Abstract
Objective
The present study investigated the predictive diseases progression value of preoperative fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) in patients with local advanced cervical cancer (LACC).
Methods
In total, 267 patients [median age 58 (range: 27–85) years old] with LACC underwent 18F-FDG PET/CT prior to any treatment. The maximum standardized uptake values (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) of the primary lesion and metastatic lymph nodes were measured on PET/CT and correlated with clinicopathological features and progression-free survival (PFS).
Results
The median follow-up was 36.52 (range: 3.09–61.29) months. During the observation period, 80 (30.0%) patients exhibited disease progression. Univariate analysis showed that FIGO stage, concurrent chemoradiotherapy (CRT), serum level of carcinoembryonic antigen (CEA) and squamous cell carcinoma antigen (SCC-Ag), primary tumor MTV (pMTV) and TLG (pTLG), lymph nodes SUVmax (nSUVmax) and TLG (nTLG), and total metabolic activity (sMTV, sTLG) were associated with PFS. nSUVmax ≥ 5.29, CEA ≥ 7.11 ng/ml and deficiency of concurrent CRT were independent risk factor for PFS (p = 0.006, p = 0.008, p = 0.014). The 3-year PFS for patients with high nSUVmax were 42.2% compared to 56.3% for low nSUVmax values.
Conclusion
Pretreatment cervical and lymph nodes metabolic parameters were associated with PFS in patients with LACC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is one of the most common malignancies and the fourth leading cause of cancer-related death in women worldwide [1, 2]. Although often curable if detected early, almost half of the patients present locally advanced cervical cancer (LACC) at the time of diagnosis. The current standard treatment modality for patients with LACC, based on International Federation of Gynecology and Obstetrics (FIGO) staging IIB–IVA, is concomitant chemoradiation therapy (CRT) using a cisplatin-based regimen with pelvic external beam radiotherapy (EBRT) and subsequent brachytherapy (BT) [3, 4]. Despite the well confirmed contribution of CRT toward improved survival outcomes with a complete clinical response achieved in 70%–90% patients [5], about one-third of patients experience disease recurrence within 2 years after therapy completion [6]. Therefore, to optimize treatment plans and improve risk-adapted treatment strategies, data regarding whether patients can achieve long-term disease-free survival through treatment are essential.
As a functional multimodality imaging system, fluorine-18 fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) has played a well-established role in the management of patients with malignant tumors, especially in clinical staging, observation of curative effect, and analysis of the prognosis for LACC based on American National Comprehensive Cancer Network guidelines [3, 7].
Recent attention has focused on the prognostic value of metabolic parameters from pretreatment 18F-FDG PET/CT in cervical cancer, including the maximum standardized uptake value (SUVmax), mean standardized uptake value (SUVmean), metabolic tumor volume (MTV) and total lesion glycolysis (TLG). Some studies reported a longer survival in patients with negative versus positive PET findings [8], and the volume-based parameters (MTV and TLG) of the primary tumor are predictors of event-free and overall survival(OS) in cervical cancer patients treated with CRT [9, 10]. Other studies revealed that lymph node metabolic activity on 18F-FDG PET/CT can help clinicians more comprehensively judge prognosis [10]. The role of 18F-FDG PET/CT as a prognostic biomarker for patients with LACC remains controversial [11], however, and a comprehensive analysis of the relationship between semi-quantitative metabolic parameters and inferior outcomes is needed [12].
In this context, we aimed to evaluate the prognostic value of multiple metabolic parameters from preoperative 18F-FDG PET/CT in patients with LACC and to investigate the prognostic values of clinicopathologic features to stratify patients with cervical cancer and determine individualized treatment.
Materials and methods
Patients
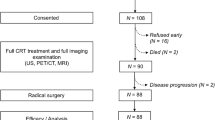
This study was approved by an Investigational Review Board of The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), the IRB number is IRB-2022-423. The requirement to acquire informed consent was waived by the Ethics Committee of Zhejiang Cancer Hospital. Consecutive patients between June 2016 and March 2019 in Zhejiang Cancer Hospital were retrospectively selected and enrolled using the following inclusion criteria: (1) histologically proven cervical cancer based on examination of biopsy specimens and staged IIB–IVA by 2014 FIGO staging; (2) determination of CRT or radical radiotherapy as the primary treatment modality; (3) no prior treatment before 18F-FDG PET/CT scan; and (4) availability of complete medical history and clinicopathological data. The exclusion criteria were as follows: (1) secondary malignant disease; (2) serious infection or inflammation(which were found out mainly based on some clinical findings, such as lab data, imaging, and their medical history.); or (3) hepatic or renal dysfunction. Data from 267 patients constituted the final clinical database. Figure 1 shows the flowchart of selection criteria.
18F-FDG PET/CT acquisitions
Whole body acquisition was performed in 2–3 min/bed using a hybrid system (GE Discovery PET/CT 710, GE Healthcare, Boston, MA, USA) that covered the area from the base of the skull to the upper thigh after intravenous injection of about 3.7 MBq/kg of 18F-FDG. All patients fasted for at least 6 h previously and presented with a blood glucose concentration < 10 mmol/L. The reconstruction used an attenuation-weighted ordered-subsets expectation maximization iterative algorithm with 4 iterations and 8 subsets, a Gaussian filter with 4.0-mm full width at half maximum, and scatter correction.
Imaging interpretation
Two experienced nuclear physicians interpreted the images and data for each patient using a GE EBW workstation. There was no significant difference between the two readers in metabolic indicators for primary tumor and positive lymph nodes, and intra class correlation coefficient (ICC) demonstrated well inter-observer agreement at 0.81 and 0.83. The SUVmax, MTV, and TLG were assessed for the primary lesion and metastatic lymph nodes, and these parameters were determined in a three-dimensional manner using the same vendor-provided software (GE). The MTV was estimated by selecting the volume of interest (VOI) on the axial image; the size of the VOI was checked on the corresponding coronal and sagittal images to ensure it included the entire active tumor in the VOI. To define the contouring margins around the target lesion, we used the percentage of SUVmax of 40% as a central value and a margin threshold to delineate the lesion edge [13]. The SUVmax was calculated with this equation: (decay-corrected activity/tissue volume)/(injected dose/body weight). The TLG was calculated by multiplying mean standardized uptake values (SUVmean) and MTV. A positive diagnostic standard for lymph node metastasis was required to meet both the lymph node short diameter > 0.5 cm and SUVmax > 2.5.
The SUVmax, MTV, and TLG of primary tumors were marked as pSUVmax, pMTV, and pTLG, respectively. The maximum SUVs of metastatic pelvic and para-aortic lymph nodes (PALN) were marked as nSUVmax; nMTV and nTLG were calculated for all metastatic lymph nodes. The sMTV was defined as the sum of pMTV and nMTV, and the sum of pTLG and nTLG was marked as sTLG.
Treatment and follow-up
All patients underwent pelvic ± lombo-aortic EBRT based the 2015 National Comprehensive Cancer Network guidelines. Delivery was by intensity-modulated radiation therapy, volumetric-modulated arc therapy, or helical tomotherapy administered to the whole pelvic region in 25–28 fractions of 1.8 Gray (Gy) for a total dose of 45–50.4 Gy. For patients with para-aortic nodal involvement, the superior border extended to the level of renal vessels or to the upper margin of the twelfth thoracic vertebra. In cases of significantly enlarged and undetected lymph nodes, additional radiotherapy with a highly conformal EBRT was required, with an additional 10–15 Gy. Concomitant chemotherapy with cisplatin 40 mg/m2 was administered weekly during EBRT for 3–6 courses. The treatment was then completed with an additional pulse dose rate BT, which was delivered with an iridium-192 source, with 24–36 Gy (biologically effective dose 38.4–57.6 Gy) in 4–6 fractions to point A. For a small number of patients, radical radiotherapy alone was administered.
Follow-up was performed 1 month after radical chemoradiotherapy, every 3–6 months for 2 years, and every 6–12 months for 3–5 years after treatment. Disease progression was determined by gynecologic examination, serum tumor markers, and enhanced abdominopelvic CT with or without follow-up 18F-FDG PET/CT(When abdominopelvic CT scan could not show suspicious lesion, PET/CT can be used in addition as for its advantage of whole-body scan and offering metabolic information.). For cases in which clinical assessment, serum tumor markers or imaging studies revealed any abnormality, additional diagnostic studies or pathological confirmation were performed to evaluate cancer progression. Progression-free survival (PFS) was calculated from the date of biopsy results to disease progression, relapse, mortality from any causes, or the most recent follow-up date.
Statistical analysis
Statistical analysis was performed using SPSS software (version 23, IBM, Armonk, NY, USA). Data were presented as median (range). The relationships between clinicopathological characteristics, PET/CT parameters, and cancer progression of were analyzed using the chi-square test. A maximally selected log-rank statistics approach was used to perform a cut-off point analysis. Univariate and multivariate analyses with clinicopathologic factors were performed to assess the association of PFS and metabolic 18F-FDG PET/CT parameters using the Kaplan–Meier method with log-rank test and Cox proportional hazards model, respectively. Survival curves were generated using Kaplan–Meier estimates, and the significance of difference between survival curves was tested using log-rank tests. All p values were 2-sided, and a value of p < 0.05 was considered to be statistically significant.
Results
Patient characteristics
A summary of patient characteristics is shown in Table 1. Based on inclusion/exclusion criteria, 267 consecutive patients were finally included in this study. The median age was 58 (27–85) years. Of the 267 cases, 255 were squamous carcinoma (SCC) and 12 were non-SCC (NSCC; adenocarcinomas or adenosquamous carcinoma).
Tumor classification by FIGO clinical stage showed stage IIB in 100 patients (37.4%), stage Ш in 161 patients (17 for ШA, 104 for ШB, 40 for IIIC), and stage IVA in 6 patients (2.2%). Almost half of the tumors (53 patients, 43.8%) showed aggressive histology (poorly). Based on 18F-FDG PET/CT, 97 patients had no lymph node metastases, whereas 170 (64.0%) patients had lymph node metastases, among whom 43 (25.3%) had both pelvic and a PALN metastasis.
Testing for serum tumor markers showed SCC antigen (SCC-Ag)-positive (≥ 10.40 ng/mL) status in 128 patients (47.9%). A high level of carcinoembryonic antigen (CEA) (≥ 7.11 ng/mL) was present in 58 patients (21.70%).
All 267 patients completed radical radiotherapy, with 21.7% receiving lombo-aortic EBRT. Most patients (94.4%) underwent high-dose-rate brachytherapy. The primary therapy was concurrent CRT for 210 patients (78.7%), and the remaining 57 patients (21.3%) received radiotherapy alone.
Disease progression prediction
The median follow-up time was 36.52 (3.09–61.29) months. At the end of follow-up, 31 patients (11.6%) had died and 49 patients (18.4%) progressed as a result of related disease during follow-up. Conversely, 187 patients remained in remission with no evidence of disease recurrence. The 3-year PFS was 51.3%.
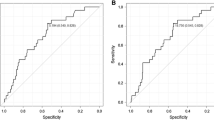
We analyzed the PET/CT images of patients with LACC and acquired the specific values of SUVmax, MTV, and TLG of the primary lesion or positive lymph nodes to predicting the PFS, considering the sensitivity and specificity for progression (Figure S1). Patient outcomes were in comparison according to the quantitative metabolic parameters of PET in Table 2. The optimal cut-off values of pSUVmax, pMTV, and pTLG were 13.70, 29.39 cm3, and 259.05, respectively. Of these metabolic parameters, only pMTV and pTLG were significantly associated with the disease progression (p = 0.001 and p = 0.013, respectively). The sensitivities and specificities of MTV were 52.5% and 69.0%, respectively, and those of TLG were 46.2% and 70.1%, respectively. For patients with lymph node metastases, nSUVmax and nTLG were significantly associated with recurrence. The best cut-off values for nSUVmax and nTLG were 5.29 and 18.66, respectively. The sMTV and sTLG also showed a significant relationship with disease progression, with cut-off values of 30.25 cm3 and 229.62, respectively. The pSUVmax and nMTV were not significant predictors of outcome in this analysis.
Based on the optimal threshold, PET parameters were dichotomized to generate Kaplan–Meier survival plots (Fig. 2). The PFS differed significantly between patients with high versus low MTV or TLG values based on the log-rank test. The 3-year PFS for patients with high versus low pMTV and pTLG values were 44.6% and 41.5% versus 55.4% and 56.6%, respectively (p = 0.001; p = 0.006). The prognostic impact of pretreatment PET with regard to 3-year PFS remained significant between the high nSUVmax and low nSUVmax groups at 42.2% and 56.3%, respectively (p = 0.001). For patients with nTLG ≥ 18.66, the 3-year PFS was significantly lower than that of those with nTLG < 18.66 (40.0% vs. 53.1%, p = 0.09). Patients with low sMTV and sTLG values had median PFS of 37.25 (3.09–61.29) months and 38.04 (3.09–61.29) months, respectively, whereas patients with high sMTV and sTLG values had median PFS of 32.93 (3.72–60.86) months and 32.59 (3.72–60.86) months respectively; this difference was statistically significant (p = 0.001; p = 0.001).
Univariate and multivariate analyses for PFS
Univariate and multivariate analyses were conducted to compare the prognostic value of histologic prognostic factors and metabolic parameters (Table 3). In univariate analysis, FIGO staging IIIA–IVA, lack of concurrent CRT experience, CEA ≥ 7.11 ng/mL, SCC-Ag ≥ 10.40 ng/mL, pMTV ≥ 29.39 cm3, pTLG ≥ 259.05, nSUVmax ≥ 5.29, nTLG ≥ 18.66, sMTV ≥ 30.25 cm3, and sTLG ≥ 229.62 were significant predictors of poor PFS (p < 0.05). Multivariate Cox regression analysis was performed to further investigate the prognostic value of all the factors just described. Results showed that concurrent CRT (HR 0.44; 95% CI 0.23–0.84; p = 0.014), CEA ≥ 7.11 ng/mL (HR 2.30; 95% CI 1.24–4.24; p = 0.008), and nSUVmax ≥ 5.29 (HR 2.46; 95% CI 1.29–4.70; p = 0.006) were independent prognostic factors for PFS (Fig. 3), whereas FIGO stage, serum SCC-Ag level, pMTV, pTLG, nTLG, sMTV, and sTLG were not independent predictors for recurrence.
Discussion
Our study aimed to explore the predictive value of PET/CT parameters and several clinicopathological features in patients with LACC for disease progression. Results showed that the SUVmax value of metastatic lymph nodes, serum CEA level, and concurrent CRT treatment may independently predict PFS in this population. The use of PET/CT combined with other clinicopathological characteristics provides a more comprehensive understanding of patient information and guidance for clinicians on formulating treatment plans.
Locally advanced cervical cancer remains a disease with a high rate of recurrence, and many patients undergo treatments that may not be beneficial. 18F-FDG PET/CT has been recognized as a useful diagnostic technique in clinical oncology. Previously an association between the intensity of FDG uptake at the cervical tumor and poor outcomes has been documented [10, 14]. Burcak et al. [15] supported that SUVmax which represents the metabolic activity of the most aggressive cells in malignant lesions, is an independent prognostic factor for disease-control survival of LACC.
Nevertheless, primary tumor SUVmax was insufficient as a reliable prognostic indicator in most studies, according to a meta-analysis [8]. Similarly, our study showed no correlation between pSUVmax and PFS in LACC. This finding may be attributed to the heterogeneity in tumor tissue, which cannot be fully reflected by SUVmax within the selected region. However, SUVmax of metastatic lymph nodes was significantly associated with PFS in our cohort.
Laurie et al. [16] reported that high SUVmax in metastatic lymph nodes was a significantly poor prognostic factor of extracervical recurrence-free survival. Won et al. [17] also suggested that SUVmax of metastatic lymph nodes as a continuous variable was a critical predictive index for disease-free survival and OS. We suspect that the high FDG uptake of cancer cells in the lymph nodes is more indicative of their high degree of invasiveness and biological activity, which leads to recurrence and poor prognosis.
Volume-based parameters, such as MTV or TLG, take into consideration both tumor volume and metabolic activity as crucial parameters of prognosis and treatment response, thus making them better prognostic factors than SUVmax. Cem et al. [18] highlighted that cervical MTV and TLG from pretreatment PET/CT were useful for predicting patient OS or PFS after chemoradiotherapy for patients with LACC. Likewise, Paulina et al. [19] demonstrated that cervical MTV was a critical prognostic factor for OS, event-free survival, locoregional control and freedom from distant metastases and should be used to guide oncologists in selecting individualized therapies. Deng et al. [20] and Paulina et al. [21] reported that total MTV was a significant predictor of recurrence and overall survival of LACC in both univariate analysis and multivariate analysis. Liang et al. [22] also proposed that total TLG measured by 18F-FDG PET/CT was obviously correlated with survival outcomes in patients with LACC. However, other studies took the opposite view and reported that further confirmation was required regarding the role of MTV and TLG to predict the prognosis of patients with cervical cancer and to develop treatment strategies [23, 24]. In our study, pMTV ≥ 29.39 cm3 (HR 2.13; 95% CI 1.37–3.31; p = 0.001), pTLG ≥ 259.05 (HR 1.84; 95% CI 1.19–2.86; p = 0.006), nTLG ≥ 18.66 (HR 2.11; 95% CI 1.19–3.74; p = 0.009), sMTV ≥ 30.25 cm3 (HR 2.08; 95% CI 1.33–3.24; p = 0.001), sTLG ≥ 229.62 (HR 2.01; 95% CI 1.30–3.13; p = 0.001) were significantly poor prognostic factors of PFS in univariate analysis. However, statistically significant associations were not found between these parameters and disease progression in multivariate analysis. We considered that the reasons for these different results may be related to the inconsistency in the definition of MTV in different studies. In addition, several study limitations may cause differences in results, such as publication bias, the small sample size of each enrolled study, and inconsistent treatment methods among different medical centers.
Several traditional clinical and pathological factors are identified as poor prognostic factors, including FIGO stage, lymph node metastasis, parametrial invasion and histological tumor type [25,26,27]. Cervical cancer contains two major histopathological types: SCC (75% of cases) and NSCC, and these two types differ in their cellularity and proliferation potential. Because of different tissue integrity and proliferation potential, SCC and NSCC may have different manifestations of the tumor metabolic burden and cell metabolic activity on PET/CT scanning [28]. Most patients in our cohort had SCC—a study characteristic that to some extent may avoid bias caused by heterogeneity of the histological subtype. In our study, high FIGO stage was associated with adverse prognosis, as indicated by Kaplan–Meier survival plots. Present of metastatic PALN was observed to have no correlation with PFS, which may be in part due to a small number of patients (16.1%) having PALN metastasis and all of these patients having undergone lombo-aortic EBRT. Meanwhile, when interpreting PET/CT results, we focused both on lymph nodes and the primary tumor and explored their role in turn. Our study indicated that nSUVmax may help identify patients at the onset of treatment who are at increased risk for relapse and death, with a threshold of 5.29.
Our study also identified serum tumor markers and radiotherapy methods for cervical cancer that may provide patient prognostic information. Results demonstrated that patients with treatment failure showed significantly higher levels of CEA or SCC-Ag than the those with long-term outcomes. The risk of recurrence was significantly increased in patients with LACC treated with radical radiotherapy alone compared with those treated with standard concurrent chemoradiotherapy and brachytherapy. These findings were consistent with those of previous research [29, 30].
Our study is unique because it includes a large number of participants (267 patients) with LACC at different disease stages, and it reviewed 12 clinicopathological indicators and 8 metabolic parameters involving metastatic lymph node information and the whole body tumor metabolic burden to explore their prognostic significance. Despite these strengths, our study has some notable limitations. First, it was a single-center study with a retrospective design, which may have biased prognostication; therefore, a prospective study in a larger cohort is necessary. Moreover, positive lymph nodes in our study were identified on PET/CT and not by histopathologic verification. In addition, aligned with the time of case inclusion, the 2014 FIGO staging classifications were used, which differ somewhat from the current new 2018 FIGO staging. Finally, combined with recent promising methods in clinical oncology, such as radiomics, PET/magnetic resonance imaging, and new imaging agents, use of 18F-FDG/PET and the clinicopathological indicators and metabolic parameters addressed in this study may yield more significant predictive values for the prognosis of cervical cancer.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49.
Buskwofie A, David-West G, Clare CA. A Review of Cervical Cancer: Incidence and Disparities. J Natl Med Assoc. 2020;112:229-32.
Abu-Rustum NR, Yashar CM, Bean S, Bradley K, Campos SM, Chon HS, et al. NCCN Guidelines Insights: Cervical Cancer, Version 1.2020. J Natl Compr Canc Netw. 2020;18:660-6.
Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv72-iv83.
Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–12.
Endo D, Todo Y, Okamoto K, Minobe S, Kato H, Nishiyama N. Prognostic factors for patients with cervical cancer treated with concurrent chemoradiotherapy: a retrospective analysis in a Japanese cohort. J Gynecol Oncol. 2015;26:12-8.
Adam JA, Loft A, Chargari C, Delgado Bolton RC, Kidd E, Schöder H, et al. EANM/SNMMI practice guideline for [(18)F]FDG PET/CT external beam radiotherapy treatment planning in uterine cervical cancer v1.0. Eur J Nucl Med Mol Imaging. 2021;48:1188-99.
Sarker A, Im HJ, Cheon GJ, Chung HH, Kang KW, Chung JK, et al. Prognostic Implications of the SUVmax of Primary Tumors and Metastatic Lymph Node Measured by 18F-FDG PET in Patients With Uterine Cervical Cancer: A Meta-analysis. Clin Nucl Med. 2016;41:34-40.
Scher N, Castelli J, Depeursinge A, Bourhis J, Prior JO, Herrera FG, et al. ((18)F)-FDG PET/CT parameters to predict survival and recurrence in patients with locally advanced cervical cancer treated with chemoradiotherapy. Cancer Radiother. 2018;22:229-35.
Han S, Kim H, Kim YJ, Suh CH, Woo S. Prognostic Value of Volume-Based Metabolic Parameters of (18)F-FDG PET/CT in Uterine Cervical Cancer: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol. 2018;211:1112-21.
Herrera FG, Breuneval T, Prior JO, Bourhis J, Ozsahin M. [(18)F]FDG-PET/CT metabolic parameters as useful prognostic factors in cervical cancer patients treated with chemo-radiotherapy. Radiat Oncol. 2016;11:43.
Akkas BE, Demirel BB, Dizman A, Vural GU. Do clinical characteristics and metabolic markers detected on positron emission tomography/computerized tomography associate with persistent disease in patients with in-operable cervical cancer? Ann Nucl Med. 2013;27:756-63.
Im HJ, Bradshaw T, Solaiyappan M, Cho SY. Current Methods to Define Metabolic Tumor Volume in Positron Emission Tomography: Which One is Better? Nucl Med Mol Imaging. 2018;52:5-15.
Han L, Wang Q, Zhao L, Feng X, Wang Y, Zou Y, et al. A Systematic Review and Meta-Analysis of the Prognostic Impact of Pretreatment Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Parameters in Patients with Locally Advanced Cervical Cancer Treated with Concomitant Chemoradiotherapy. Diagnostics (Basel). 2021;11.
Yilmaz B, Dağ S, Ergul N, Çermik TF. The efficacy of pretreatment and after treatment 18F-FDG PET/CT metabolic parameters in patients with locally advanced squamous cell cervical cancer. Nucl Med Commun. 2019;40:219-27.
Brunette LL, Bonyadlou S, Ji L, Groshen S, Shuster D, Mehta A, et al. Predictive Value of FDG PET/CT to Detect Lymph Node Metastases in Cervical Cancer. Clin Nucl Med. 2018;43:793-801.
Lee WK, Chong GO, Jeong SY, Lee HJ, Park SH, Ryu JM, et al. Prognosis-Predicting Model Based on [(18)F]fluorodeoxyglucose PET Metabolic Parameters in Locally Advanced Cervical Cancer Patients Treated with Concurrent Chemoradiotherapy: Multi-Center Retrospective Study. J Clin Med. 2020;9.
Onal C, Guler OC, Reyhan M, Yapar AF. Long-term outcomes of cervical cancer patients with complete metabolic response after definitive chemoradiotherapy. J Gynecol Oncol. 2021;32:e74.
Cegla P, Hofheinz F, Cholewiński W, Czepczyński R, Kubiak A, van den Hoff J, et al. Prognostic Value of Pretherapeutic Primary Tumor MTV from [(18)F]FDG PET in Radically Treated Cervical Cancer Patients. Metabolites. 2021;11.
Deng C, Ding D, Wang M. The predictive recurrence value of MTV-s as an 18F-FDG PET/CT index in patients with IIB-IVA cervical cancer. Postgrad Med. 2021;133:436-43.
Cegla P, Burchardt E, Roszak A, Czepczynski R, Kubiak A, Cholewinski W. Influence of Biological Parameters Assessed in [18F]FDG PET/CT on Overall Survival in Cervical Cancer Patients. Clin Nucl Med. 2019;44:860-3.
Liang Y, Li X, Wan H, Fang Y, Zheng R, Zhang W, et al. Prognostic Value of Volume-Based Metabolic Parameters Obtained by 18F-FDG-PET/CT in Patients With Locally Advanced Squamous Cell Cervical Carcinoma. J Comput Assist Tomogr. 2018;42:429-34.
Pinho DF, King B, Xi Y, Albuquerque K, Lea J, Subramaniam RM. Value of Intratumoral Metabolic Heterogeneity and Quantitative (18)F-FDG PET/CT Parameters in Predicting Prognosis for Patients With Cervical Cancer. AJR Am J Roentgenol. 2020;214:908-16.
Guler OC, Torun N, Yildirim BA, Onal C. Pretreatment metabolic tumour volume and total lesion glycolysis are not independent prognosticators for locally advanced cervical cancer patients treated with chemoradiotherapy. Br J Radiol. 2018;91:20170552.
Li X, Wei LC, Zhang Y, Zhao LN, Li WW, Ping LJ, et al. The Prognosis and Risk Stratification Based on Pelvic Lymph Node Characteristics in Patients With Locally Advanced Cervical Squamous Cell Carcinoma Treated With Concurrent Chemoradiotherapy. Int J Gynecol Cancer. 2016;26:1472-9.
Chen CC, Wang L, Lin JC, Jan JS. The prognostic factors for locally advanced cervical cancer patients treated by intensity-modulated radiation therapy with concurrent chemotherapy. J Formos Med Assoc. 2015;114:231-7.
Teh J, Yap SP, Tham I, Sethi VK, Chua EJ, Yeo R, et al. Concurrent chemoradiotherapy incorporating high-dose rate brachytherapy for locally advanced cervical carcinoma: survival outcomes, patterns of failure, and prognostic factors. Int J Gynecol Cancer. 2010;20:428-33.
Rahman T, Tsujikawa T, Yamamoto M, Chino Y, Shinagawa A, Kurokawa T, et al. Different Prognostic Implications of 18F-FDG PET Between Histological Subtypes in Patients With Cervical Cancer. Medicine (Baltimore). 2016;95:e3017.
Huang G, Chen R, Lu N, Chen Q, Lv W, Li B. Combined Evaluation of Preoperative Serum CEA and CA125 as an Independent Prognostic Biomarker in Patients with Early-Stage Cervical Adenocarcinoma. Onco Targets Ther. 2020;13:5155-64.
Fu J, Wang W, Wang Y, Liu C, Wang P. The role of squamous cell carcinoma antigen (SCC Ag) in outcome prediction after concurrent chemoradiotherapy and treatment decisions for patients with cervical cancer. Radiat Oncol. 2019;14:146.
Funding
This work was supported by Zhejiang Province Major Medical Health Science and Technology Plan (WKJ-ZJ-1814), Zhejiang Province Public Welfare Technology Application Research Project (LGF22H180013), National Natural Science Foundation of China (81902722), and Zhejiang Province Public Welfare Technology Application Research Project (GF19H160028).
Author information
Authors and Affiliations
Contributions
WQP mainly took charge of quality control of PET imaging. JLS, the first author, collected, analyzed and interpreted PET data; drafted the manuscript. HQY, JFJ and XMY collected clinical data and followed-up LACC patients. LFL, the corresponding author, responsible for the designing of the study and obtaining grants and funding to support the trial; revised the manuscript and enhanced the intellectual content of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB number: IRB-2022-423) of Zhejiang Cancer Hospital approved this study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 1, 2, 3.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
261_2023_4158_MOESM1_ESM.jpg
The ROC curve analysis of different metabolic parameters of PET for predicting disease progression in LACC. Supplementary file1 (JPG 3156 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, J., Pang, W., Yi, H. et al. Tumor and metastatic lymph nodes metabolic activity on 18F-FDG-PET/CT to predict progression-free survival in locally advanced cervical cancer. Abdom Radiol 49, 975–984 (2024). https://doi.org/10.1007/s00261-023-04158-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-04158-8