Abstract
Intervertebral disc (IVD) degeneration (IDD) is considered as one of the major causes for low back pain (LBP). However, conventional surgical approaches for treating LBP do not aim to counter the degeneration. Biological interventions have been investigated with an attempt to regenerate the IVD by restoring its matrices and cell activities. This review summarizes the current clinical trials that explore the efficacy of covering cell-, growth factor-, and small molecule-based approaches. While investigations of growth factor- and small molecule-based therapies are still preliminary, intradiscal delivery of mesenchymal stromal cells has been more widely adopted and shown positive results in addressing the pain and the associated physical disability, albeit to a lower extent than observed in previous animal studies. Strategies that potentiate the endogenous disc progenitors may offer a valid alternative to the exogenous cell transplantation. Identification of the novel biologics to arrest IDD phenotype may potentiate disc repair in future. Large-scale, high-quality long-term trials should be conducted to clarify the safety and efficacy of these therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
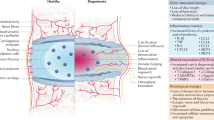
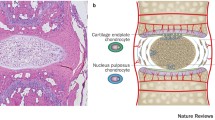
Intervertebral disc degeneration (IDD) is one of the most debilitating medical conditions and considered a major cause for low back pain in addition to radicular symptoms [1,2,3]. Intervertebral discs (IVDs) are fibrocartilaginous tissues between vertebrae contributing to spinal mobility and shock absorption. The IVD is composed of an inner gelatinous nucleus pulposus (NP) core and an outer ring of annulus fibrosus (AF), sandwiched between the cartilaginous endplates (CEP) of the rostral and caudal vertebral bodies. The NP consists of primarily proteoglycans and collagen II in the extracellular matrix (ECM). This matrix meshwork is established collectively by a heterogenous NP cell population including notochordal and chondrocyte-like cells [4]. Loss of NP cells, particularly the resident progenitors, and consequently the disrupted balance of synthesis and degradation of ECM, especially proteoglycans which play an essential role in maintaining hydration of the IVD, is thought to be one of the leading causes of IDD [4]. Abnormal expression of growth factors (e.g., TGF β [5, 6], IGF [6, 7]) and pro-inflammatory cytokines (e.g., IL-1 [8], TNF α [9]) is also associated with the degenerative process. These changes ultimately compromise the anatomical and mechanical properties of IVD, resulting in a loss of disc height, disc deformation, and spinal disability under load.
Surgical procedures, such as spinal fusion or disc arthroplasty, are the last resort for treating IDD if conservative therapies fail to relieve the symptoms [10, 11]. However, spinal fusion needs long recovery time and may cause adjacent level degeneration [12]. In addition to substantial health care-related expenses, the surgery may also result in adverse complications [13]. New biological approaches are therefore investigated aiming to control disc degeneration progression and preserve the spinal kinematics in a minimally invasive manner.
These approaches, often referred as regenerative medicine, include growth factor-, gene-, and cell-based therapies [6, 14, 15]. They have been demonstrated to induce the repair of IVD, primarily via NP regeneration, in various in vitro and preclinical studies. The efficacy of some of these approaches in clinical settings have been demonstrated, therefore offering hope to low back pain patients [16]. This review aims to revisit the completed and undergoing clinical trials of the state-of-the-art biologics for treating IDD and attempt to discuss the direction of future regenerative strategies in light of the findings.
Cell-based therapy
Cell-based therapies in clinical trial
Cell-based therapies, mostly by intradiscal delivery, have drawn considerable attention over past decades [15,16,17,18]. The major cell sources include notochordal [17], chondrocyte-like NP cells [18], and mesenchymal stromal cells (MSCs) [15, 19]. In particular, initial clinical studies have utilized MSCs due to their advantages over the other cell sources in terms of production and possible auxiliary effects on suppressing inflammation [15, 16, 20,21,22,23,24,25,26,27] (Table 1).
Out of 12 reports, ten are related to MSCs, of which seven have been completed with data disclosed, while the other two propose the use of NP cell derivatives (clinicaltrials.gov: NCT01640457 [20], NCT03347708). In contrast to adipose-derived MSCs (NCT02338271 [21], NCT02097862 [22], NCT03461458), bone marrow-derived (BM-) MSCs have been more widely investigated (NCT01290367 [23], NCT01860417 [24, 25], NCT03011398 [28], NCT03692221, NCT03340818, etc. [26, 27]). Although allogeneic MSCs might induce weak and transient immune response, indicated by anti-HLA antibodies at a detectable level in serum within 12-month post-intradiscal injection, the therapeutic efficacy appeared not dependent on HLA matching [24, 25]. This may be presumably due to the suppressed host immune responses by transplanted MSCs [29]. Indeed, allogenic BM-MSC may be of valid alternative as it allows one-step treatment for patients. BM-MSCs implantation was explored in five trials and generally reported to provide significant benefits in terms of pain relief for 12 and up to 36 months and increased IVD hydration [23, 25,26,27,28]. Interestingly, IVD height was usually not restored. Patient mobility and quality of life was improved for up to a six year post-treatment [27]. It is also noteworthy that a higher dosage of MSCs appeared to result in better outcome, where fewer patients needed further surgical intervention [23].
However, cautions should be taken when interpreting the results due to the limited sample size and noncontrolled or nonrandomized design. Two phase II trials have been completed using allogenic BM-MSC with up to 36 months follow-up in 125 patients [23,24,25]. They are in blinded, randomized, and controlled setup. However, the other five completed trials using autologous MSCs were open-label and single-arm with attempt to clarify the safety and tolerability of the treatment [21, 22, 26,27,28]. To date, there are two undergoing autologous MSCs trials in a total of 84 patients involving a more robust design based on a double-blinded, randomized, and controlled setup (NCT03692221, NCT03340818).
Limitations and future developments
Despite the encouraging findings from the clinical trials, the limitations should be carefully considered in treating human IDD via the cell-based therapy. For instance, inflammation and endplate destruction have been reported in a goat degeneration model after injection of adipose-derived stromal vascular fractions or purified stromal cells [30]. Although this could be species (goat)- and source (adipose)-dependent and no resembling observations were reported in current clinical trials, such outcomes deserve attentions in future long-term studies. Interestingly, platelet-rich plasma (PRP) supplemented with MSCs could induce disc repair in a rabbit IDD model without severe adverse effects [31]. Comella et al. also reported no adverse effect in the clinical trial of PRP supplemented with adipose-derived MSCs in a short-term (6 months) and small-scale (n = 15) study [22]. This may suggest a synergic effect of PRP and adipose-derived MSCs in treating IDD.
Human IVDs have distinct cellular compositions, biomechanics, and nutritional supply compared to various preclinical models such as rodents, rabbits, and other large quadrupeds [32]. However, insights obtained from these preclinical studies may improve the therapies. Studies in animal models suggested that the efficacy of MSCs in IVD repair may be largely based on their intrinsic chondrogenic potential and inhibitory effect on inflammatory cascades or endogenous cell apoptosis [33,34,35]. However, IVD is an avascular tissue. Either the implanted cells or newly regenerated cells need to adapt to the low glucose supply, hypoxia, acidic, and hypertonic environment. Otherwise, they may suffer from suppressed metabolic activity or even cell death [36]. Consistent with the limited nutritional supply in the IVD, our previous study in rabbit lumbar discs indicated that delivering a large quantity of MSCs could be detrimental [34]. Studies have attempted to test other sources of stem cells for the reparative effect, such as umbilical cord-derived MSCs [37]. Moreover, priming of MSCs before implantation was also investigated, such as genetically modified hTIMP-expressing BM-MSCs which might elicit additional inhibitory effects on matrix degradation [38] and MSC preconditioned by pentosan polysulphate for better chondrogenic differentiation potentials [39]. However, their efficacy awaits to be compared to the unmodified MSCs.
On the other hand, preventing cell loss after implantation is also a key concern. A study indicated that cell loss could reach up to 90% after implantation due to the annulus failure [40]. However, injection at high doses appeared to avoid the issue, and that injection at early stage might minimize cell loss [41]. Various cell carriers have been developed to facilitate the MSCs implantation as well as chondrogenic differentiation [42,43,44]. For example, Zhou et al. generated a genepin cross-linked type II collagen/chondroitin sulfate composite hydrogel for MSCs delivery in a mouse IDD model [42]. Other examples were self-assembly peptide nanofibers [43] and collagen-low MW (150-300KDa) hyaluronic acid hydrogel [44]. An alternative is to develop deliver approaches that minimize damage to the annulus, such as intravenous injection [45] and transpedicular approach [46].
Altogether intradiscal injection of MSCs seems to be safe and able to relieve the IDD symptoms in initial human trials. However, long-term safety and efficacy are awaited to be clarified by larger-scale and well-controlled studies. Preclinical findings supported the use of cell sources alternative to MSCs or disc cells, benefits of preconditioned or functionally enhanced MSCs, and strategies that maximize cell engraftment. Their safety and efficacy warrant further investigation in humans.
Endogenous progenitors-based therapy
Strategies that can activate endogenous progenitors may be an alternative approach to exogenous cell-based therapies. Accumulative evidences have suggested the existence of disc progenitors in NP, AF, and EP regions and their reduced activity in aging and IDD [47, 48]. Cells clusters observed in NP and AF lesions in different animal models and clinical lumbar degenerative discs are indicative of an attempted self-repair by resident stem cells [49]. These progenitors could offer an opportunity to overcome the practical and regulatory hurdles related to cell implantation mentioned above. For example, NP progenitors were in vitro differentiated and transplanted for sciatic regeneration [50]. While Ishii et al. showed that, in contrast to the MSCs, these disc progenitors presented a lower proliferative capacity and differentiation potential [51]; further study is required to understand their metabolic activities in vivo and how these progenitors may be activated and migrate to injury sites. The progenitor function and regulation in IVDs has been reviewed by Clouet et al. [15]. In particular, studies have highlighted the role of SDF1 in stem cell migration and GDF5/6 in progenitor differentiation, providing potential implications for harnessing disc progenitor activity.
Growth factor-based therapy
Several growth factors have been reported to restore the balance of anabolic and catabolic activities in both in vitro and animal studies (reviewed by Kennon et al. [6]). In particular, studies have focused on the use of TGF β family members or their modifiers [52, 53] (Table 2).
Kwon et al. evaluated a total of 50 subjects with symptomatic lumbar disc degeneration (Thompson grade 2–3) and VAS > 40 mm and ODI >30% at first visit [53]. YH14618 is biglycan fragment that binds to TGF β1 and arrest IDD in a rabbit model [53]. YH14618 were intradiscally injected into the IVDs at three different dosages of 1, 3, and 6 mg/disc. Adverse effects were reported by 27 patients in this study. Fifty percent of the subjects were responsive to YH14618 treatment and VAS scored − 2.18 from baseline after six months. ODI was reduced by 12.38 while placebo group by 6.67. Two patients (6 mg/disc) with MRI improvement were noted albeit no statistical significance. Peniel 2000 is another biglycan-derived peptide that regulates TGF signaling and reported to attenuate IDD in a rabbit model [54]. These suggest that moderating TGF signaling may have a role in modifying IDD.
Three ongoing phase II trials (NCT01158924, NCT01124006, NCT01182337) investigating the efficacy of GDF5 are documented. The outcomes are yet to be released.
Small molecule-based therapy
Compared to cell- or growth factor-based therapy, small molecules are barely degradable in vivo and commonly considered as a relatively economic approach.
Abaloparatide is parathyroid hormone (PTH)-related protein analog drug for treating osteoporosis (NCT03708926). PTH has been shown to effectively attenuate disc degeneration in aged mice [55]. A phase II clinical trial is being conducted to investigate its effect in improving pain and physical function in lumbar disc degeneration patients.
SM04690 is a Wnt pathway inhibitor capable to induce chondrogenic differentiation of both MSCs and disc cells and reported to regenerate disc structure in a rat IDD model [56]. Samumed initiated this clinical trial to test its therapeutic potential in alleviating pain and improving disc health (NCT03246399). Three different dosages (0.03 mg, 0.07 mg, and 0.15 mg per disc) were intradiscally injected, and the subjects monitored up to six months.
Small molecule therapy is broadly applied to treat various pathological conditions, including osteoarthritis [56, 57] and IDD [58,59,60,61] (Table 3). Several small molecules with their molecular targets known or unknown have been proposed, including IL17A inhibitor [58], epigallocatechin 3-gallate [60], resveratrol [59], and nicotinamide phosphoribosyltransferase inhibitor (APO866) [61] to inhibit matrix degradation in animal models. Resveratrol could also inhibit NP cells apoptosis [62]. Polyphenol epigallocatechin 3-gallat was shown with anti-inflammatory and anti-catabolic activities and could reduce radiating pain [60]. Urolithin A could inhibit inflammatory responses of NP cells and alleviate IDD in rat [63]. However, as the key pathological events/molecules for IDD and the target of these small molecules are yet to be elucidated, their application in humans is debatable. Identification of effective small molecules with defined molecular target is not only highly desirable for management of IDD but also the understanding of IDD aetiology.
Conclusion
In summary, this review collects and summarizes the clinical evidences for IVDs’ regeneration using cell-based, growth factor-based, and small molecule-based therapies. MSCs-based therapy has been more widely investigated in clinical trials. Encouraging results have been obtained albeit at a lower extent of efficacy than expected. Therapies that rely on eliciting self-repair mechanism, such as endogenous progenitor activation may be a valid alternative strategy. Small molecule-based therapy is an area relatively underdeveloped presumably due to the limited understanding of the degenerative mechanism, and hence difficulty in pinpointing the regulatory targets. A combination of above regeneration strategies as well as identifying degeneration stage-specific therapeutic windows may be one of the ways to enhance disc repair efficacy.
IDD is a chronic disorder with predeposition from aging [64], genetic [65], and environmental risk factors, including smoking [66], obesity [67], physical loading [68], etc. As the regulatory target/pathway is still largely unknown [69], the strategies likely require repeated administration to effectively control the progression. Moreover, not all IDD patients have LBP [1]. Whether IVD regeneration may effectively prevent LBP and other associated IDD symptoms in long term, needs are to be addressed by large-scale randomized controlled studies.
References
Cheung KM, Samartzis D, Karppinen J, Luk KD (2012) Are “patterns” of lumbar disc degeneration associated with low back pain?: new insights based on skipped level disc pathology. Spine (Phila Pa 1976) 37(7):E430–E438
Samartzis D, Karppinen J, Mok F, Fong DY, Luk KD, Cheung KM (2011) A population-based study of juvenile disc degeneration and its association with overweight and obesity, low back pain, and diminished functional status. J Bone Joint Surg Am 93(7):662–670
Cheung KM (2010) The relationship between disc degeneration, low back pain, and human pain genetics. Spine J 10(11):958–960
Zhao CQ, Wang LM, Jiang LS, Dai LY (2007) The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev 6(3):247–261
Bian Q, Ma L, Jain A, Crane JL, Kebaish K, Wan M, Zhang Z, Edward Guo X, Sponseller PD, Seguin CA et al (2017) Mechanosignaling activation of TGFbeta maintains intervertebral disc homeostasis. Bone Res 5:17008
Kennon JC, Awad ME, Chutkan N, DeVine J, Fulzele S (2018) Current insights on use of growth factors as therapy for Intervertebral Disc Degeneration. Biomol Concepts 9(1):43–52
Liu ZQ, Zhao S, Fu WQ (2016) Insulin-like growth factor 1 antagonizes lumbar disc degeneration through enhanced autophagy. Am J Transl Res 8(10):4346–4353
Risbud MV, Shapiro IM (2014) Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 10(1):44–56
Wang C, Yu X, Yan Y, Yang W, Zhang S, Xiang Y, Zhang J, Wang W (2017) Tumor necrosis factor-alpha: a key contributor to intervertebral disc degeneration. Acta Biochim Biophys Sin Shanghai 49(1):1–13
Karppinen J, Shen FH, Luk KD, Andersson GB, Cheung KM, Samartzis D (2011) Management of degenerative disk disease and chronic low back pain. Orthop Clin North Am 42(4):513–528 viii
Gamradt SC, Wang JC (2005) Lumbar disc arthroplasty. Spine J 5(1):95–103
Ghiselli G, Wang JC, Bhatia NN, Hsu WK, Dawson EG (2004) Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am 86-A(7):1497–1503
Eliasberg CD, Kelly MP, Ajiboye RM, SooHoo NF (2016) Complications and rates of subsequent lumbar surgery following lumbar total disc arthroplasty and lumbar fusion. Spine (Phila Pa 1976) 41(2):173–181
Sampara P, Banala RR, Vemuri SK, Av GR, Gpv S (2018) Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: a review. Gene Ther 25(2):67–82
Clouet J, Fusellier M, Camus A, Le Visage C, Guicheux J (2018) Intervertebral disc regeneration: from cell therapy to the development of novel bioinspired endogenous repair strategies. Adv Drug Deliv Rev. https://doi.org/10.1016/j.addr.2018.04.017
Basso M, Cavagnaro L, Zanirato A, Divano S, Formica C, Formica M, Felli L (2017) What is the clinical evidence on regenerative medicine in intervertebral disc degeneration? Musculoskelet Surg 101(2):93–104
Potier E, Ito K (2014) Using notochordal cells of developmental origin to stimulate nucleus pulposus cells and bone marrow stromal cells for intervertebral disc regeneration. Eur Spine J 23(3):679–688
Wang W, Deng G, Qiu Y, Huang X, Xi Y, Yu J, Yang X, Ye X (2018) Transplantation of allogenic nucleus pulposus cells attenuates intervertebral disc degeneration by inhibiting apoptosis and increasing migration. Int J Mol Med 41(5):2553–2564
Yim RL, Lee JT, Bow CH, Meij B, Leung V, Cheung KM, Vavken P, Samartzis D (2014) A systematic review of the safety and efficacy of mesenchymal stem cells for disc degeneration: insights and future directions for regenerative therapeutics. Stem Cells Dev 23(21):2553–2567
Tschugg A, Diepers M, Simone S, Michnacs F, Quirbach S, Strowitzki M, Meisel HJ, Thome C (2017) A prospective randomized multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART disk plus autologous disk chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disks to avoid secondary disease: safety results of Phase I-a short report. Neurosurg Rev 40(1):155–162
Kumar H, Ha DH, Lee EJ, Park JH, Shim JH, Ahn TK, Kim KT, Ropper AE, Sohn S, Kim CH et al (2017) Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study. Stem Cell Res Ther 8(1):262
Comella K, Silbert R, Parlo M (2017) Effects of the intradiscal implantation of stromal vascular fraction plus platelet rich plasma in patients with degenerative disc disease. J Transl Med 15(1):12
Hyun W. Bae KA, Coric D, McJunkin TL, Pettine KA, Hong HJ, DePalma MJ, Kim KD, Beckworth WJ, Oehme D, Goldschlager T, Brown RD (2014) A phase II study demonstrating efficacy and safety of mesenchymal precursor cells in low back pain due to disc degeneration. Spine J 14(11):31–32
Garcia-Sancho J, Sanchez A, Vega A, Noriega DC, Nocito M (2017) Influence of HLA matching on the efficacy of allogeneic mesenchymal stromal cell therapies for osteoarthritis and degenerative disc disease. Transplant Direct 3(9):e205
Noriega DC, Ardura F, Hernandez-Ramajo R, Martin-Ferrero MA, Sanchez-Lite I, Toribio B, Alberca M, Garcia V, Moraleda JM, Sanchez A et al (2017) Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation 101(8):1945–1951
Pettine KA, Suzuki RK, Sand TT, Murphy MB (2017) Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up. Int Orthop 41(10):2097–2103
Elabd C, Centeno CJ, Schultz JR, Lutz G, Ichim T, Silva FJ (2016) Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study. J Transl Med 14:253
Centeno C, Markle J, Dodson E, Stemper I, Williams CJ, Hyzy M, Ichim T, Freeman M (2017) Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy. J Transl Med 15(1):197
Le Blanc K, Davies LC (2015) Mesenchymal stromal cells and the innate immune response. Immunol Lett 168(2):140–146
Detiger SE, Helder MN, Smit TH, Hoogendoorn RJ (2015) Adverse effects of stromal vascular fraction during regenerative treatment of the intervertebral disc: observations in a goat model. Eur Spine J 24(9):1992–2000
Wang SZ, Jin JY, Guo YD, Ma LY, Chang Q, Peng XG, Guo FF, Zhang HX, Hu XF, Wang C (2016) Intervertebral disc regeneration using platelet-rich plasma-containing bone marrow-derived mesenchymal stem cells: a preliminary investigation. Mol Med Rep 13(4):3475–3481
Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, Melrose J, Ralphs J, Stokes I, Wilke HJ (2008) Are animal models useful for studying human disc disorders/degeneration? Eur Spine J 17(1):2–19
Yuan M, Yeung CW, Li YY, Diao H, Cheung KM, Chan D, Cheah K, Chan PB (2013) Effects of nucleus pulposus cell-derived acellular matrix on the differentiation of mesenchymal stem cells. Biomaterials 34(16):3948–3961
Leung VY, Aladin DM, Lv F, Tam V, Sun Y, Lau RY, Hung SC, Ngan AH, Tang B, Lim CT et al (2014) Mesenchymal stem cells reduce intervertebral disc fibrosis and facilitate repair. Stem Cells 32(8):2164–2177
Cheng X, Zhang G, Zhang L, Hu Y, Zhang K, Sun X, Zhao C, Li H, Li YM, Zhao J (2018) Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J Cell Mol Med 22(1):261–276
Wang F, Shi R, Cai F, Wang YT, Wu XT (2015) Stem cell approaches to intervertebral disc regeneration: obstacles from the disc microenvironment. Stem Cells Dev 24(21):2479–2495
Beeravolu N, Brougham J, Khan I, McKee C, Perez-Cruet M, Chaudhry GR (2018) Human umbilical cord derivatives regenerate intervertebral disc. J Tissue Eng Regen Med 12(1):e579–e591
Yi Z, Guanjun T, Lin C, Zifeng P (2014) Effects of transplantation of hTIMP-1-expressing bone marrow mesenchymal stem cells on the extracellular matrix of degenerative intervertebral discs in an in vivo rabbit model. Spine (Phila Pa 1976) 39(11):E669–E675
Oehme D, Ghosh P, Goldschlager T, Itescu S, Shimon S, Wu J, McDonald C, Troupis JM, Rosenfeld JV, Jenkin G (2016) Reconstitution of degenerated ovine lumbar discs by STRO-3-positive allogeneic mesenchymal precursor cells combined with pentosan polysulfate. J Neurosurg Spine 24(5):715–726
Omlor GW, Bertram H, Kleinschmidt K, Fischer J, Brohm K, Guehring T, Anton M, Richter W (2010) Methods to monitor distribution and metabolic activity of mesenchymal stem cells following in vivo injection into nucleotomized porcine intervertebral discs. Eur Spine J 19(4):601–612
Maidhof R, Rafiuddin A, Chowdhury F, Jacobsen T, Chahine NO (2017) Timing of mesenchymal stem cell delivery impacts the fate and therapeutic potential in intervertebral disc repair. J Orthop Res 35(1):32–40
Zhou X, Tao Y, Chen E, Wang J, Fang W, Zhao T, Liang C, Li F, Chen Q (2018) Genipin-cross-linked type II collagen scaffold promotes the differentiation of adipose-derived stem cells into nucleus pulposus-like cells. J Biomed Mater Res A 106(5):1258–1268
Wu Y, Jia Z, Liu L, Zhao Y, Li H, Wang C, Tao H, Tang Y, He Q, Ruan D (2016) Functional self-assembled peptide nanofibers for bone marrow mesenchymal stem cell encapsulation and regeneration in nucleus pulposus. Artif Organs 40(6):E112–E119
Tsaryk R, Gloria A, Russo T, Anspach L, De Santis R, Ghanaati S, Unger RE, Ambrosio L, Kirkpatrick CJ (2015) Collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres for cell and growth factor delivery for nucleus pulposus regeneration. Acta Biomater 20:10–21
Tam V, Rogers I, Chan D, Leung VY, Cheung KM (2014) A comparison of intravenous and intradiscal delivery of multipotential stem cells on the healing of injured intervertebral disk. J Orthop Res 32(6):819–825
Le Fournier L, Fusellier M, Halgand B, Lesoeur J, Gauthier O, Menei P, Montero-Menei C, Guicheux J, Clouet J (2017) The transpedicular surgical approach for the development of intervertebral disc targeting regenerative strategies in an ovine model. Eur Spine J 26(8):2072–2083
Wu H, Shang Y, Yu J, Zeng X, Lin J, Tu M, Cheang LH, Zhang J (2018) Regenerative potential of human nucleus pulposus resident stem/progenitor cells declines with ageing and intervertebral disc degeneration. Int J Mol Med 42(4):2193–2202
Tekari A, Chan SC, Sakai D, Grad S, Gantenbein B (2016) Angiopoietin-1 receptor Tie2 distinguishes multipotent differentiation capability in bovine coccygeal nucleus pulposus cells. Stem Cell Res Ther 7(1):75
Brown S, Matta A, Erwin M, Roberts S, Gruber HE, Hanley EN Jr, Little CB, Melrose J (2018) Cell clusters are indicative of stem cell activity in the degenerate intervertebral disc: can their properties be manipulated to improve intrinsic repair of the disc? Stem Cells Dev 27(3):147–165
Ishii T, Sakai D, Schol J, Nakai T, Suyama K, Watanabe M (2017) Sciatic nerve regeneration by transplantation of in vitro differentiated nucleus pulposus progenitor cells. Regen Med 12(4):365–376
Wu H, Zeng X, Yu J, Shang Y, Tu M, Cheang LH, Zhang J (2017) Comparison of nucleus pulposus stem/progenitor cells isolated from degenerated intervertebral discs with umbilical cord derived mesenchymal stem cells. Exp Cell Res 361(2):324–332
Colombier P, Clouet J, Boyer C, Ruel M, Bonin G, Lesoeur J, Moreau A, Fellah BH, Weiss P, Lescaudron L et al (2016) TGF-beta1 and GDF5 act synergistically to drive the differentiation of human adipose stromal cells toward nucleus pulposus-like cells. Stem Cells 34(3):653–667
Young-Joon Kwon ESK, Kim S-M, Park H, Byun HM, Nam S-Y (2015) Intradiscal injection of YH14618, a first-in-class disease-modifying therapy, reduces pain and improves daily activity in patients with symptomatic lumbar degenerative disc disease. Spine J 15(10):S119
Kwon YJ, Lee JW, Moon EJ, Chung YG, Kim OS, Kim HJ (2013) Anabolic effects of Peniel 2000, a peptide that regulates TGF-beta1 signaling on intervertebral disc degeneration. Spine (Phila Pa 1976) 38(2):E49–E58
Zheng L, Cao Y, Ni S, Qi H, Ling Z, Xu X, Zou X, Wu T, Deng R, Hu B et al (2018) Ciliary parathyroid hormone signaling activates transforming growth factor-beta to maintain intervertebral disc homeostasis during aging. Bone Res 6:21
Deshmukh V, Hu H, Barroga C, Bossard C, Kc S, Dellamary L, Stewart J, Chiu K, Ibanez M, Pedraza M et al (2018) A small-molecule inhibitor of the Wnt pathway (SM04690) as a potential disease modifying agent for the treatment of osteoarthritis of the knee. Osteoarthr Cartil 26(1):18–27
Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L, Hsu C, Ali SA, Alman BA (2009) Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med 15(12):1421–1425
Suyama K, Sakai D, Hirayama N, Nakamura Y, Matsushita E, Terayama H, Qu N, Tanaka O, Sakabe K, Watanabe M (2018) Effects of interleukin-17A in nucleus pulposus cells and its small-molecule inhibitors for intervertebral disc disease. J Cell Mol Med 22(11):5539–5551
Kwon YJ (2013) Resveratrol has anabolic effects on disc degeneration in a rabbit model. J Korean Med Sci 28(6):939–945
Krupkova O, Sekiguchi M, Klasen J, Hausmann O, Konno S, Ferguson SJ, Wuertz-Kozak K (2014) Epigallocatechin 3-gallate suppresses interleukin-1beta-induced inflammatory responses in intervertebral disc cells in vitro and reduces radiculopathic pain in rats. Eur Cell Mater 28:372–386
Shi C, Wu H, Du D, Im HJ, Zhang Y, Hu B, Chen H, Wang X, Liu Y, Cao P et al (2018) Nicotinamide phosphoribosyltransferase inhibitor APO866 prevents IL-1beta-induced human nucleus pulposus cell degeneration via autophagy. Cell Physiol Biochem 49(6):2463–2482
Li K, Li Y, Mi J, Mao L, Han X, Zhao J (2018) Resveratrol protects against sodium nitroprusside induced nucleus pulposus cell apoptosis by scavenging ROS. Int J Mol Med 41(5):2485–2492
Liu H, Kang H, Song C, Lei Z, Li L, Guo J, Xu Y, Guan H, Fang Z, Li F (2018) Urolithin a inhibits the catabolic effect of TNFalpha on nucleus pulposus cell and alleviates intervertebral disc degeneration in vivo. Front Pharmacol 9:1043
Buckwalter JA (1995) Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 20(11):1307–1314
Chan D, Song Y, Sham P, Cheung KM (2006) Genetics of disc degeneration. Eur Spine J 15(Suppl 3):S317–S325
Fogelholm RR, Alho AV (2001) Smoking and intervertebral disc degeneration. Med Hypotheses 56(4):537–539
Liuke M, Solovieva S, Lamminen A, Luoma K, Leino-Arjas P, Luukkonen R, Riihimaki H (2005) Disc degeneration of the lumbar spine in relation to overweight. Int J Obes 29(8):903–908
Seidler A, Euler U, Bolm-Audorff U, Ellegast R, Grifka J, Haerting J, Jager M, Michaelis M, Kuss O (2011) Physical workload and accelerated occurrence of lumbar spine diseases: risk and rate advancement periods in a German multicenter case-control study. Scand J Work Environ Health 37(1):30–36
Amelot A, Mazel C (2018) The intervertebral disc: physiology and pathology of a brittle joint. World Neurosurg 120:265–273
Funding
This research was supported by the RGC General Research Fund (GRF 17104815), the Health and Medical Research Fund (#2132206), and the AOSpine Asia Pacific (AOSEA-R-2017-08).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sun, Y., Leung, V.Y. & Cheung, K.M. Clinical trials of intervertebral disc regeneration: current status and future developments. International Orthopaedics (SICOT) 43, 1003–1010 (2019). https://doi.org/10.1007/s00264-018-4245-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-018-4245-8