post-operatively via MIS posterior approach which was similar to that of the direct lateral approach [14]. Another electromyography study documented 53.3% of the SGN to GMED muscle partially compromised through posterior approach, incorporating acute and chronic changes, probably as the result of blunt laceration of the nerve [24]. Furthermore, late SGN palsy with the potential consequence of GMED denervation induced by the entrapment and pull of extensive scar tissues developed from the detached short external rotators on account of the normal anatomic variation that SGN run on the ventral surface of piriformis is definitely another unexpected hidden factor via posterior approach [25]. In addition to the SGN injury, violent pull by retractors may also cause varying extent of blunt damage to the involved muscles [21]. The MIS posterior approach in our research, as with previous reports, GMED and gluteus minimus being retracted anteriorly roughly for the better exposure of acetabulum, and with the cicatricial tissues generated from the detached short external rotators could result in the GMED muscle blunt injury and SGN impairment. For the mDL approach, the partial GMED tendon insertion was released from the greater trochanter which might reduce the necessity of strenuous retraction to abductors or SGN. Moreover, the short external rotators remained intact thereby minimizing the GMED damage caused by these factors. Beyond that, the post-operative joint stability is equally important for the abductor muscles recovery [26]. Tetsu Yamaguchi et al. discovered that the posterolateral reconstruction of the posterior capsule, piriformis tendon, and other external rotators in MIS posterior THA had significantly higher abductor muscle strength than the non-reconstruction group due to the augmentation of the hip joint stability and the stabilization of central bearing [27, 28]. In our study, although there were no patients with prosthesis dislocation in both approaches, the stability of the hip joint after the MIS posterior THA might be more worrying due to the lack of posterior structural integrity. Hence, the comparable GMED degeneration via MIS posterior THA appeared to be reasonable. Additionally, for the mDL approach, it is not difficult to understand the degenerative performance of GMED morphology at the very beginning of the post-operative period for the detachment of the partial abductors tendon. However, due to the in-situ reconstruction of the GMED tendon, the changes of the muscle atrophy and the fatty infiltration were not as obvious as previously reported during the entire follow-up. As Jiří Chomiak et al. concluded in their study, the main factor of adequate abductor recovery was probably a careful reattachment of the origins of the gluteal muscles to the greater trochanter [24].
Abductor muscle strength is another important parameter for assessing the GMED injury. The whole-muscle force which was generally dependent on its muscle quality decreased due to muscle atrophy and intramuscular fatty accumulation [29,30,31]. In this study, with the improvement of GMED muscle quality, the abductor strength on the surgical side gradually increased in both groups while it was not completely parallel to the GMED morphological changes. One of the important reasons for this was the distinct post-operative pain on the surgical side until 52 weeks post-operatively. VAS pain score did increase at these time points, and the decrease of the VAS pain score was consistent with the recovery of the abductor strength. In addition, the hip joint function was also affected by the post-operative pain. For the mDL approach, the hip external rotation function was worse than that of the contralateral side in the early post-operative period, and with the reduction of post-operative pain, the function gradually returned to normal. Therefore, the muscle strength and the joint function were determined by multiple factors, especially the pain or the fear of pain [26, 32].
A limitation of this study was that patients did not complete all the post-operative rehabilitation exercises at the same rehabilitation centre after the hospital discharge. Although the same protocol was established for every patient involved at each follow-up visit, it could not fully ensure that each patient accomplished the program as planned with quality and quantity. In addition, factors such as pain management, quotidien diet, and mental health after the hospital discharge were beyond our control, and these might ultimately affect the results. However, these are determined by the national medicare system that is hard to make a change at the individual level. Another limitation was the sample size. It was closely related to the number of patients undergoing THA in our department each year. Furthermore, only the patients diagnosed with primary osteoarthritis were included, and we have demanded that all operations of one THA approach must be performed by the same surgeon. Nevertheless, the sample size still met the minimum requirement for this study. Of course, a larger sample size would undoubtedly increase the repeatability of the results. There are several classic approaches for THA, and each approach has its own advantages and disadvantages, so it is meaningless to simply compare them. We hope to identify more problems that have long been ignored by orthopedists and improve the post-operative results of each approach. And, the further researches are in progress.
Conclusion
In conclusion, surgical approach in THA is an area of debate among orthopedic surgeons, especially on the release of the hip abductors. After analyzing the results of this study, we cannot support the contention that a MIS posterior THA is done reliably without compromising any abductors, at least not on the GMED. The blunt surgical injury of abductors should not be underestimated. In addition, the planned detachment combined with the in-situ reconstruction in mDL approach may not necessarily cause the irreversible damage to the GMED. It is also worth noting that postoperative rehabilitation of abductor muscle strength is conjunct results of multiple factors, especially the pain, rather than determined by the muscle morphological changes or a certain surgical approach alone.
Data availability
All data is stored in the clinical trial registry. And the datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- THA:
-
Total hip arthroplasty
- GMED:
-
Gluteus medius
- MIS:
-
Minimally invasive
- mDL:
-
Modified direct lateral
- EMG:
-
Electromyography
- SGN:
-
Superior gluteal nerve
References
Bernard J, Razanabola F, Beldame J, Van Driessche S, Brunel H, Poirier T, Matsoukis J, Billuart F (2018) Electromyographic study of hip muscles involved in total hip arthroplasty: surprising results using the direct anterior minimally invasive approach. Orthop Traumatol Surg Res 104(8):1137–1142
de Jong L, Klem TMAL, Kuijper TM, Roukema GR (2018) The minimally invasive anterolateral approach versus the traditional anterolateral approach (Watson-Jones) for hip hemiarthroplasty after a femoral neck fracture: an analysis of clinical outcomes. Int Orthop 42(8):1943–1948
Mjaaland KE, Svenningsen S, Fenstad AM, Havelin LI, Furnes O, Nordsletten L (2017) Implant survival after minimally invasive anterior or anterolateral vs. conventional posterior or direct lateral approach: an analysis of 21,860 total hip arthroplasties from the Norwegian Arthroplasty Register (2008 to 2013). J Bone Joint Surg Am 99(10):840–847
Pospischill M, Kranzl A, Attwenger B, Knahr K (2010) Minimally invasive compared with traditional transgluteal approach for total hip arthroplasty—a comparative gait analysis. J Bone Joint Surg Am 92(2):328–337
Müller M, Tohtz S, Dewey M, Springer I, Perka C (2011) Age-related appearance of muscle trauma in primary total hip arthroplasty and the benefit of a minimally invasive approach for patients older than 70 years. Int Orthop 35(2):165–171
Repantis T, Bouras T, Korovessis P (2015) Comparison of minimally invasive approach versus conventional anterolateral approach for total hip arthroplasty: a randomized controlled trial. Eur J Orthop Surg Traumatol 25(1):111–116
Agten CA, Sutter R, Dora C, Pfirrmann CWA (2017) MR imaging of soft tissue alterations after total hip arthroplasty: comparison of classic surgical approaches. Eur Radiol 27(3):1312–1321
Abitbol JJ, Gendron D, Laurin CA, Beaulieu MA (1990) Gluteal nerve damage following total hip arthroplasty. J Arthroplast 5(4):319–322
Miguel-Pérez M, Ortiz-Sagristà JC, López I, Pérez-Bellmunt A, Llusá M, Alex L, Combalia A (2010) How to avoid injuries of the superior gluteal nerve. Hip Int 20(S7):26–31
Petis S, Howard JL, Lanting BL, Vasarhelyi EM (2014) Surgical approach in primary total hip arthroplasty: anatomy, technique and clinical outcomes. Can J Surg 58(2):128–139
Kolk S, Minten MJ, van Bon GE, Rijnen WH, Geurts AC, Verdonschot N, Weerdesteyn V (2014) Gait and gait-related activities of daily living after total hip arthroplasty: a systematic review. Clin Biomech (Bristol, Avon) 29(6):705–718
Agostini V, Ganio D, Facchin K, Cane L, Moreira Carneiro S, Knaflitz M (2014) Gait parameters and muscle activation patterns at 3, 6 and 12 months after total hip arthroplasty. J Arthroplast 29(6):1265–1272
Bahl JS, Nelson MJ, Taylor M, Solomon LB, Arnold JB, Thewlis D (2018) Biomechanical changes and recovery of gait function after total hip arthroplasty for osteoarthritis: a systematic review and meta-analysis. Osteoarthr Cartil 26(7):847–863
Gibson A (1950) Posterior exposure of the hip joint. J Bone Joint Surg 32-B(2):183–186
Hardinge K (1982) The direct lateral approach to the hip. J Bone Joint Surg 64-B(1):17–19
Daguet E, Jolivet E, Bousson V, Boutron C, Dahmen N, Bergot C, Vicaut E, Laredo J-D (2011) Fat content of hip muscles: an anteroposterior gradient. J Bone Joint Surg 93(20):1897–1905
Gladstone JN, Bishop JY, Lo IK, Flatow EL (2007) Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 35(5):719–728
Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB (2002) Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 50(5):897–904
Meneghini RM, Pagnano MW, Trousdale RT, Hozack WJ (2006) Muscle damage during MIS total hip arthroplasty: Smith-Peterson versus posterior approach. Clin Orthop Relat Res 453:293–298
Mardones R, Pagnano MW, Nemanich JP, Trousdale RT (2005) Muscle damage after total hip arthroplasty done with the two-incision and mini-posterior techniques. Clin Orthop Relat Res 441:63–67
Rasch A, Byström AH, Dalén N, Martinez-Carranza N, Berg HE (2009) Persisting muscle atrophy two years after replacement of the hip. J Bone Joint Surg (Br) 91(5):583–588
Apaydin N, Kendir S, Loukas M, Tubbs RS, Bozkurt M (2012) Surgical anatomy of the superior gluteal nerve and landmarks for its localization during minimally invasive approaches to the hip. Clin Anat 26(5):614–620
Odak S, Ivory J (2013) Management of abductor mechanism deficiency following total hip replacement. Bone Joint J 95-B(3):343–347
Chomiak J, Huráček J, Dvořák J, Dungl P, Kubeš R, Schwarz O, Munzinger U (2015) Lesion of gluteal nerves and muscles in total hip arthroplasty through 3 surgical approaches. An electromyographically controlled study. Hip Int 25(2):176–183
Güleç A, Büyükbebeci O (1996) Late superior gluteal nerve palsy following posterior fracture-dislocation of the hip. Acta Orthop Belg 62(4):218–221
Grimaldi A (2011) Assessing lateral stability of the hip and pelvis. Man Ther 16(1):26–32
Yamaguchi T, Naito M, Asayama I, Kambe T, Fujisawa M, Ishiko T (2003) The effect of posterolateral reconstruction on range of motion and muscle strength in total hip arthroplasty. J Arthroplast 18(3):347–351
Kiyama T, Naito M, Shinoda T, Maeyama A (2010) Hip abductor strengths after total hip arthroplasty via the lateral and posterolateral approaches. J Arthroplast 25(1):76–80
Strasser EM, Draskovits T, Praschak M, Quittan M, Graf A (2013) Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Interface. 35(6):2377–2388
Mithal A, Bonjour J-P, Boonen S, Burckhardt P, Degens H, El Hajj Fuleihan G, Josse R, Lips P, Morales Torres J, Rizzoli R, Yoshimura N, Wahl DA, Cooper C, Dawson-Hughes B (2013) Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos Int 24(4):1555–1566
Rahemi H, Nigam N, Wakeling JM (2015) The effect of intramuscular fat on skeletal muscle mechanics: implications for the elderly and obese. Interface. 12(109):109–116
Berry DJ, Berger RA, Callaghan JJ, Dorr LD, Duwelius PJ, Hartzband MA, Lieberman JR, Mears DC (2003) Minimally invasive total hip arthroplasty-development, early results, and a critical analysis. J Bone Joint Surg 85-A(11):2235–2246
Acknowledgments
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: Wei Huang, Hong Chen, Ting Wang.
Data curation: Ting Wang, Long Shao.
Formal analysis: Ting Wang, Long Shao.
Investigation and measurement: Ting Wang, Long Shao.
Methodology: Ting Wang, Long Shao, Wei Xu.
Project administration: Ting Wang, Long Shao, Wei Xu.
Writing-original draft: Ting Wang, Long Shao.
Writing-review and editing: Hong Chen, Wei Huang.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest. No specific funding was received.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The paper is NOT based on a previous communication to a society or meeting.
Rights and permissions
About this article
Cite this article
Wang, T., Shao, L., Xu, W. et al. Comparison of morphological changes of gluteus medius and abductor strength for total hip arthroplasty via posterior and modified direct lateral approaches. International Orthopaedics (SICOT) 43, 2467–2475 (2019). https://doi.org/10.1007/s00264-019-04331-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-019-04331-z