Abstract
Purpose
This study evaluated the feasibility of computed magnetic resonance imaging (MRI) volumetry in conventional osteosarcomas. Secondly, we investigated whether computed volumetry provides new prognostic indicators for histological response of osteosarcomas after neoadjuvant chemotherapy.
Methods
In a retrospective cohort study, data from the Vienna Bone Tumour Registry was used. MR images from 14 patients (male:female = 1.8, mean age 19 years) were analysed prior to and after neoadjuvant chemotherapy according to current therapy regimens. Histological response to chemotherapy was graded according to the Salzer-Kuntschik classification. Computed volumetry was performed for the intraosseous part, as well as the soft-tissue component and the tumour as a whole.
Results
In a setting of appropriate radiological equipment, the method has been considered to be well implementable into clinical routine. The mean tumour volume prior to chemotherapy was 321 (±351) ml. In good responders (n = 6), overall tumour volume decreased by 47 % (p = 0.345), whereas poor responders (n = 8) showed a 19 % decrease (p = 0.128). Neoadjuvant multidrug therapy remarkably changed the tumour composition. This is seen in a decrease of the mean ratio of soft-tissue to intraosseous tumour volume from 8.67 in poor responders and 1.15 in good responders to 1.26 and 0.45 (p = 0.065), respectively. Interestingly, the bony compartment of good responders showed a volume increase during neoadjuvant chemotherapy (p = 0.073). However, we did not find prognostic markers for histological tumour response to pre-operative chemotherapy.
Conclusions
Separated volumetry of tumour segments revealed interesting insights into therapy-induced growth patterns. If verified in a larger study population, these results should be taken into account when planning ablative surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With an estimated incidence of four to five cases per million people per year, osteosarcoma is the most common primary bone tumour [1]. Before the introduction of (neo-)adjuvant chemotherapy in the late 1970s, the long-term cure rate of non-metastatic osteosarcoma was 10–25 % [2]. Today’s multimodal treatment approach has yielded a number of improvements, especially in terms of disease-free survival as well as long-term survival [3]. The impact of neoadjuvant chemotherapy on the surgical margins to be safely chosen is currently investigated [4, 5].
Unfortunately, in recent decades the long-time event-free survival of osteosarcoma patients has reached a plateau at 60–70 % [6]. Amongst other available factors [7, 8], the histological response to pre-operative chemotherapy has evolved to the most critical prognostic factor [9, 10]. Therefore, the percentage of total necrosis is routinely assessed after surgery and graded according to the Salzer-Kuntschik classification [11]. The necrosis rate is considered to reflect either the effectiveness of the neoadjuvant chemotherapy regimen or intrinsic properties of the tumour that compromise its responsiveness [12]. With the administration of neoadjuvant chemotherapy, the local disease as well as existing micrometastases (about 20 % of cases [13]) can be controlled in good responders, but a potential disease progression has to be accepted in poor responders. The latter is true for as many as 20 % of patients, who are resistant to this treatment [14]. Thus, the endeavour to salvage poor responders by postoperative therapy adaptation belongs to the major challenges in osteosarcoma treatment today and finds its expression in current therapy-optimisation trials. An earlier identification of poor responders by surrogate markers for response prediction would avoid unnecessary exposure to ineffectual chemotherapy and would reduce or even prevent disease progression during early treatment phases.
For the non-invasive evaluation of tumour necrosis, magnetic resonance imaging (MRI) has been in the focus of researchers for quite some time now. The tumour volume of osteosarcomas has often been assessed in MR images and has a well-known prognostic significance [15]. Bajpai et al. [12] recently undergirded the assumption that conventional MRI is the method of choice for tumour volumetry of osteosarcomas and proved a direct relation of baseline tumour volumetric parameters and histological necrosis patterns, though a post-chemotherapeutic change in tumour volume did not show a correlation. Nevertheless, we observed that—to our best knowledge—all volumetric studies either used tumour diameters as described by Bieling et al. [15, 16] or proceeded on the assumption of an ellipsoidal or cylindrical lesion using standard mathematical formulae [12, 17]. We designed this retrospective cohort study to appraise the feasibility of computed volumetry as a routine procedure and to look for new prognostic indicators of histological response.
Materials and methods
Patient selection
The Vienna Bone Tumour Registry, founded in 1969, provided the basis for our analysis. We included patients with biopsy-proven high-grade central osteosarcoma with diagnostic biopsy between 2004 and 2009, neoadjuvant chemotherapy, MRI of the lesion before and after neoadjuvant chemotherapy and surgical tumour resection with subsequent histopathological evaluation. The desirable availability of digital MRI copies made a further expansion of the recruitment period unreasonable. Patients with any contraindication against chemotherapy, neoadjuvant chemotherapy according to other than the COSS-96, EURAMOS I or EURO-B.O.S.S. protocols or with contraindication against surgical tumour resection, as well as MRI data not applicable due to poor image quality or ineligible MRI sequence, were excluded. Radiation-induced and recurrent osteosarcomas have also been excluded due to different tumour biology and treatment procedures.
We desired T2-weighted fat-suppressed coronal slices of the lesion with gadolinium-DTPA contrast-enhancement. In 17 cases we obtained the required image data at both time points and in coronal orientation, whereas in two cases the image quality was degraded due to patient movements. Unfortunately, only printed MR images have been obtainable from five patients. After scanning of analogue images and import to the local PACS workstation, the principal investigator blinded all data by assignment of consecutive patient numbers only known to himself. The characteristics of the final data set are provided in Table 1. It comprises 14 patients (five female, nine male; male:female = 1.8) with a mean age at diagnosis of 19 years (±13 years). Femur and tibia are equally affected. A good histological response is considered as Salzer-Kuntschik grades I–III and the ratio of good and poor responders according to the histopathological evaluation is balanced with six good and eight poor responders.
Radiological analysis using MIPAV
MIPAV (Medical Image Processing, Analysis and Visualisation) is a Java-based application designed by the Center for Information Technology of the National Institutes of Health, Bethesda, MD, USA. We used version 5.2.0 released on 21 January 2011 run on a Microsoft Windows 7-based workstation. All files have been exported in DICOM format.
Tumour volumetry
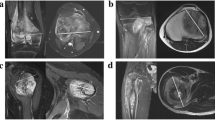
For the definition of volumes of interest (VOIs), we first drew a rough demarcation of the tumour using a polygonal VOI line. After smoothening the tentative VOI in order to generate more anchor points for the subsequent fine-tuning, the investigator moved every anchor point onto the supposed true tumour margin. If available, we also took additional imaging sequences and triplanar views into account. Figure 1a shows a single VOI line of the same tumour before and after chemotherapy.
a A single VOI line in a Salzer-Kuntschik grade I tumour located in the right tibial head before (left) and after neoadjuvant chemotherapy (right) b Working steps for a VOI split: two preliminary straight lines split the VOI contour on both sides of the bone (left) and then are aligned to the bony contour after adding new anchor points (right) c The VOI of the defective slice was generated by the mean area of the neighbouring VOIs and then added to the total tumour volume d The tumour in the distal femur is depicted incompletely in the pre-treatment image series; thus, the VOI drawn in the post-treatment series is cut alike, also taking the leg positioning into account
After grouping all VOIs of a series, the third dimension of the VOI and the tumour volume can be calculated. This worked out for all digital image data using the built-in statistics calculator of MIPAV.
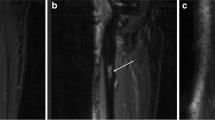
However, digital image data were not obtainable from five patients pre-chemotherapeutically or post-chemotherapeutically, or both. This complicated the volume calculations substantially. On the one hand, high-resolution scanning is necessary for exact analyses and is inseparably accompanied by large file sizes and high computing effort. On the other hand, printed media only provide information of two dimensions and a mathematical reconstruction of the third dimension impairs the final results. Figure 2 illustrates this problem and the reconstruction of the third dimension.
Differentiation of intraosseous and extraosseous masses
In order to ascertain the volumes of the intraosseous and the soft-tissue component separately, firstly the VOI line was split straight-lined and then the VOI contour was readapted to the bone contour manually. The musculoskeletal radiologist also reviewed this working step as the anatomical bone structure often is destroyed by the tumour and the bony margins are difficult to demarcate. Figure 1b points out the working steps of a VOI split. The volume calculation of the separated VOIs is performed in the exact same manner as elucidated before.
Complications
In one patient, one single slice was defective and the area within the VOI line was generated by the mean area of the neighbouring VOIs (Fig. 1c). The investigator proceeded equally when generating intraosseous and extraosseous VOIs for this slice.
In another patient, the tumour was depicted incompletely in the pre-treatment series. As a solution, we cut the VOI of the post-treatment series adapted to the way the defective series was depicted (Fig. 1d). Thus, changes in tumour volume as well as the relationship of intraosseous to soft-tissue masses were not impaired in their scale.
Statistical analysis
For significance testing in independent samples not following a normal distribution the Mann–Whitney- U test was applied, whereas the Wilcoxon test was used for significance testing in non-parametric paired samples. A p value of <0.05 was considered statistically significant. The data were analysed using IBM SPSS Statistics 19.0 (SPSS, Chicago, IL, USA).
Results
Figure 3 graphically displays the key relationships between the pre-chemotherapeutic and post-chemotherapeutic data. The mean tumour volume in good responders (calculated as sum of intrachemotherapeutic and extraosseous compartmental volumes) did decrease by 47 % from 353 to 188 ml (p = 0.345). In tumours with Salzer-Kuntschik grades IV–VI, the change in average tumour volume showed a decrease by 19 % from 337 to 273 ml (calculated as sum of intraosseous and extraosseous compartmental volumes; p = 0.128). The mean soft-tissue tumour volume in the therapy-naive state was 218 ml in good responders and 198 ml in poor responders. The initial mean volume of the bony component was 135 and 138 ml, respectively. After multi-drug therapy, the extraosseous site shrank down to 72 ml (−67 %) in good responders and less strongly down to 169 ml (−15 %) in poor responders. Interestingly, good responders on average presented a weaker decrease in intraosseous tumour volume after initial chemotherapy (−14 % to 116 ml) than poor responders (−25 % to 105 ml). No difference in relative or absolute tumour volume change (Fig. 3c, d) could be determined between good and poor responders or between single Salzer-Kuntschik grades.
a Tumour composition in good and poor responders prior to neoadjuvant chemotherapy b Tumour composition in good and poor responders after neoadjuvant chemotherapy c Absolute change in tumour volume after neoadjuvant chemotherapy did not reveal a significant difference related to histological response or Salzer-Kuntschik grade (p = 0.331 in each case) d Relative change in tumour volume after neoadjuvant chemotherapy did not reveal a significant difference related to histological response (p = 0.272) or Salzer-Kuntschik grade (p = 0.109)
Prior to chemotherapy, the mean ratio of soft-tissue tumour volume to intraosseous tumour volume was 1.15 in good responders and 8.67 in poor responders, respectively (p = 0.833). The comparison of the ratios after neoadjuvant chemotherapy, however, suggests a relevant difference, such that good responders provide a considerably lower compartmental ratio (mean ratio 0.45) than poor responders (mean 1.26) (p = 0.065). This result can be brought into accordance with the fact that 80 % of good responders featured a decrease in the soft-tissue section and 60 % of good responders simultaneously showed an increase in the bony parts. In contrast, the vast majority (88 %) of tumours with insufficient response reacted with an intraosseous volume decrease. The soft-tissue parts of poor responding tumours did not show any characteristic volume change.
The mean percentage change in soft-tissue volume was clearly less in poor responders than in good responders (−13 % vs -39 %). Here as well, tumours with adequate response to neoadjuvant chemotherapy show a positive mean change of intraosseous tumour volume (18 %) in contrast to poor responding tumours which shrink in the intraosseous compartment on average (−29 %) (p = 0.073). This underpins the observation from above and is independently hinting at the same unexpected behaviour of the intraosseous part of Salzer-Kuntschik grade I––III tumours, which clearly increases while showing more than 90 % tumour necrosis.
Discussion
In this study, we were not able to find significant prognostic markers in the MRI of patients prior to neoadjuvant chemotherapy predicting histological tumour response.
The most substantial limitation in our study is the small sample size, even though this is not unusual in studies on osteosarcoma due to its low incidence. However, we needed a minimum homogeneity in terms of image quality and MRI standards. Only about one-third of the patients we collected based on disease-related criteria did meet the image requirements. From these circumstances we deduce that the call for centralisation and standardisation is steadily growing louder in modern medicine. In our centre, many patients from abroad already received imaging in an external institution. To not further delay the treatment onset, we did not repeat imaging according to our protocols in all cases. This, however, would have been desirable when faced with the present results. Thus, we need to emphasise the importance of the full diagnostic procedure as well as the subsequent treatment to be conducted in a specialised institution with sufficient caseload. For our study, this would have meant a larger study population with a higher degree of comparability.
When compared with large representative studies [18], we did not find critical differences in the distribution of sex or age. Merely, the distribution of the primary location of the lesion was a little untypical, in that we did not provide any location other than the knee area (0 % vs 24 %). This can be referred to the small study population and the fact that the knee area is by far most frequently involved.
The restraint availability of digital data issued a particular challenge. We reconstructed the tumour from two-dimensional data at the expense of inter-patient comparability. This has even been aggravated in patients who provided different modalities before and after chemotherapy, as it compromises intra-patient comparability. The magnitude of the error that arises when comparing reconstructed data to three-dimensional data is incalculable and leaves room for discussion and improvement. However, this problem does not occur using standard formulas for geometrical cylindrical or spherical forms [19], but even our reconstruction method from two-dimensional data takes more tumour data into account than these formulae do. Although we did not compare the accuracy of the two methods statistically, we admittedly considered the reconstruction from two-dimensional data to be less precise than original three-dimensional data, but more reasonable than geometrically calculated data.
The issue of whether MRI is capable of defining the true tumour extent has been addressed by several studies [20, 21]. Recent data suggest that over 80 % of abnormal MR signals surrounding the tumour are free of malignant cells in the pathohistological examination [22]. The reliability of our method would have been worthwhile to evaluate. The comparison of maximum tumour diameters as determined by the pathologist and the radiologist is quite meaningful but has been abandoned, because these specified dimensions do not have direct influence on the computed method of volumetry. In addition, a second radiologist has not independently repeated the tumour measurements and, thus, information on inter-observer reliability of the method used in this study is unavailable. Due to the elaborate preparation of two-dimensional images, the radiological analyst also abandoned repeating the volumetric measurements on his own. Therefore, this study is also lacking intra-observer reliability information, which made a methodological evaluation inappropriate.
The foremost achievement of this study clearly is the accomplishment of a computed tumour volumetry separated by tumour compartments. To our best knowledge, this has not been performed before. This offered the opportunity of not only evaluating the exact growth behaviour as a whole but also attributing the changes to the specific tumour segment. Despite the small study population, the calculations admit an assumption relating to the growth behaviour of histologically good responding tumours. The comparison of the post-treatment intraosseous compartmental volume to both the post-treatment soft-tissue volume and the therapy-naive intraosseous volume yielded surprising results in good responders. Even though not statistically significant, these two parameters independently show strong tendencies suggesting that the intraosseous volume of good responders is mounting up during chemotherapy, while malignant cells are necrotising at the same time. As a consequence, the compartmental ratio changes from greater than 1 prior to chemotherapy to less than 1 after chemotherapy. As presented by the compartmental change variables, this happens due to extraosseous tumour decay as well as intraosseous tumour growth.
These effects are unexpected in tumours graded as therapeutic success by a well-established classification. One way to interpret these results could be necrosis-related oedema in osteosarcomas of good responders. Because in these tumours more than 90 % of malignant cells decay, a relevant tumour oedema seems reasonable. Then again, tumours of the good response group initially show a large soft-tissue component, even larger than in the poor response group (218 ml vs 198 ml). So, why would necrosis-related oedema in good responders only increase the comparatively small intraosseous volume? A number of theories have been proposed on the aetiology of bone marrow oedema in primary bone lesions, including increased prostaglandin levels and COX-2 expression [23–25]. At all events, extracellular fluid is clearly more restrained in the bony compartment compared with the soft-tissue parts, where excess fluid is drained more rapidly. Although one cannot necessarily assume that the restraint is also true for tumorous altered bone, this constitutes an explanatory approach for the growth pattern observed. Interestingly, in the series by Masrouha et al. [22] all MR areas of suspected oedema were free of malignant cells in the pathohistological examination. This is contradictive to the prevalent view on perineoplastic oedema in musculoskeletal sarcomas saying that sarcoma cells can be histologically detected beyond the tumour margins [26]. However, Masrouha et al. [22] used post-treatment MR images for evaluation, which makes those results more valuable for the present series and the discussion on MR appearance of bone tumours treated with neoadjuvant chemotherapy. Sclerotic changes as cause for a specific intraosseous volume increase might explain the predisposition of the bony compartment, but seem unlikely to induce such noticeable size differences between the two tumour compartments in good responders.
The decrease in the bony tumour parts in 88 % of poor responders (mean volume change, -29 %) has to be seen against the background that tumours which are necrotic for the most part can still be classified as poorly responding tumours according to the Salzer-Kuntschik grading (Salzer-Kuntschik IV: necrosis in more than 50 %, but less than 90 % of the entire tumour mass). In this series, this is true for six out of eight tumours classified as tumour with histologically poor response after all. In all likelihood, this circumstance can be blamed for the high percentage of tumour mass reduction in the poor response group. The imbalance within the two response groups can definitely be seen as a weak point in our analysis and particular attention should be paid to the case selection when using this classification. However, Huvos’ classification system only admits even less precise distinction between the subgroups.
On the soft-tissue side, the most remarkable difference can be found in the mean volume change. While showing comparable therapy-naive volumes, well responding lesions showed a mean decrease in the soft-tissue portion about three times as large as the poor responding counterparts. This is less surprising if it is reflected that 80 % of tumours responsive to chemotherapy react with a decrease in the soft-tissue component, which is the site of predominant change in 75 % of these tumours.
Eventually, it is not pertinent to reflect on subgroup relations in a study population of the present size. However, the analysis of these variables yielded strong tendencies, tempting speculation that an only slightly larger sample size might confirm the proposed theories. From the clinical point of view, these results emphasise the importance of radiological re-evaluation when ascertaining therapy-induced changes in tumour composition and for appropriate surgical planning.
Conclusions
In summary, we did point out that separated volumetry of tumour segments is worth a more detailed inspection in osteosarcoma. Interesting and pre-eminently unexpected growth characteristics in tumours with sufficient histological regression underlined the importance of radiological tumour evaluation after initial chemotherapy. If these observations can be verified in a larger study population, the growth behaviour of single tumour segments should be taken into account when planning surgery and diagnosing MR images obtained after neoadjuvant chemotherapy.
References
Fletcher C, Unni K, Mertens F (2002) World Health Organization classification of tumours. Pathology and genetics of tumours of soft tissue and bone. IARC Press, Lyon, pp 264–284
Carrle D, Bielack SS (2006) Current strategies of chemotherapy in osteosarcoma. Int Orthop 30:445–451. doi:10.1007/s00264-006-0192-x
Jaffe N (2010) Osteosarcoma: review of the past, impact on the future. The american experience. Cancer Treat Res 152:239–262. doi:10.1007/978-1-4419-0284-9_12
Li X, Moretti VM, Ashana AO, Lackman RD (2012) Impact of close surgical margin on local recurrence and survival in osteosarcoma. Int Orthop 36:131–137. doi:10.1007/s00264-011-1230-x
Hayashi K, Tsuchiya H, Yamamoto N et al (2008) Functional outcome in patients with osteosarcoma around the knee joint treated by minimised surgery. Int Orthop 32:63–68. doi:10.1007/s00264-006-0289-2
Marina N, Bielack S, Whelan J et al (2010) International collaboration is feasible in trials for rare conditions: the EURAMOS experience. Cancer Treat Res 152:339–353. doi:10.1007/978-1-4419-0284-9_18
Funovics PT, Edelhauser G, Funovics MA et al (2011) Pre-operative serum C-reactive protein as independent prognostic factor for survival but not infection in patients with high-grade osteosarcoma. Int Orthop 35:1529–1536. doi:10.1007/s00264-011-1208-8
Rastogi S, Kumar R, Sankineani SR et al (2012) Role of vascular endothelial growth factor as a tumour marker in osteosarcoma: a prospective study. Int Orthop 36:2315–2321. doi:10.1007/s00264-012-1663-x
Davis AM, Wright JG, Williams JI et al (1996) Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res 5:508–516
Picci P, Sangiorgi L, Rougraff BT et al (1994) Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol 12:2699–2705
Salzer-Kuntschik M, Delling G, Beron GR (1983) Morphological grades of regression in osteosarcoma after polychemotherapy—study COSS 80. J Cancer 106:21–24
Bajpai J, Gamnagatti S, Kumar R et al (2011) Role of MRI in osteosarcoma for evaluation and prediction of chemotherapy response: correlation with histological necrosis. Pediatr Radiol 41:441–450. doi:10.1007/s00247-010-1876-3
Bacci G, Lari S (2001) Adjuvant and neoadjuvant chemotherapy in osteosarcoma. Chir Organi Mov 86:253–268
Kong C, Hansen M (2009) Biomarkers in osteosarcoma. Expert Opin Med 3:13–23. doi:10.1517/17530050802608496.Biomarkers
Bieling P, Rehan N, Winkler P et al (1996) Tumor size and prognosis in aggressively treated osteosarcoma. J Clin Oncol 14:848–858
Kim MS, Lee S-Y, Cho WH et al (2008) Initial tumor size predicts histologic response and survival in localized osteosarcoma patients. J Surg Oncol 97:456–461. doi:10.1002/jso.20986
Holscher H, Bloem J, Van der Woude H et al (1995) Can MRI predict the histopathological response in patients with osteosarcoma after the first cycle of chemotherapy? Clin Radiol 50:384–390
Bielack S, Jürgens H, Jundt G et al (2010) Osteosarcoma: the COSS experience. Cancer Treat Res 152:289–308. doi:10.1007/978-1-4419-0284-9_15
Denecke T, Hundsdörfer P, Misch D et al (2010) Assessment of histological response of paediatric bone sarcomas using FDG PET in comparison to morphological volume measurement and standardized MRI parameters. Eur J Nucl Med Mol Imaging 37:1842–1853. doi:10.1007/s00259-010-1484-3
Schima W, Amann G, Stiglbauer R et al (1994) Preoperative staging of osteosarcoma: efficacy of MR imaging in detecting joint involvement. AJR Am J Roentgenol 163:1171–1175
Hoffer FA, Nikanorov AY, Reddick WE et al (2000) Accuracy of MR imaging for detecting epiphyseal extension of osteosarcoma. Pediatr Radiol 30:289–298
Masrouha KZ, Musallam KM, Samra AB et al (2012) Correlation of non-mass-like abnormal MR signal intensity with pathological findings surrounding pediatric osteosarcoma and Ewing’s sarcoma. Skeletal Radiol 41:1453-1461. doi:10.1007/s00256-012-1383-8
James SLJ, Panicek DM, Davies AM (2008) Bone marrow oedema associated with benign and malignant bone tumours. Eur J Radiol 67:11–21. doi:10.1016/j.ejrad.2008.01.052
Yamamura S, Sato K, Sugiura H et al (1997) Prostaglandin levels of primary bone tumor tissues correlate with peritumoral edema demonstrated by magnetic resonance imaging. Cancer 79:255–261
Shinmura K, Ishida T, Goto T et al (2004) Expression of cyclooxygenase-2 in chondroblastoma: immunohistochemical analysis with special emphasis on local inflammatory reaction. Virchows Arch 444:28–35. doi:10.1007/s00428-003-0889-9
White LM, Wunder JS, Bell RS et al (2005) Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 61:1439–1445. doi:10.1016/j.ijrobp.2004.08.036
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Laux, C.J., Berzaczy, G., Weber, M. et al. Tumour response of osteosarcoma to neoadjuvant chemotherapy evaluated by magnetic resonance imaging as prognostic factor for outcome. International Orthopaedics (SICOT) 39, 97–104 (2015). https://doi.org/10.1007/s00264-014-2606-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-014-2606-5