Abstract
To investigate the frequency and mechanism of the peritumoral inflammatory reaction in chondroblastoma, we evaluated the relationship between clinicoradiological findings and immunohistochemical expression of cyclooxygenase-2 (COX-2) in excised tumors. Twenty-one cases of chondroblastoma were studied. Imaging analysis was performed with radiographs and T1- and T2-weighted magnetic resonance images in all cases and with computed tomography scan and bone scintigraphy in some cases. Immunohistochemical study for COX-2 was carried out using formalin-fixed paraffin-embedded tissues. Periosteal reaction was observed in 6 cases (29%) and bone marrow edema in 15 cases (71%). Soft-tissue edema, joint effusion, and synovitis were found in 10 cases (48%), in 7 cases (33%), and in 9 cases (43%), respectively. Immunohistochemical expression of COX-2 in chondroblastoma cells was found in 15 of 21 cases (71%). The intensity of COX-2 immunoreactivity was correlated statistically with the presence of periosteal reaction, bone-marrow edema, soft-tissue edema, and synovitis. Our results indicate that activation of eicosanoid synthesis by COX-2 expression in the tumor itself is probably an important factor, inducing peritumoral inflammatory changes in chondroblastomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chondroblastoma is a relatively rare benign cartilage tumor, representing approximately 1% of all primary bone tumors [7, 21]. It most frequently occurs in the second decade of life and arises in the epiphysis or apophysis of long tubular bone, especially of the proximal and distal femur, proximal tibia, and proximal humerus. Chondroblastoma is typically a well-demarcated lytic lesion with or without calcification on radiographs.
In some chondroblastoma cases, synovitis and inflammatory reactions around the tumor may be found, and these findings are well demonstrated by different imaging modalities, including magnetic resonance imaging (MRI). MRI is a sensitive imaging modality for demonstrating these inflammatory reactions; thus, MRI may lead to overestimation of the aggressiveness and extent of the tumor. Knowledge of the potential pitfalls may help to avoid misplaced reliance on MRI for benign bone tumor diagnosis [12]. Some chondroblastoma tissues contain high levels of prostaglandins (PGs), which are considered to play an important role in the development of a peritumoral inflammatory reaction revealed by imaging studies [25]. PGs are produced from phospholipids via arachidonic acid metabolism by cyclooxygenase-2 (COX-2). Thus, we sought to examine the expression of COX-2 in chondroblastoma tissue and to evaluate the relationship between COX-2 expression and the inflammatory reaction of the peritumoral tissues in chondroblastomas.
Materials and methods
Tumor samples
We retrieved 21 cases of chondroblastoma from the files of Division Surgical Pathology, Tokyo Medical University Hospital; Department of Pathology, Teikyo University School of Medicine; and Department of Pathology, The University of Tokyo Hospital. Treatment was curettage in all but two of these cases, and it was en-block excision in these two cases. The histopathological diagnosis of each tumor was re-confirmed by two of the authors (T. Is. and T. Im.) according to the criteria described in the bone tumor textbooks [7, 21].
Clinical and imaging studies
Clinical symptoms were evaluated for signs and symptoms of local inflammation, including the presence of local pain, swelling, limitation in range of motion in the adjacent joint, and muscle atrophy of affected limb. In all cases, plain radiographs and T1- and T2-weighted MRI were available for review. Tomogram, computed tomography (CT) scan, and bone scintigram were also examined in some cases. MRI was obtained using either a 0.5- or 1.5-T unit prior to surgery. MRI techniques, however, were not tightly controlled because of the different scanners available at the different institutes examined in this study. A range of spin-echo pulse sequences was obtained for T1- and T2-weighted images. Gadolinium-labeled diethylene triamine pentaacetate (Gd-DTPA)-enhanced T1-weighted images were obtained in 15 cases. The presence of a periosteal reaction was evaluated by radiographs, tomograms, and CT scans. Bone-marrow edema, soft-tissue swelling and edema, joint effusion, and synovitis were evaluated on MRI. Bone marrow demonstrating lower signal intensity than normal marrow intensity on T1-weighed images as well as high signal intensity on T2-weighed images was considered positive for intramedullary bone-marrow edema [19, 22]. For joint effusion, accumulation of joint fluid that is clearly demonstrated in the joint space adjacent to the tumor as high signal intensity on T2-weighed images was considered a positive finding. Synovitis and soft-tissue swelling and edema were defined as tissue swelling with high signal intensity on T2-weighted images or on Gd-DTPA-enhanced T1-weighted images [1, 9].
Histological and immunohistochemical studies
Tumor tissues were fixed in 10% formalin and embedded in paraffin. Histological sections were cut at 3 μm thickness and stained with hematoxylin and eosin. Immunohistochemical study was carried out by the labeled streptavidin biotin (LSAB) method using LSAB kit (Dako, Capinteria, CA). Polyclonal antibody for COX-2 (IBL; Fujioka, Japan, dilution 1:50) was used for primary antibody. Deparaffinized sections were incubated in methanol with 0.3% hydrogen peroxide to eliminate endogenous peroxidase activity. Antigen retrieval method was applied with autoclaving at 110°C for 10 min in 10 mM citrate buffer (pH 6.0). Colorectal adenocarcinoma tissue known to express COX-2 was used as a positive control. As the negative control, the primary antibody was replaced with Tris-NaCl buffer. The results of immunoreactivity were divided into four grades according to the number of positive cells, i.e., negative staining (0–9%), 0; weak staining (10–29%), 1+; moderate staining (30–49%), 2+; strong staining (50%>), 3+. In three cases, the synovial tissue adjacent to the tumor was also examined histologically and immunohistochemically. Immunohistochemical evaluation in 20 cases of giant cell tumor (GCT) (14 male and 6 female, age range 20–75 years, mean age 33 years) of bone in the epiphysis of the long bones (location as follows: proximal tibia, 7 cases; distal femur, 6 cases; distal radius, 3 cases; proximal humerus, 2 cases; sacrum and acetabulum, 1 case each) other than two cases was performed in comparison with chondroblastomas.
Statistical analysis
Correlation between the grading of COX-2 expression and inflammatory reactions was tested by the Spearman's correlation coefficient by rank test. Statistical significance was defined as P<0.05.
Results
In the 21 cases examined (7 male and 14 female; age range 11–35 years), the location of the tumor was the proximal femur (7 cases), distal femur (3 cases), proximal humerus (7 cases), proximal tibia (2 cases), and acetabulum and calcaneus (1 case each). Although local pain was a common complaint, details of pain relief from NSAIDs were unknown.
Clinical symptoms and radiological features
Clinical data are summarized in Table 1. Symptoms considered indicative of inflammation were observed. Local pain and limitation in range of motion in the affected joint were observed in 20 (95%) and 15 (71%) cases, respectively. Muscle atrophy of affected limb was recognized in 10 cases (48%), which had a substantial impairment of movement caused by pain. In preoperative laboratory data, a slight increase of white blood cells was seen in only one case. C-reactive protein was within normal limits in all cases.
Imaging findings of tumors studied were all well-circumscribed radiolucent lesion with a sclerotic rim (Fig. 1, Fig. 2). Calcification, punctuated or flocculant, was found in 12 cases (57%). Cystic changes demonstrated by MRI were found in 12 cases (57%). Interruption of the cortex was noted on CT scan in 10 cases (48%).
A plain radiograph of the humerus shows a well-circumscribed calcified tumor of the epiphysis with conspicuous periosteal reaction of solid buttressing type along the diametaphyseal shaft far from the tumor (case no. 8). B Coronal T1-weighted magnetic resonance (MR) image [repetition time (TR)/echo time (TE): 450/25] reveals a protruding tumor showing intermediate to low signal intensity. Bone-marrow edema is also identified as a longitudinal area of low signal intensity descending into the diaphyseal shaft. C Coronal T2-weighted MR image (TR/TE: 3000/100) reveals a low signal intensity tumor and bone-marrow edema showing high signal intensity. D Gadolinium-labeled diethylene triamine pentaacetate-enhanced T1-weighted image reveals bone-marrow edema as an area of high signal intensity and soft-tissue edema in the rotator cuff and deltoid muscle around the tumor. E Bone scintigram shows intense uptake in the proximal humerus and also less intense uptake along the lateral shaft far beyond the tumor itself
Coronal T1- weighted image (TR/TE: 500/17) reveals a well-demarcated tumor with isointensity and bone-marrow edema showing low signal intensity in the intertrochanteric area (case no. 18). B Coronal T2-weighted image (TR/TE: 3000/96) reveals bone-marrow edema with high signal intensity in the corresponding area of T1-weighted image and obvious joint effusion (large arrows) and synovitis (small arrows) in affected side. The tumor shows low signal intensity
On plain radiograph, a periosteal reaction was observed in 6 cases (29%). The affected anatomical locations of these periosteal reaction-positive cases were all long tubular bone. Specifically, the location of the tumor in 4 of 6 cases was the proximal humerus. The femur and tibia were the site of the lesion in one case each. In 5 cases, a distinctive layered-type periosteal reaction along the diametaphyeal shaft distant from the tumor was recognized. In the remaining 1 case, remarkable periosteal reaction of solid buttressing type was seen along the proximal shaft of the humerus (Fig. 1). Bone-marrow edema was revealed in 15 cases (71%). The extent of edema varied from slight to prominent, and tended to localize to marrow space distant from original tumors (Fig. 1B, C, D, Fig. 2A, B). Soft-tissue edema was revealed, extending to peritumoral muscles in 10 cases (48%) and was seen in 5 of the 7 cases affecting in the proximal humerus. Joint effusion had various patterns from slight to extreme in 7 cases (33%) and was seen in 3 of the 5 cases affecting in the femoral head (Fig. 2A, B). Synovitis was found in only 9 cases (43%) (Fig. 2A, B).
Immunohistochemical study
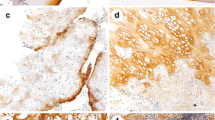
The histology of the tumors demonstrated findings typical of chondroblastoma, with sheet-like proliferation of round-shaped chondroblasts and foci of chondroid matrix production associated with occasional chicken-wire calcification and scattered osteoclast-like giant cells (Fig. 3A). These findings were observed in all cases in most areas, or at least within the focal area of tumors studied. Synovial tissue studied in three cases showed reactive synovitis with hyperemia, edema, and lymphoplasmacytic infiltration (Fig. 3B).
Histology of the tumor shows sheet-like proliferation of chondroblasts and chondroid matrix production (case no. 18) (hematoxylin and eosin, ×100). B Histology of synovium shows reactive synovitis with edema, hyperemia, and lymph follicle (case no. 21) (hematoxylin and eosin, ×50). C–D Immunohistochemical staining for cyclooxygenase-2 (COX-2). C The tumor cells are diffusely positive for COX-2 in (3+) case (case no. 8) (×100). D COX-2 expression is seen in (1+) case (case no. 2) (×100)
The results of immunohistochemical study are summarized in Table 1. Of 21 tumors, 15 (71%) were positive for COX-2 (Fig. 3C, D). The staining intensity was unequivocally positive among all COX-2-positive cases. In contrast, three samples of synovial tissue of the affected joint and all 20 GCT did not express COX-2. In 15 COX-2-positive cases, 7 cases showed only weak staining (1+), but 3 cases showed moderate staining (2+). In the remaining 5 cases, multiple foci of COX-2-positive tumor cells were observed, and they were classified as strong staining (3+). The expression of COX-2 was found in both areas of cellular sheet of tumor cells and tumor cells embedded in the chondroid matrix. There was no change of staining property among chondroblasts and cells within the cartilage matrix. Osteoclast-like giant cells were consistently negative for COX-2. There was no correlation of histological features (mitotic activity, apoptosis, tumor vessel formation, and tumor size) except inflammatory reactions and COX-2 expression.
Statistical analysis
The grading of COX-2 immunoreactivity was correlated with the presence of periosteal reaction (P=0.035), bone-marrow edema (P<0.01), soft-tissue edema (P=0.037), and synovitis (P=0.015).
Discussion
In general, radiological images correspond well with the aggressiveness of bone tumors. Radiographically, non-aggressive tumors showed bland-looking appearances without periosteal reaction, cortical violation, and adjacent tissue inflammation. These entities usually contain benign tumors and tumor-like lesions. However, aggressive lesions, such as malignant bone tumors, some osteomyelitis, and eosinophilic granuloma, induce tissue reactions adjacent to the lesions. These reactions include a periosteal reaction, bone-marrow and soft-tissue edema, synovial fluid accumulation, and soft-tissue swelling. In some benign tumors, however, these prominent tissue reactions, which are normally associated with aggressive or malignant bone lesions, have been described [12]. Osteoid osteoma is a typical example of such a benign tumor [17, 18]. Recent imaging advances, especially MRI, have revealed that other benign tumors, e.g., chondroblastomas, demonstrate tissue reactions around them[3, 12, 16, 23, 25].
In general, chondroblastoma shows a well-circumscribed radiolucent lesion with or without calcification and sclerotic rim. In addition to these findings, inflammatory responses, including periosteal reaction, bone-marrow and soft-tissue edema, and synovitis, have been described in some chondroblastomas by plain film or MRI. A thick-solid or layered-type periosteal reaction distant from the lesion itself has been found in 20–90% of cases of chondroblastoma on radiography or MRI [2, 3, 4, 8, 12, 13, 15, 16, 23, 25]. Bone-marrow edema extending to the circumference of the tumor is observed in 82–92% of cases on MRI, and soft-tissue edema is also found in 58–82% of cases [15, 16, 23]. Approximately 30% of cases are associated with joint effusion as demonstrated by MRI [15, 16, 25]. In our study, the frequency of inflammatory reaction was similar to previously reported data. Periosteal reaction was noted in 29%, bone-marrow edema in 71%, soft-tissue edema in 48%, joint effusion in 33%, and synovitis in 43% of cases examined. Therefore, the frequencies of inflammatory reactions found in chondroblastoma cases appear to be consistent across institutions.
The mechanism of inflammatory reactions that are sometimes observed in benign bone tumors remains unclear. Recent studies suggest, however, that high levels of PGs within the lesion, such as osteoid osteoma and chondroblastoma, may play an important role for the development or maintenance of inflammatory reactions [5, 10, 11, 24, 26].
COX is the essential regulatory enzyme of the eicosanoid biosynthetic pathway and catalyzes the conversion of arachidonic acid into PGG2 and PGH2. PGH2 is subsequently converted into various eicosanoids including PGE2, PGF2α, PGI2, and the like. COX has two different isoforms, COX-1 and COX-2, derived from distinct genes, respectively. COX-1 is constitutively expressed and mediates many of the housekeeping functions of COX in nearly all tissues under basal conditions [6]. Conversely, COX-2 is inducible and is considered to be a key mediator of inflammation via the eicosanoid biosynthetic pathway.
In osteoid osteomas, PGs are thought to be responsible for inflammatory reactions around the nidus and for nocturnal pain that is characteristically relieved by non-steroidal anti-inflammatory drugs (NSAIDs). Osteoblastic tumor cells within the osteoid osteoma nidus have shown diffuse immunoreactivity for COX-2 [17, 18, 20]. Bone-marrow edema around the lesion detected by MRI is greatly reduced or eliminated after surgical removal of the nidus [18]. Taken together, these data suggest that COX-2 expression in osteoid osteoma cells may play a major role in activating the eicosanoid biosynthetic pathway leading to bone-marrow edema and other inflammatory reactions found in osteoid osteoma cases.
In our study, immunohistochemical expression of COX-2 in chondroblastoma cells was found in 15 of 21 cases (71%). Osteoclastic giant cells and synovial tissue and osteoblasts around the lesion were all negative for COX-2. Bone-marrow edema was observed in 14 of 15 COX-2-positive cases; however, only 1 case was observed in which COX-2 staining was negative and bone-marrow edema was present. COX-2 expression is seen in all periosteal reaction-, joint effusion-, synovitis-, and soft-tissue edema-positive cases. No cases were seen with negative COX-2 staining that were positive for periosteal reaction, joint effusion, synovitis, or soft-tissue edema. These observations indicate that COX-2 expression in tumor tissue and inflammatory reactions are closely related and are statistically significant. In addition, a small COX-2-positive chondroblastoma (case no.13) was also associated with these inflammatory reactions [14]. Conversely, in relatively large, COX-2-negative chondroblastomas, the inflammatory reactions are not found. Thus, it appears that the most important factor for inducing inflammatory reactions in chondroblastoma is COX-2 expression in the lesion itself, most likely via activation of eicosanoid biosynthetic pathway. The study of PG concentration in chondroblastoma is limited; however, Wold et al. demonstrated that one chondroblastoma tumor sample contained increased levels of PGE2 relative to the adjacent bone [24]. Another study reported by Yamamura et al. showed higher levels of PGs in eight cases of chondroblastoma than that of other cartilage tumors and GCT [26].
In osteoid osteoma, diffuse intense immunoreactivity for COX-2 was found in osteoblastic tumor cells [17, 18, 20]. COX-2 immunoreactivity in chondroblastomas was less intense than in typical cases of osteoid osteoma [14]. Among chondroblastoma cases, intensity of COX-2 expression may also correlate with the intensity of peritumoral inflammatory reactions, but does not correlate with tumor size.
Bone-marrow edema is more frequently seen than other peritumoral inflammatory changes. This could be related to the fact that bone marrow is adjacent to the tumor and that MRI is a very sensitive imaging modality. The periosteum, synovium, and joint cavity are not close to the tumor itself; therefore, the concentration of PGs in these tissues is likely lower than within the bone marrow immediately surrounding the lesion. In addition, plain radiography and CT are less sensitive imaging modalities for the detection of periosteal reaction.
GCT is an aggressive tumor that generally occurs in the epiphysis like chondroblastoma. Despite histological similarities, these two entities must be distinguished because of their radically different biological behavior. Although local aggressiveness is frequently observed in giant cell tumor, peritumoral inflammatory changes have not been noted in giant cell tumor. Interestingly, all 20 cases of giant cell tumor studied show neither COX-2 immunoreactivity nor inflammatory reactions. Thus, peritumoral inflammatory reactions are not related to tumor histology, size, location, and aggressiveness, but are related to COX-2 expression in the tumor tissue.
In summary, there is the possibility that increased COX-2 expression is only an associated finding with inflammation; nevertheless, our study suggests that COX-2 expression is likely an important factor for inducing peritumoral inflammatory changes in chondroblastomas. The same mechanism, activation of eicosanoid biosynthetic pathway by COX-2 within the tumor, functions in these secondary inflammatory reactions, such as bone-marrow edema and periosteal reaction both in the chondroblastoma and osteoid osteoma.
References
Beltran J, Chandnani V, McGhee RA Jr, Kursunoglu-Brahme S (1991) Gadopentetate dimeglumine-enhanced MR imaging of the musculoskeletal system. Am J Roentgenol 156:457–466
Bloem JL, Mulder JD (1985) Chondroblastoma: a clinical and radiological study of 104 cases. Skeletal Radiol 14:1–9
Braunstein E, Martel W, Weatherbee L (1979) Periosteal bone apposition in chondroblastoma. Skeletal Radiol 4:34–36
Brower AC, Moser RP, Kransdorf MJ (1990) The frequency and diagnostic significance of periostitis in chondrobalstoma. Am J Roentgenol 154:309–314
Ciabattoni G, Tramburrelli F, Greco F (1991) Increased prostacyclin biosynthesis in patients with osteoid osteoma. Eicosanoids 4:165–167
Crofford LJ (1997) COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol 24:15–19
Dorfman HD, Czerniak B (eds) (1998) Benign cartilage tumors. In: Bone tumors. Mosby, St. Louis, pp 253–352
Edel G, Ueda Y, Nakanishi J, Brinker KH, Roessner A, Blasius S, Vestring T, Muller-Miny H, Erlemann R, Wuisman P (1992) Chondroblastoma of bone. Virchows Archiv 421:355–366
Erlemann R, Reiser MF, Peters PE, Vasallo P, Nommensen B, Kusnierz-Glaz CR, Ritter J, Roessner A (1989) Musculoskeletal neoplasms: static and dynamic Gd-DTPA-enhanced MR imaging. Radiology 171:767–773
Greco F, Tamburrelli F, Ciabattoni G (1991) Prostaglandins in osteoid osteoma. Int Orthopaedics 15:35–37
Hasegawa T, Hirose T, Sakamoto R, Seki K, Ikata T, Hizawa K (1993) Mechanism of pain in osteoid osteomas: an immunohistochemical study. Histopathology 22:487–491
Hayes CW, Conway WF, Sundaram M (1992) Misleading aggressive MR imaging appearance of some benign musculoskeletal lesions. Radiographics 12:1119–1134
Hudson TM, Hawkins IF Jr (1981) Radiological evaluation of chondroblastoma. Radiology 139:1–10
Ishida T, Goto T, Motoi N, Mukai K (2002) Intracortical chondroblastoma mimicking intra-articular osteoid osteoma. Skeletal Radiol 31:603–607
Jee WH, Park YK, McCauley TR, Choi KH, Ryu KN, Suh JS, Suh KJ, Cho JH, Lee JH, Park JM, Lee YS, Ok IY, Kim JM (1999) Chondroblastoma: MR characteristics with pathologic correlation. J Comput Assist Tomogr 23:721–726
Kaim AH, Hugli R, Bonel HM, Jundt G (2002) Chondroblastoma and clear cell chondrosarcoma: radiological and MRI characteristics with histopathological correlation. Skeletal Radiol 31:88–95
Kawaguchi Y, Sato C, Hasegawa T, Oka S, Kawahara H, Norimatsu H (2000) Intraarticular osteoid osteoma associated with synovitis: a possible role of cyclooxygenase-2 expression by osteoblasts in the nidus. Mod Pathol 13:1086–1091
Kawaguchi Y, Hasegawa T, Oka S, Sato C, Arima N, Norimatsu H (2001) Mechanism of intramedullary high intensity area on T2-weighted magnetic resonance imaging in osteoid osteoma: a possible role of COX-2 expression. Pathol Int 51:933–937
Moore SG, Bisset GS 3rd, Siegel MJ, Donaldson JS (1991) Pediatric musculoskeletal MR imaging. Radiology 179:345–360
Mungo DV, Zhang X, O'Keefe RJ, Rosier RN, Puzas JE, Schwarz EM (2002) COX-1 and COX-2 expression in osteoid osteomas. J Orthopaedic Res 20:159–162
Unni KK (ed) (1996) Benign chondroblastoma. In: Dahlin's bone tumors: general aspects and data on 11,087 cases, 5th edn. Lippincott-Raven, Philadelphia, pp 47–57
Vogler JB 3rd, Murphy WA (1988) Bone marrow imaging. Radiology 168:679–693
Weatherall PT, Maale GE, Mendelsohn DB, Sherry CS, Erdman WE, Pascoe HR (1994) Chondroblastoma: classic and confusing appearance at MR imaging. Radiology 190:467–474
Wold LE, Pritchard DJ, Bergert J, Wilson DM (1988) Prostaglandin synthesis by osteoid osteoma and osteoblastoma. Mod Pathol 1:129–131
Yamamura S, Sato K, Sugiura H, Iwata H (1996) Inflammatory reaction in chondroblastoma. Skeletal Radiol 25:371–376
Yamamura S, Sato K, Sugiura H, Katagiri H, Ando Y, Futatsu H, Iwata H (1997) Prostaglandin levels of primary bone tumor tissues correlate with peritumoral edema demonstrated by magnetic resonance imaging. Cancer 79:255–261
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shinmura, K., Ishida, T., Goto, T. et al. Expression of cyclooxygenase-2 in chondroblastoma: immunohistochemical analysis with special emphasis on local inflammatory reaction. Virchows Arch 444, 28–35 (2004). https://doi.org/10.1007/s00428-003-0889-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-003-0889-9