Abstract
Local delivery of antibacterial substances is an effective and promising option to prevent infection. A dissolving dip-coating for implants for fracture repair was developed. The aminoglycoside Gentamicin showed promising potential. This coating leads to antibiotic concentrations lying well above the minimal inhibitory concentration of the most commonly encountered bacterial strains in the immediate surroundings of the implant. This coating has been tested in multiple pre-clinical studies in rats and rabbits. In 2005, an intramedullary tibia nail with antibiotic coating was developed for human use and proved to be a useful tool in the treatment of tibia fractures in patients with a higher risk of developing implant-related infections. Since 2011, a tibia nail of the latest generation with the same coating is available. The use of a Gentamicin coated tibia nail could reduce the risk of deep infections and prevent long-term external fixation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Advances in surgical techniques, implant-design and surface modifications changed fracture management. Regaining full mobility and quality of life is nowadays the ultimate goal of treatment in the vast majority of patients. Nevertheless, complex fractures and fractures in high-risk patients remain a challenge for surgeons and still have a high potential for complications.
Among these, deep infections and osteomyelitis are especially alarming due to the need of additional operative interventions and long-lasting antibiotic therapies, the risk of life-threatening septic conditions, impaired fracture healing, reduced limb function which all compromise the quality of the patient’s life [1, 2]. Furthermore, the socio-economic burden of infections is significant; patients can be partially or completely excluded from social life as well as from work for weeks or even months [3].
Important measures to avoid infection are appropriate prehospital care of the fractured extremity, aseptic conditions in the operating theatre, short surgery time, stable fracture fixation and careful soft tissue handling. Additionally, antibiotic prophylaxis is applied in all osteosynthetic procedures [4, 5]. Despite this, the rate of infections after surgical fixation of long bone fractures remains high in certain patient populations. Some patient-related factors are known, which increase the risk for a patient to develop an infection, but are not directly related to the fracture itself. These are, among others: polytrauma, diabetes, obesity, abuse of alcohol and nicotine, immunosuppression, circulatory diseases, allergies [6–8]. The local conditions at the fracture site also contribute substantially to the likelihood for an infection to occur: severe soft-tissue damage, a problematic soft tissue envelope, contamination of the fracture site, and status after revision surgery [9, 10]. High-energy open tibial fractures resulting in severe soft-tissue damage are especially prone to infection, non-union and other complications [4, 11, 12]. Due to general vascular disturbances or poor blood supply by scar or traumatized tissue, the antibiotic agent doesn’t reach the site of interest in a sufficient manner.
2 Scientific Background
To optimize the bioavailability at the site of interest, local delivery of antibacterial substances is an effective and promising option. Various methods for local delivery of antibiotics exist, such as rinsing with antibiotic solutions, implantation of antibiotic-impregnated PMMA bead chains, collagen sponges, Calcium-Phosphate or –Sulphate materials impregnated with antibiotics, and others [13–16]. These methods can be effective to a certain degree but have not found wide acceptance, mainly due to lack of proof of evidence or to the fact that their implementation represents a significant deviation from the standard surgical technique.
The presence of an implant enhances the risk of infection due to its surface, which is prone for pathogens to adhere. Already during insertion of the implant, its surface is covered by a protein-layer, which serves as a point of attack for the body’s own cells and bacterial pathogens. This contest is described as ‘race to the surface’ [17]. In case of victory of the pathogens, a biofilm is formed. This makes removal of the implant necessary for eradication of the infection.
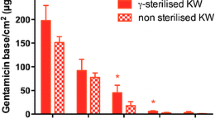
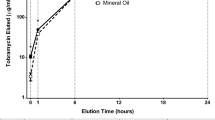
To improve prophylaxis against bacterial colonization of the implant surface, and furthermore to deliver required drugs at the site of interest, a dissolving dip-coating for surgically used implants was developed at the Institute for Oncology and Therapy-Research of the Technical University Munich. Originally, Hirudin was included in the coating of endovascular implants to inhibit the formation of neointima [18, 19]. This coating technology was further modified at the Clinic for Trauma and Reconstructive Surgery Charité of the Humboldt University of Berlin. In particular, the concept was applied to implants for fracture repair. Its usefulness as a delivery device for growth factors and antibiotics was shown in several animal studies [20–22]. Subsequent investigations demonstrated the usefulness of this coating technology to deliver other active substances directly to the fracture site. In particular, substances, which may support fracture healing, such as BMP-2, Zoledronic Acid or Simvastatin were successfully included in the coating [23–25]. From an anti-infective point of view, several agents were evaluated. The use of the aminoglycoside Gentamicin showed promising potential. This coating leads to antibiotic concentrations lying well above the minimal inhibitory concentration of the most commonly encountered bacterial strains in the immediate surroundings of the implant. Moreover, it is highly abrasion-resistant and has the potential to significantly reduce the bacterial colonization of the implant surface at the time of implantation and in the first following days [26]. This coating has been tested in multiple pre-clinical studies in rats and rabbits. Implants coated with polylactid acid containing Gentamicin-sulfate were inserted into the medullary canal previously inoculated with bacteria. Compared to control groups of uncoated implants, the coating successfully prevented the occurrence of infection at 42 days of implantation without exception [20]. This working mechanism addresses the first step in the chain of colonization, biofilm formation, encapsulation and finally infection, which can occur upon the implantation of a foreign body [6, 27].
These experiments demonstrate the flexibility of this dip-coating technology as a method to combine the biomechanical properties of metal implants with biologically active substances, which support the fracture healing process or reduce the risk of implant-related infection.
Based on these promising data, the coating technology, which equips the traditional metal implant with additional properties, was adopted by industry and product development initiated. An intramedullary tibia nail with antibiotic coating was developed and approved for clinical use in European countries. The first such implant (UTN PROtect®, Synthes, Solothurn, CH) was released in 2005 and subsequently investigated in a prospective case series, where it proved to be a useful tool in the treatment of tibia fractures in patients with a higher risk of developing implant-related infections [28]. Since 2011, a tibia nail of the latest generation with the same coating is available (Expert Tibial Nail PROtect®, Synthes, Solothurn, CH) and is being used on a broader scale.
Animal studies report about alternative coating methods like titanium oxide and siloxane polymer doped with silver [29], but they have not left the experimental stage.
3 Indications and Limits
Coated nails can push the indications for nailing of tibia fractures from closed and open grade I and II [30] towards grade III fractures, in which uncoated nailing is currently not the treatment of choice [31]. The use of a Gentamicin coated tibia nail could reduce the risk of deep infections and prevent long-term external fixation [26]. Coated nails are implanted using the identical instruments and techniques used for uncoated nails. Surgery is not prolonged and the surgeon can exclusively focus on fracture reduction and fixation.
Case Description
An 18-year-old man was hit as a pedestrian by a car. The car drove over his right lower leg. The patient suffered a complex grade III open fracture of the right tibia (AO/OTA 42.C3) including a compartment syndrome. The peripheral perfusion, motoric and sensation were intact at the time of admission (Figs. 32.1, 32.2 and 32.3).
The fracture was primarily stabilized with a medioventral external fixator with two Schanz screws inserted in the proximal and distal segments of the tibia. The wound was extensively debrided and cleaned by jet-lavage. Approximately 7 cm of devascularized tibia needed to be resected (Figs. 32.4 and 32.5).
The compartment syndrome was treated by extension of the open wound and fasciotomy. At day six post trauma, a secondary skin closure was performed (Figs. 32.6 and 32.7).
Over the following 3.5 weeks, the fracture and wound healed to such an extent that intramedullary nailing with an antibiotic-coated tibia nail (Expert TN PROtect®, Synthes, Solothurn, CH) could be performed. The fibula was treated with a seven hole locking compression plate to restore the correct length of the lower limb. The external fixator was removed simultaneously. The Gentamicin-coated nail (9 mm × 375 mm) was implanted and locked statically (Figs. 32.8 and 32.9).
Nine days later, the patient was discharged from hospital for rehabilitation. The soft tissues were in a good condition. There were at no time point any hints for infection neither at the skin nor in the blood samples. Partial weight bearing of 20 kg on the injured leg was allowed.
Eleven weeks after implantation of the antibiotic coated nail and smoking cessation, an Ilizarov ring fixator was applied for callus distraction to bridge the defect in the tibia. A proximal osteotomy was performed in the proximal third of the tibia. Five days after this operation, bone transport was started using the monorail-technique [32] (Figs. 32.10 and 32.11).
During the next 3 months, bone transport was continued until the segment reached the docking site (Figs. 32.12 and 32.13). Consecutively, bone grafts from the iliac crest together with BMP-2 were inserted in the docking zone, which was additionally secured with a LC-DCP. The ring fixator was removed (Figs. 32.14 and 32.15).
One year after trauma, bone healing was achieved (Figs. 32.16 and 32.17).
There were several situations during the treatment of this patient, where the use of an antibiotic coated nail served well:
-
Severe III grade open tibia fracture with bone loss
-
One stage exchange from external fixation to an intramedullary device
-
Osteotomy of an injured leg after soft tissue damage
-
Bone transport by monorail-technique [32] using an ILIZAROV ring fixator
The availability of this Gentamicin-coated nail and the knowledge that bacterial growth is suppressed from the surface gave us the confidence to proceed with intramedullary nailing in this patient.
4 Clinical Evidence
The clinical use of antibiotic coated tibia nails was investigated in two prospective case series [20, 25]. In both series, no implant-related infections were observed in spite of the fact that the patient population was prone to develop an implant-related infection. Of the 27 patients, 20 presented with open fractures, 7 of which had a grade III° open fracture. At 6 months follow-up, 19 patients had healed fractures while fracture healing was progressing in the remaining 8.
It is difficult to show superiority of antibiotic coated nails when compared to uncoated nails in prospective studies. But all collected data confirm that the antibiotic coating has no negative side effects on bone healing [20, 24, 25].
5 Conclusion
Antibiotic coated implants may be beneficial in the treatment of patients at risk of developing implant-related infections: patients with severe open fractures, with revisions after failed osteosynthesis, with non-union or patients with a history of infection and with other risk factors.
Severe open tibia fractures are prone to bone infection despite the systemic administration of antibiotics [30]. A systemically applied antibiotic is disseminated haematogenously in the respective compartments. For various reasons, however, penetration and diffusion barriers may exist, such as tissue changed due to inflammation or necrosis. High and potentially toxic doses of active substances cannot always assure adequate levels of activity. If, however, the antibiotic is implanted directly into the site of possible infection by means of an adequate carrier, high concentrations of the active substance can be achieved without any toxicity [28]. So far, the only coated product for intramedullary nailing on the market is the Expert Tibia Nail PROtect®. There is a need for additional antibiotic coated implants.
References
Tsukayama DT. Pathophysiology of posttraumatic osteomyelitis. Clin Orthop Relat Res. 1999;360:22–9.
Young EJ, Sugarman B. Infections in prosthetic devices. Surg Clin North Am. 1988;68(1):167–80.
Darouiche R, Mosier M, Voigt J. Antibiotics and antiseptics to prevent infection in cardiac rhythm management device implantation surgery. Pacing Clin Electrophysiol. 2012;35(11):1348–60.
Zimmerli W. Antibiotic prophylaxis. In: AO principles of fracture management. Rüedi TP, Murphy WM, eds. New York: Thieme Stuttgart; 2000, pp. 699–707.
Patzakis MJ, Bains RS, Lee J, Shepherd L, Singer G, Ressler R, et al. Prospective, randomized, double-blind study comparing single-agent antibiotic therapy, ciprofloxacin, to combination antibiotic therapy in open fracture wounds. J Orthop Trauma. 2000;14(8):529–33.
Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151–7.
Ochsner PE, Müller U. Acute infection. In: AO principles of fracture management. Rüedi TP, Murphy WM, eds. New York: Thieme Stuttgart; 2000, pp. 729–747.
Schmidt AH, Swiontkowski MF. Pathophysiology of infections after internal fixation of fractures. J Am Acad Orthop Surg. 2000;8(5):285–91.
Schaser KD, Vollmar B, Menger MD, Schewior L, Kroppenstedt SN, Raschke M, et al. In vivo analysis of microcirculation following closed soft-tissue injury. J Orthop Res. 1999;17(5):678–85.
Yokoyama K, Itoman M, Uchino M, Fukushima K, Nitta H, Kojima Y. Immediate versus delayed intramedullary nailing for open fractures of the tibial shaft: a multivariate analysis of factors affecting deep infection and fracture healing. Indian J Orthop. 2008;42(4):410–9.
Malik MH, Harwood P, Diggle P, Khan SA. Factors affecting rates of infection and nonunion in intramedullary nailing. J Bone Joint Surg Br. 2004;86(4):556–60.
Gaebler C, Berger U, Schandelmaier P, Greitbauer M, Schauwecker HH, Applegate B, et al. Rates and odds ratios for complications in closed and open tibial fractures treated with unreamed, small diameter tibial nails: a multicenter analysis of 467 cases. J Orthop Trauma. 2001;15(6):415–23.
Cai X, Han K, Cong X, Cai J, Tong D, Han D, et al. The use of calcium sulfate impregnated with vancomycin in the treatment of open fractures of long bones: a preliminary study. Orthopedics. 2010;33(3).
Walenkamp GH, Kleijn LL, de Leeuw M. Osteomyelitis treated with gentamicin-PMMA beads: 100 patients followed for 1–12 years. Acta Orthop Scand. 1998;69(5):518–22.
Stemberger A, Grimm H, Bader F, Rahn HD, Ascherl R. Local treatment of bone and soft tissue infections with the collagen-gentamicin sponge. Eur J Surg Suppl. 1997;578:17–26.
Ostermann PA, Seligson D, Henry SL. Local antibiotic therapy for severe open fractures. A review of 1085 consecutive cases. J Bone Joint Surg Br. 1995;77(1):93–7.
Gristina AG, Naylor PT, Webb LX. Molecular mechanisms in musculoskeletal sepsis: the race for the surface. Instr Course Lect. 1990;39:471–82.
Alt E, Haehnel I, Beilharz C, Prietzel K, Preter D, Stemberger A, et al. Inhibition of neointima formation after experimental coronary artery stenting: a new biodegradable stent coating releasing hirudin and the prostacyclin analogue iloprost. Circulation. 2000;101(12):1453–8.
Herrmann R, Schmidmaier G, Markl B, Resch A, Hahnel I, Stemberger A, et al. Antithrombogenic coating of stents using a biodegradable drug delivery technology. Thromb Haemost. 1999;82(1):51–7.
Lucke M, Schmidmaier G, Sadoni S, Wildemann B, Schiller R, Haas NP, et al. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone. 2003;32(5):521–31.
Raschke M, Wildemann B, Inden P, Bail H, Flyvbjerg A, Hoffmann J, et al. Insulin-like growth factor-1 and transforming growth factor-beta1 accelerates osteotomy healing using polylactide-coated implants as a delivery system: a biomechanical and histological study in minipigs. Bone. 2002;30(1):144–51.
Schmidmaier G, Wildemann B, Bail H, Lucke M, Fuchs T, Stemberger A, et al. Local application of growth factors (insulin-like growth factor-1 and transforming growth factor-beta1) from a biodegradable poly(D, L-lactide) coating of osteosynthetic implants accelerates fracture healing in rats. Bone. 2001;28(4):341–50.
Back DA, Pauly S, Rommel L, Haas NP, Schmidmaier G, Wildemann B, et al. Effect of local zoledronate on implant osseointegration in a rat model. BMC Musculoskelet Disord. 2012;13:42.
Pauly S, Back DA, Kaeppler K, Haas NP, Schmidmaier G, Wildemann B. Influence of statins locally applied from orthopedic implants on osseous integration. BMC Musculoskelet Disord. 2012;13:208.
Greiner SH, Wildemann B, Back DA, Alidoust M, Schwabe P, Haas NP, et al. Local application of zoledronic acid incorporated in a poly(D, L-lactide)-coated implant accelerates fracture healing in rats. Acta Orthop. 2008;79(5):717–25.
Schmidmaier G, Lucke M, Wildemann B, Haas NP, Raschke M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: a review. Injury. 2006;37 Suppl 2:S105–12.
Gristina AG, Shibata Y, Giridhar G, Kreger A, Myrvik QN. The glycocalyx, biofilm, microbes, and resistant infection. Semin Arthroplasty. 1994;5(4):160–70.
Fuchs T, Stange R, Schmidmaier G, Raschke MJ. The use of gentamicin-coated nails in the tibia: preliminary results of a prospective study. Arch Orthop Trauma Surg. 2011;131(10):1419–25.
Tran N, Tran PA, Jarrell JD, Engiles JB, Thomas NP, Young MD, et al. In vivo caprine model for osteomyelitis and evaluation of biofilm-resistant intramedullary nails. Biomed Res Int. 2013;2013:674378.
Petrisor B, Anderson S, Court-Brown CM. Infection after reamed intramedullary nailing of the tibia: a case series review. J Orthop Trauma. 2005;19(7):437–41.
Raschke MJ, Mann JW, Oedekoven G, Claudi BF. Segmental transport after unreamed intramedullary nailing. Preliminary report of a “Monorail” system. Clin Orthop Relat Res. 1992;282:233–40.
Raschke M, Oedekoven G, Ficke J, Claudi BF. The monorail method for segment bone transport. Injury. 1993;24 Suppl 2:S54–61.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag London
About this chapter
Cite this chapter
Raschke, M.J., Rosslenbroich, S.B., Fuchs, T.F. (2015). Antibiotic Coated Nails. In: Rommens, P., Hessmann, M. (eds) Intramedullary Nailing. Springer, London. https://doi.org/10.1007/978-1-4471-6612-2_32
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6612-2_32
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6611-5
Online ISBN: 978-1-4471-6612-2
eBook Packages: MedicineMedicine (R0)