Abstract
Purpose
Implant-associated osteomyelitis still represents a demanding challenge due to unfavourable biological conditions, bacterial properties and incremental resistance to antibiotic treatment. Therefore different bactericide or bacteriostatic implant coatings have been developed recently to control local intramedullary infections. Controlled local release of gentamicin base from a highly lipophilic gentamicin palmitate compound achieves extended intramedullary retention times and thus may improve its bactericide effect.
Methods
Forty male Sprague-Dawley rats were divided into two groups receiving an intramedullary femoral injection of 102 colony-forming units (CFU) of a common methicillin susceptible Staphylococcus aureus strain (MSSA Rosenbach) and either an uncoated femur nail (Group I) or a nail coated with gentamicin palmitate (Group II). Animals were observed for 28 and 42 days. Serum haptoglobin and relative weight gain were assessed as well as rollover cultures of explanted femur nails and histological scores of periprosthetic infection in dissected femurs.
Results
Implants coated with gentamicin palmitate significantly reduced periprosthetic bacterial growth as well as signs of systemic inflammation compared with uncoated implants.
Conclusions
Gentamicin palmitate appears to be a viable coating for the prevention of implant-associated infections. These findings will have to be confirmed in larger animal models as well as in clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteomyelitis, a bone marrow infection, is still one of the most feared late complications after excessive bone and soft-tissue surgery, open fractures or implant-associated infections. It is divided into an acute and a chronic form. Chronic osteomyelitis evolves over years with persistent low-grade inflammation, impaired circulation and subsequent bone destruction with formation of sequesters [1]. Most common pathogens of chronic osteomyelitis are Gram-positive bacteria, especially Staphylococcus aureus (MSSA) and Coagulase-negative Staphylococci (CoNS), which together make up 75 % of all infections [2]. Moreover Gram-positive bacteria are also the most common pathogens causing periprosthetic joint infections [3, 4] as well as infections of incorporated biomaterials in general [5]. Due to their pyogenous potential these pathogens induce early osteonecrosis as well as bone resorption by stimulation of osteoclastic activity [1]. Therapy strategies are scarce and mainly focus on implant removal—if applicable—and radical surgical debridement of the infection site in combination with local and systemic application of antibiotics [6–8]. Today the “gold standard” for local application of antimicrobial agents is polymethylmethacrylate (PMMA) loaded with various antibiotics [3, 9, 10]. Delivery from PMMA remains the only local clinical application technique for antibiotics, however it may impair tissue healing itself and therefore bears a considerably high risk of reinfection in the long run [11]. Antibiotic protection of cementless implants, however, has not been possible in clinical practice until today [12].
Challenges of local antibiotic delivery are a high antibiotic release within the first hours exceeding minimum inhibitory concentrations (MICs) of the selected pathogen, as well as a sufficient long-term antibiotic release thereafter. For infections caused by MSSA, failure rates of local therapy strategies are around 20 %. Recent research has therefore focused on the development of new implant designs or coatings to prevent bacterial colonisation and biofilm formation and thus win the so-called “race for the surface” in an early stage [13]. This can be achieved either by implant design itself or by the help of bioactive substances like antibiotics [14]. Gentamicin is an aminoglycoside antibiotic with a relatively wide spectrum of activity, including MSSA as it is seen in acute osteomyelitis. Unfortunately, side effects of a systemic therapy with gentamicin are ototoxicity and severe nephrotoxicity, which limits its use especially in critically ill patients. Local application (e.g. PMMA beads) instead does not seem to cause these adverse reactions [3]. Furthermore, it does not seem to interfere with the formation of new vital bone, as is desired in infected defect situations [15]. Several formulations of gentamicin are already in clinical use. Gentamicinpalmitate has already been shown to have a good activity against MSSA and is also thought to remain intramedullary due to its highly lipophilic fraction [12, 16].
The aim of the present study was to evaluate the efficacy of an implant coating with a novel gentamicinpalmitate (GP) formulation in vivo using an established rat model of acute implant-associated osteomyelitis [17].
Materials and methods
Implants
Coating and sterilisation
Gentamcinpalmitate (GP) coating solutions were prepared by inverse salt exchange (Heraeus Medical, Wehrheim, Germany) [18]. In doing so the sulphate ions of gentamicinsulphate (GS) are replaced by palmitic acid anions. The protonised gentamicin base (GB) remained unchanged. A methanolic solution of GP was then sprayed homogenously on 1.5-mm Kirschner wires (k-wires) (Hemoteq, Aachen, Germany).
All wires had a surface of circa 0.97 cm2 and were coated with circa 1 mg GP. The total amount of applied GP was determined by analytic weighing directly after the coating procedure. Afterwards the wires were dried for one hour at room temperature (23 °C, 55 % rH) and weighed again. Mean applied GP was 1.0088 g, with a standard deviation of 57.7 μg per wire (5.42 % relative standard deviation). Optimal spraying time was determined at 13 seconds in preparation for this study. Coated and uncoated k-wires were sterilised by γ-radiation with 25 kGy (BGS Beta-Gamma-Service, Wiehl, Germany).
Elution-experiments with sterilised and non-sterilised implants
Coated sterilised and non-sterilised k-wires were incubated in phosphate-buffered saline (PBS) (Biochrom, Berlin, Germany) at 37 °C and samples were taken on five consecutive days. To determine the amount of eluted gentamicin, 100 μl of the eluate were analysed via fluorescent polarisation in the TDx system (Figureott TDx, Figureott Park, IL, USA). Wires stored for four years at room temperature (23 °C, 55 % rH) were analysed again during the experiments to determine stability of the GP coating.
Kinetics of bacterial reduction
An overnight culture of MSSA was diluted in pre-warmed media (37 °C) to a bacterial number of 106 colony-forming units (CFU)/ml resulting in a final volume of 10 ml. This bacterial suspension was then incubated at 37 °C and shaken at 96 rpm for two hours. Subsequently antibiotics and k-wires were added. Samples were drawn after 15, 30, 45 and 60 minutes and then by the hour. The last sample was taken after 24 hours. Aliquots of the samples were plated on agar plates to determine germ number. GS at 50 μg/ml served as a control.
HPLC analysis of eluates
To ascertain the amount of released antibiotic over time, eluates were analysed on high-performance liquid chromatography (HPLC). Ten calibration standards from 100 to 7,500 ng/ml gentamicin as well as a blank sample (with internal standard) were prepared by spiking 200 μl of working solution with internal standard working solutions (18 μl gentamicin).
The study samples were diluted by factor 20 and prepared according to the calibration standards by adding internal standard working solution. Every sample was analysed three times in a row. LC-MS/MS chromatographic separation was performed on a modular HPLC 1200 Series (Agilent Technologies, Waldbronn, Germany) using a Luna C18 (II) column, 150 × 2 mm, with two C18 guard columns, 4 × 2 mm (Phenomenex, Aschaffenburg, Germany) at 25 °C. Injection volume was 2 μl. The mobile phase A was 0.11 M trifluoroacetic acid/methanol (50:50) and mobile phase B was acetonitrile. An isocratic separation for gentamicin was achieved with an A:B ratio of 95:5 at a flow rate of 0.25 ml/min. The runtime was 2.5 min and the total cycle time was less than three minutes. Under these conditions the four gentamicin components C1, C2, C2a and C1a co-eluted. This HPLC method has been used previously to determine gentamicin levels in biopsy samples [19]. The detection of the co-eluted gentamicin components was carried out using an API 4000 QTrap (Applied Biosystems, Darmstadt, Germany). Ionisation was carried out with an electrospray interface (positive polarity) using the mass selective detector in the multiple reaction monitoring mode (MRM). The extracted ion chromatograms of the following ion transitions were stored and calculated: 478.4 → 322.3 m/z (gentamicin C1), 464.4 → 322.3 m/z (gentamicin C2 and C2a), 450.3 → 322.3 m/z (gentamicin C1a.) and 468.4 → 163.1 m/z (internal standard). The three ion transitions of gentamicin components were summed by the software Analyst 1.4.2 (Applied Biosystems, Darmstadt, Germany) and calculated with Excel (Microsoft Deutschland GmbH, Unterschleißheim, Germany).
Pathogen
Induction of osteomyelitis was achieved by inoculation of 102 CFU of methicillin-susceptible Staphylococcus aureus (MSSA) namely Staphylococcus aureus subsp aureus Rosenbach [20] dissolved in PBS. This facultative anaerobe has a known sensitivity to gentamicin.
Animals
Forty male five month-old Sprague-Dawley rats (Harlan Winkelmann, Borchen, Germany) were used in the experiment. The animals were kept in individual plastic cages (Macrolon Type III) in a room maintained at a constant temperature of 22.1 °C, 55 % rH with a 12 hour light/dark cycle. They had free access to drinking water and standard laboratory pellets LASQCdiet® Rod16 Rad (LASvendi, Soest, Germany). All experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the local Animal Ethics Committee Regierungspräsidium Giessen under reference number V 54 – 19 c 20 15 (1) MR 20/21 – No. 45/2010.
Surgery
Animals were divided into two groups of 20 rats prior to surgery. Group I served as a negative control, receiving an uncoated implant, whereas Group II received a coated implant. Subgroups of ten animals were killed after observation times of 28 and 42 days in each group.
Surgery was performed under general anaesthesia by weight-adjusted intraperitoneal injection of xylazine 2 % (Rompun®, 10 mg/kg body weight; Bayer CropScience, Leverkusen, Germany) and ketamine hydrochloride (Ketamin WDT, 100 mg/kg body weight; WDT eG, Garbsen, Germany). The left hindleg was shaved around the knee and aseptically prepared with phenoxyethanol (Schülke & Mayr, Norderstedt, Germany). An approximately 10-mm long ventral parapatellar arthrotomy was made to open up the knee joint. The patella was everted, exposing both femoral condyles and the trochlea. The femoral intramedullary cavity was opened in the trochlear groove and carefully reamed up using a small k-wire. After reaching the desired measures, a Hamilton microlitre syringe was used to administer the previously defined amount of inoculum into the intramedullary canal. Afterwards a sterilised k-wire (Group I, uncoated/control, or Group II, coated) was applied avoiding contact to every surrounding tissue. After implantation, the site was closed by suturing up the overlying tissue and skin with fast resorbing Vicryl rapide 4–0 (Ethicon, Norderstedt, Germany). All surgical procedures were performed by experienced surgeons. Due to the infectious nature of these experiments no additional antibiotics were used to prevent wound infection perioperatively.
Radiographs
Radiographic evaluation was performed seven, 14, 28 and 42 days after surgery using digital plain film imaging (Siemens Healthcare Diagnostics, Eschborn, Germany) in anterior-posterior (AP) and axial views. All animals were anaesthetised as described above for the duration of the radiographic scans. Images were transferred to a Leonardo image analysis workstation (Siemens Healthcare Diagnostics, Eschborn, Germany) for evaluation.
Blood serum samples and body weight
Blood serum samples were collected by retrobulbar venous plexus puncture under general anaesthesia following radiographic evaluation after seven, 14, 28 and 42 days. All samples were screened for the acute phase protein, haptoglobin, to assess early systemic inflammation [21].
Body weight of all animals was checked after four, seven, ten, 14, 17, 21, 28, 34 and 42 days and expressed in relation to the baseline weight (Δg) to assess weight gain or loss.
Harvesting of tissue and sectioning
Animals were killed by CO2 asphyxiation. The condyles at the implantation site were carefully removed using a bone forceps to expose the intramedullary nails. These were pulled out and kept under sterile conditions for direct processing. The whole remaining femur was resected and immediately fixed in 4 % buffered formalin for three days and then decalcified in Osteosoft® EDTA-solution (Merck, Darmstadt, Germany) over a period of 28 days. After that specimens were dehydrated in graded alcohol solution and cedar wood oil and embedded in paraffin. Sections were cut at 5 μm with a 40° stainless-steel blade on a rotation microtome RM2055 (Leica Microsystems, Bensheim, Germany).
Microbiological evaluation
Intramedullary nails were explanted as described above and immediately rolled over a blood agar plate under sterile working conditions. Inoculated blood agar plates were incubated for 48 hours at a temperature of 37 °C. Colony counts were carried out following the incubation time using an indirect viable cell count method. Confluent bacterial growth was set at 500 CFU per plate.
Histology and histomorphometric analysis
Histological staining was performed with a haematoxylin-eosin (HE) (Merck, Darmstadt, Germany) formulation according to standard protocols. Histomorphometric analysis was performed at a primary magnification of fivefold using a digital microscope DM5000 (Leica Microsystems, Bensheim, Germany) and QUIPS analysis software (Leica Microsystems, Bensheim, Germany). Fifty images per specimen were captured and assembled into a montage displaying the whole femur. Four regions of interest (ROIs) were defined dividing the femur into proximal epiphyseal/metaphyseal, proximal diaphyseal, distal diaphyseal and distal epiphyseal/metaphyseal.
Presence of a periprosthetic infection or osteomyelitis was detected for each ROI using a modified Petty Score with evaluation of abscess formation (0–1), bone sequesters (0–1), cortical thickening or bone formation (0–1), cortical destruction (0–1) and overall appearance (0–2). Total scores (0–6) were calculated as means of all four ROIs.
Statistical analysis
Paired t-tests or multiple t-tests with a post hoc Holm-Sidak correction were used to evaluate the differences between groups using Graph Pad Prism 6 (Graph Pad, La Jolla, CA, USA). Data are given as means ± standard deviation (SD) if not indicated otherwise. The level of significance was set at p < 0.05.
Results
Release kinetics of coated implants
Elution experiments on freshly coated k-wires
Figure 1 gives an overview on the release of GB of freshly coated y-sterilised and non-sterilised k-wires. The data of the eluted GB were calculated in μg per cm2 of the surface (measurements were carried out with five k-wires). The amount of the released GB was around 200 μg/cm2 on the first day and decreased steadily until day five. Release rates of the non-sterilised k-wires were similar.
Measurement of elution over 21 days of long-stored k-wires
Long-stored k-wires were kept in a dry place for two years and ten months. Figure 2 gives an overview on the release of GB of freshly coated k-wires. The release rates of the long-stored k-wires were similar to the release rates of the freshly coated k-wires. The initial burst was similar to that of freshly coated k-wires. The investigated k-wires showed 25–30 μg of GB release on days seven, 14 and 21.
Cumulative released amounts of GB from 1 h to 21 days of long-stored k-wires. There were no significant differences between long-stored and freshly coated k-wires regarding antibiotic release kinetics as presented in Fig. 1
Elimination kinetics of GP compared with GS
Bacterial elimination kinetics of k-wires compared with GS. Both the GP (two years and ten months old) and the GS show similar elimination kinetics in vitro (Fig. 3).
Weight and general condition
Six animals were lost perioperatively or preoperatively due to complications of intraperitoneal anaesthesia. These animals were replaced and respective groups were filled up. No animals were lost postoperatively or in the course of the experiment.
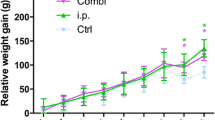
Relative weight gain in Group I (control) showed an increase from 7.00 ± 1.81 g after four days to 84.86 ± 11.83 g after 42 days, whereas Group II (coated) showed an increase from 6.76 ± 12.45 g after four days to 184.66 ± 28.78 g after 42 days (Fig. 4).
Radiographic evaluation
Radiographs did not show any misplaced k-wires. No specific radiographic signs of acute osteomyelitis could be seen in course of the experiment in any of the groups.
Blood serum analyses
Serum Hp levels started from 1.67 ± 0.63 mg/dl (day seven) in Group I (control) falling to 1.24 ± 0.51 mg/dl (day 13) and rising again to 1.64 ± 0.81 mg/dl (day 28) to remain at 1.66 ± 0.46 mg/dl (day 42). Group II (coated) showed a steady decrease of serum Hp levels starting from 1.29 ± 0.53 mg/dl (day seven) over 0.98 ± 0.65 mg/dl (day 13) to 0.78 ± 0.33 mg/dl (day 28) and finally 0.57 ± 0.23 mg/dl (day 42). Coated implants yielded significantly lower Hp levels after 28 and 42 days compared with uncoated implants.
Microbiological evaluation
Rollover cultures of the explanted k-wires revealed confluent bacterial growth in Group I (control) and disseminated punctiform bacterial growth in Group II (coated) after 28 and 42 days. Bacterial counts revealed a mean of 380.60 ± 134.60 CFU for Group I (control) and 35.10 ± 47.65 CFU for Group II (coated) after 28 days as well as 356.0 ± 142.60 CFU for Group I (control) and 21.10 ± 28.94 CFU for Group II (coated) after 42 days. Differences between both groups were statistically significant at both timepoints (Fig. 5).
Histology
Mean Petty-Scores in Group I (control) were 4.2 ± 0.55 indicating an acute osteomyelitis, whereas Group II (coated) yielded mean scores of 1.17 ± 0.65, which holds for one characteristic of implant-associated infection after 28 days. Group I (control) scored 4.2 ± 0.77, again indicating acute osteomyelitis, whereas Group II (coated) scored 1.27 ± 0.34, again showing present but locally controlled infection after 42 days (Fig. 6).
H&E stained slide (10×) from Group I (control) showing disseminated abscess formation in transition from cortical to metaphyseal bone with consecutive destruction. Mean Petty Scores for each group indicating an acute osteomyelitis after 28 and 42 days in Group I (control) and significantly different signs of suppressed infection in Group II (coated)
Discussion
Implant-associated MSSA osteomyelitis could be successfully prevented throughout the whole period of investigation using a novel GP coating. The present study has focused on a model of intramedullary nailing as described by Lucke et al. [17] previously, only choosing the femur instead of the tibia as implantation site. According to the results of Lucke et al., no soft tissue infections could be noted near the entry sites of the distal femoral condyles [17]. Intramedullary injection of only 102 CFU led to induction of histologically and systemically verifiable acute osteomyelitis; however, the radiographic findings of Lucke et al. [17] could not be reproduced in this case. This may be due to the different anatomical shape and greater intramedullary volume of the rat femur in comparison to the tibia [22]. Inoculum concentrations of 102 CFU may thus be enough to induce osteomyelitis in a rat femoral model, but do not lead to such bony destruction that it can be seen on plain radiographies though. Colonisation of all explanted uncoated k-wires (Group I) could be verified by rollover cultures, whereas all coated k-wires (Group II) remained sterile after explantation.
Type of surface and its composition affect interaction between orthopaedic implants and tissue. Inert surfaces which are not integrated within healthy tissue are most prone to bacterial colonisation and thus—in the long run—biofilm formation [23]. Local application of antibiotics—like from a GP coating, as shown in this study—provides high levels and avoids systemic toxic effects of antimicrobial agents [10]. High local concentrations of antibiotics have been shown to be beneficial in relatively avascular sites and in cases of biofilm [24, 25]. Titanium implants coated with biodegradable PDLLA containing 10 % gentamicin have been investigated already [5, 26] and local antibiotic delivery has also successfully been achieved with cancellous bone chips, hydroxyapatite (HA) or PMMA beads [27–29]. Release profiles for different antibiotics range from a few hours to ten days there. To date, however, only antimicrobial silver-coatings are being used on endoprostheses or implants in the clinical setting, but resistance of bacteria against silver has been described already and there are a lot of unfavourable characteristics for this coating as well (e.g. systemic toxicity). PMMA, however, has a major disadvantage when used as a carrier, since the material is not degradable and may cause severe foreign body reactions. Systemic concentrations of antibiotics were below toxic levels when released from PMMA and elution curves showed levels above MIC until six weeks after implantation in another study [29]. High doses of drug release may interfere with bone ingrowth though and may cause a temporarily induced formation of fibrous tissue without inflammation [11, 30]. Small colony variants of MSSA have been found in osteomyelitis despite gentamicin application, but none were observed in another study [28], which might have been due to higher concentrations of gentamicin compared with the first one. In the present study, punctiform colonies of MSSA were found on the gentamicin-coated titan implants. Nonetheless, bacterial numbers counted were less than 10 % of the uncoated group after 28 and 42 days of infection. Histology showed signs of osteomyelitis only in one case treated with a gentamicin-coated wire. Unfortunately, both HA and PMMA cannot provide primary stability in bone surgery if used alone. Covalently bound antibiotics on metal surfaces like titanium may be a valuable alternative here [9].
Antimicrobial coatings in general, however, are of enormous clinical interest, as the increasing number of joint replacements will inevitably lead to a growing number of septic loosening or chronic septic complications such as osteomyelitis. Today the costs for arthroplasty revisions are several times above those for primary surgery, although results are not regularly satisfying.
With 102 CFU, the present study used the lowest known dose of bacterial inoculum to induce infection [31], since a high bacterial inoculation should not be expected in standard operating theatres. In a later study of Lucke et al., only 30 % of implants remained sterile, since the coating of PDLLA contained 10 % of gentamicin and 103 CFU/10 μl of bacteria were inoculated [26]. The rate of sterile implants increased to 85 % if lower concentrations of inoculum were used, pointing out a general need for local application of antibiotics, especially for smaller concentrations of inoculum which come close to clinical situations [31]. The reduction of infections related to the application of local antibiotics have also been described in other models applying doses between 102 and 103 CFU [5, 17, 31, 32].
GP concentrations reached MIC within 30 min after application [12]. Bone cements containing GS and titan discs coated with GP showed a similar effectiveness against MSSA, both eliminating bacterial numbers ranging between 100 and 100,000 within 24 hours. Coatings of PDLLA, in contrast, as used by Lucke et al., degrade via hydrolysis within three to six months and are metabolised in the citric acid cycle [31]. However, high concentrations of antibiotics can be delivered for a short time. Hydrophobic forces are exerted over distances of 15 nm [23] and the strongly lipophilic GP coating used in the present study prevents or at least reduces colonisation of bacteria from the early seeding around six weeks. Although there is a high early burst release of gentamicin, the concentration remains above MIC until six weeks after implantation.
The effectiveness of antibiotic coatings seems to depend on the concentrations of bacterial inoculum used. Even after inoculation of only 102 CFU there is no complete prevention of bacterial colonisation. This does not jeopardise prophylaxis of infection during implantation of metal devices, since the number of bacteria inoculated in operating theatres should be expected to be even lower. Therefore, careful surgical debridement seems necessary to allow the antimicrobial coatings to work properly. The early release of the antibiotic within 30 minutes should be sufficient to avoid biofilm formation and the hydrophobic surface should additionally contribute to prevention of biofilm formation. Lipophilic coatings on the other hand can be expected to remain longer intramedullary than hydrophilic substances.
Conclusions
Gentamicin-palmitate coating significantly reduces bacterial colonisation by MSSA and diminishes a corresponding intramedullary infection. The local concentration of gentamicin appears to be sufficient to prevent biofilm formation for at least six weeks postoperatively in this animal model of implant-associated osteomyelitis. Further studies will have to confirm these results in larger animal models and clinical trials. They will also have to clarify the question if additional systemic antibiotics as used in clinical routines can boost the effects of such a gentamicin-palmitate coating.
References
Uçkay I, Jugun K, Gamulin A et al (2012) Chronic osteomyelitis. Curr Infect Dis Rep 14:566–575
Li G-Q, Guo F-F, Ou Y et al (2013) Epidemiology and outcomes of surgical site infections following orthopedic surgery. Am J Infect Control 41:1268–1271
Chang Y, Tai C-L, Hsieh P-H, Ueng SWN (2013) Gentamicin in bone cement: a potentially more effective prophylactic measure of infectionin joint arthroplasty. Bone Joint Res 2:220–226
Seghrouchni K, van Delden C, Dominguez D et al (2012) Remission after treatment of osteoarticular infections due to Pseudomonas aeruginosa versus Staphylococcus aureus: a case-controlled study. Int Orthop 36:1065–1071
Schmidmaier G, Lucke M, Wildemann B et al (2006) Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: a review. Injury 37(Suppl 2):S105–S112
Lew DP, Waldvogel FA (2004) Osteomyelitis. Lancet 364:369–379
Rod-Fleury T, Dunkel N, Assal M et al (2011) Duration of post-surgical antibiotic therapy for adult chronic osteomyelitis: a single-centre experience. Int Orthop 35:1725–1731
Betz M, Abrassart S, Vaudaux P et al (2014) Increased risk of joint failure in hip prostheses infected with Staphylococcus aureus treated with debridement, antibiotics and implant retention compared to Streptococcus. Int Orthop. doi:10.1007/s00264-014-2510-z
Antoci V, Adams CS, Parvizi J et al (2008) The inhibition of Staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infection. Biomaterials 29:4684–4690
Joosten U, Joist A, Gosheger G et al (2005) Effectiveness of hydroxyapatite-vancomycin bone cement in the treatment of Staphylococcus aureus induced chronic osteomyelitis. Biomaterials 26:5251–5258
Dion A, Langman M, Hall G, Filiaggi M (2005) Vancomycin release behaviour from amorphous calcium polyphosphate matrices intended for osteomyelitis treatment. Biomaterials 26:7276–7285
Kittinger C, Marth E, Windhager R et al (2011) Antimicrobial activity of gentamicin palmitate against high concentrations of Staphylococcus aureus. J Mater Sci Mater Med 22:1447–1453
Gracia E, Laclériga A, Monzón M et al (1998) Application of a rat osteomyelitis model to compare in vivo and in vitro the antibiotic efficacy against bacteria with high capacity to form biofilms. J Surg Res 79:146–153
Ozturk AM, Tabak AY, Aktekin CN et al (2008) Alendronate enhances antibiotic-impregnated bone grafts in the treatment of osteomyelitis. Int Orthop 32:821–827
Fassbender M, Minkwitz S, Kronbach Z et al (2013) Local gentamicin application does not interfere with bone healing in a rat model. Bone 55:298–304
Matl FD, Obermeier A, Repmann S et al (2008) New anti-infective coatings of medical implants. Antimicrob Agents Chemother 52:1957–1963
Lucke M, Schmidmaier G, Sadoni S et al (2003) A new model of implant-related osteomyelitis in rats. J Biomed Mater Res B Appl Biomater 67:593–602
Vogt S, Kühn K-D, Gopp U, Schnabelrauch M (2005) Resorbable antibiotic coatings for bone substitutes and implantable devices. Materwiss Werksttech 36:814–819
Heller DN, Peggins JO, Nochetto CB et al (2005) LC/MS/MS measurement of gentamicin in bovine plasma, urine, milk, and biopsy samples taken from kidneys of standing animals. J Chromatogr B Anal Technol Biomed Life Sci 821:22–30
Cowan ST, Shaw C, Williams RE (1954) Type strain for Staphylococcus aureus Rosenbach. J Gen Microbiol 10:174–176
Giffen PS, Turton J, Andrews CM et al (2003) Markers of experimental acute inflammation in the wistar han rat with particular reference to haptoglobin and C-reactive protein. Arch Toxicol 77:392–402
Welch JM, Weaver CM, Turner CH (2004) Adaptations to free-fall impact are different in the shafts and bone ends of rat forelimbs. J Appl Physiol 97:1859–1865
Gristina AG (1987) Biomaterial-centered infection: microbial adhesion versus tissue integration. Science 237:1588–1595
Lewis CS, Supronowicz PR, Zhukauskas RM et al (2012) Local antibiotic delivery with demineralized bone matrix. Cell Tissue Bank 13:119–127
Hanssen AD (2005) Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin Orthop Relat Res 437:91–96
Lucke M, Schmidmaier G, Sadoni S et al (2003) Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone 32:521–531
Lewis CS, Katz J, Baker MI et al (2011) Local antibiotic delivery with bovine cancellous chips. J Biomater Appl 26:491–506
Joosten U, Joist A, Frebel T et al (2004) Evaluation of an in situ setting injectable calcium phosphate as a new carrier material for gentamicin in the treatment of chronic osteomyelitis: studies in vitro and in vivo. Biomaterials 25:4287–4295
Cornell CN, Tyndall D, Waller S et al (1993) Treatment of experimental osteomyelitis with antibiotic-impregnated bone graft substitute. J Orthop Res 11:619–626
Hamanishi C, Kitamoto K, Tanaka S et al (1996) A self-setting TTCP-DCPD apatite cement for release of vancomycin. J Biomed Mater Res 33:139–143
Lucke M, Wildemann B, Sadoni S et al (2005) Systemic versus local application of gentamicin in prophylaxis of implant-related osteomyelitis in a rat model. Bone 36:770–778
Helbig L, Simank HG, Lorenz H et al (2014) Establishment of a new methicillin resistant Staphylococcus aureus animal model of osteomyelitis. Int Orthop 38:891–897
Acknowledgments
The present study was supported by Synthes GmbH, Umkirch, Germany. The authors thank Mr. Guido Schemken and his staff at the Central Animal Housing Facility in Marburg, as well as Prof. Dr. Markus Schofer and Dr. Stefan Lakemeier for their kind support in performing this study.
Conflict of interest
All authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fölsch, C., Federmann, M., Kuehn, K.D. et al. Coating with a novel gentamicinpalmitate formulation prevents implant-associated osteomyelitis induced by methicillin-susceptible Staphylococcus aureus in a rat model. International Orthopaedics (SICOT) 39, 981–988 (2015). https://doi.org/10.1007/s00264-014-2582-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-014-2582-9