Abstract
Purpose
The aim of this prospective study was to assess the usefulness of 18F-FDG PET/CT performed before and during treatment for predicting treatment failure in patients with advanced squamous cell carcinoma of the uterine cervix treated with concurrent chemoradiotherapy (CCRT).
Methods
Patients with cervical squamous cell carcinoma, International Federation of Gynecology and Obstetrics stage III/IVA or positive pelvic or paraaortic lymph node (LN) metastasis without other distant metastasis on PET/CT entering a randomized trial of CCRT (AGOG 09-001) were eligible. PET/CT scans were performed at baseline, during week 3 of CCRT and 2 − 3 months after CCRT. PET/CT parameters were correlated with sites of failure and overall survival (OS). The resulting predictors developed from the study cohort were validated on two independent datasets using area under the curve values, sensitivities and specificities.
Results
With a median follow-up of 54 months for survivors, 20 (36 %) of the 55 eligible patients were proven to have treatment failure. Sites of failure were local in five, regional in 11, and distant in 11. Four predictors for local failure, three for regional failure, and four for distant failures were identified. After validation with two independent cohorts of 31 and 105 patients, we consider the following as clinically useful predictors: pretreatment metabolic tumour volume (MTV) and during-treatment cervical tumour MTV for local failure; during-treatment SUVnode (maximum standardized uptake value of LNs) for regional and distant failure, and during-treatment MTV for distant failure. During-treatment SUVnode (P = .001) and cervical tumour MTVratio (P = .004) were independent significant predictors of OS by stepwise Cox regression.
Conclusion
PET/CT imaging before and during treatment is useful for predicting failure sites and OS, making tailored therapeutic modifications feasible with potential outcome improvement during primary therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is the fourth most common cancer in women, with approximately 266,000 deaths worldwide in 2012 [1]. Concurrent chemoradiotherapy (CCRT) has become the mainstay of treatment for advanced cervical cancer. Unfortunately, about one-third of patients treated with CCRT will have persistent or recurrent disease following CCRT [2, 3]. PET/CT with 18F-FDG is increasingly recognized for its potential clinical utility in patients with cervical cancer [4]. In a previous prospective study, the 3-year progression-free survival rates in patients with complete metabolic response, partial metabolic response and progressive disease on posttreatment 18F-FDG PET/CT imaging were 78 %, 33 % and 0 %, respectively [5]. A pretreatment 18F-FDG scan is useful in staging and prognostic evaluation in advanced cervical cancer [4, 6, 7]. Serial 18F-FDG scans during treatment have shown a gradual decrease in cervical tumour maximum standardized uptake value (SUVmax) over time [8]. In this scenario, the monitoring of tumour response by 18F-FDG imaging before and during treatment may be clinically useful for identifying patients at high risk of treatment failure.
A phase II clinical trial has shown that the addition of gemcitabine to standard CCRT may improve clinical outcomes in patients with advanced cervical cancer [9]. A phase III clinical trial has shown that the addition of gemcitabine to cisplatin CCRT significantly improves survival compared with current standard of care [10]. Patients were treated with a combination of gemcitabine and cisplatin weekly for 6 weeks, with concurrent external-beam radiotherapy (XRT) then brachytherapy (BCT), followed by two adjuvant 21-day cycles of cisplatin plus gemcitabine, cisplatin and concurrent XRT, and BCT only without adjuvant therapy. Two years before the results of the study by Dueñas-González et al. [10] were known, a prospective, randomized phase III Asian Gynecologic Oncology Group trial (AGOG 09-001, ClinicalTrials.gov identifier NCT00842660) was designed to investigate whether the addition of gemcitabine to cisplatin in the CCRT phase (without adjuvant chemotherapy after CCRT) can improve survival in patients with advanced cervical squamous cell carcinoma [11]. Both treatment arms in the latter trial showed similar survival results. Moreover, a parallel imaging study with PET/CT imaging during treatment was devised to assess response to treatment in the AGOG 09-001 participants. Although accrual to the AGOG 09-001 trial was prematurely closed in March 2013 because of slow recruitment, overly optimistic assumption of benefit and an unexpectedly small difference in survival, the aim of examining the clinical usefulness of serial PET/CT scans in a parallel imaging study could be achieved, and the results are reported here.
Materials and methods
Patient eligibility
Inclusion criteria for the AGOG 09-001 trial included a diagnosis of primary squamous cell carcinoma of the uterine cervix and a clinical International Federation of Gynecology and Obstetrics (FIGO) stage III/IVA or clinical FIGO stage I/II with suspicious pelvic or paraaortic lymph node (LN) metastasis on MRI and verified on PET/CT imaging. The exclusion criteria were as follows: distant metastases at sites other than the paraaortic LNs; age less than 35 years or more than 70 years; inadequate bone marrow, pulmonary, liver or renal function; Eastern Collaborative Oncology Group performance status >1; or a history of chemotherapy or pelvic radiotherapy (RT). The patients randomized in the AGOG 09-001 trial were invited to participate in the parallel imaging study, which was approved by the local institutional review boards. All participants gave their written informed consent.
Concurrent chemoradiotherapy
The CCRT protocol has been previously described [11–14]. In general, patients were initially treated with XRT, delivered 5 days per week, one fraction per day and 1.8 Gy per fraction. Doses of large-field radiation to the whole pelvis were 45 Gy delivered using a conventional four-field box technique. Patients with parametrial extension or metastases to pelvic LNs received nodal and parametrial boost doses (5.4 − 12.6 Gy) using parallel-opposed anterior/posterior fields with a midline block of width 4 cm. In patients with lower vaginal tumour extension or with persistent bulky tumour after receiving a dose of 45 Gy, external beam doses to the lower pelvis were either increased to 50 − 54 Gy without a central block if followed by BCT or to 68 − 72 Gy when BCT was not used. Intracavitary high dose-rate 192Ir BCT was delivered at 4.3 Gy per fraction for six fractions, two fractions per week. In the presence of metastases to common iliac or paraaortic LNs, the irradiation field was extended to include the abdominal paraaortic region up to the T12/L1 intervertebral space. Gross nodal lesions outside the parametrial boost field were treated to a total dose of 54 to 57.6 Gy. Intensity-modulated RT was optionally offered to patients who did not receive BCT or who were treated with paraaortic irradiation.
Chemotherapy consisted of weekly intravenous infusion of cisplatin (40 mg/m2) either with or without gemcitabine (125 mg/m2) administered during the course of RT up to six cycles (arm C vs. arm CG in the AGOG 09-001 trial). Reductions in dose or withholding of chemotherapy were considered upon development of haematological toxicity (granulocyte count <1,500/mL or platelet count <100,000/mL). Completion of six courses of chemotherapy was not mandatory in patients who had completed their RT course.
MR imaging
MR images were acquired using a 1.5-T (Magnetom Vision; Siemens Medical Systems, Erlangen, Germany) or a 3.0-T system (Trio Tim; Siemens Medical Systems) using phased-array body coils and parameters as previously reported [15]. In brief, we applied T1-weighted (repetition time, TR, echo time, TE, 626/11 ms; number of signal averages 2; matrix 256 × 320; field of view, FOV, 20 cm) and T2-weighted turbo spin-echo sequences (TR/TE 5,630/87 ms; number of signal averages 3; matrix, matrix 256 × 320; FOV 20 cm) to scan the pelvis in 5-mm axial and sagittal planes. The anatomical cervical tumour volume was determined by an experienced radiologist (G. Lin) as the sum of irregular tumour regions on transaxial T2-weighted MRI slices multiplied by the slice thickness.
Serial 18F-FDG PET/CT imaging
In addition to the pretreatment PET/CT scan, PET/CT scans during and after treatment were arranged during week 3 of CCRT and between 2 and 3 months after completion of CCRT. Serial PET/CT scans in each patient were performed using the same PET/CT machine (Discovery ST16; GE Healthcare, Milwaukee, WI; or Biograph mCT; Siemens Healthcare, Erlangen, Germany) in the institution where CCRT was carried out. Each participating institution had to conform to the standardized PET/CT imaging protocol. Patients were required to fast for 6 h before examination. Imaging was started at 50 min after intravenous injection of 18F-FDG (370 MBq ±10 %). A nonenhanced CT scan from the head to the thighs was initially acquired, followed by PET imaging. All images were acquired with the patient in the supine position. PET scans were corrected for attenuation using the CT data with an ordered subsets expectation maximization algorithm.
Image interpretation and parametric quantification
All PET/CT images were interpreted and analysed centrally. Parameters were quantified on a Syngo MI Workplace software platform (Siemens Healthcare, Forchheim, Germany). SUV was defined as the image-derived radioactivity concentration normalized to the whole-body concentration (injected dose divided by patient body weight). SUVmax was defined as the maximum SUV in the volume of interest (VOI). Metabolic tumour volume (MTV) is the volume of tumour cells with increased radioactivity uptake. VOIs for the primary tumour and positive LNs were drawn manually on the images displayed on the workstation with SUVmax recorded for each VOI. The MTVs of the cervical tumours were measured using a fixed boundary SUV of 3.0. SUVnode was defined as the maximum SUVmax of all positive LNs. The SUV and MTV ratios between the studies before and during treatment were defined as:
If there were multiple positive LNs, the highest SUVratio of all positive LNs was taken as the maximum LN SUVratio. All these imaging parameters were analysed as continuous variables.
Patient follow-up and classification of failure sites
The first patient follow-up was arranged for 2 months after CCRT completion. Posttreatment MRI was routinely arranged and PET/CT was performed in patients entering the parallel study. During follow-up, biopsies were arranged in patients with incomplete regression of tumour, suspicious progression, or positive findings on posttreatment MRI or PET/CT. Further follow-up was based on the protocol used in our previous studies [11–14]. The failure sites were categorized as local (cervix, bladder or rectum), regional (pelvic or paraaortic LN), and distant (other distant sites) based on the posttreatment imaging and biopsy findings. The failure sites in a patient with treatment failure could be of one or more categories.
Independent patient cohorts for validation
Due to the limited number of patients in the study, we decided to validate the resultant predictors developed from the study in two independent cohorts. The first cohort came from a prospective study performed in Chang Gung Memorial Hospital (Linkou) with three PET/CT examinations during treatment (IRB 99-0834C) and the following inclusion criteria: (1) diagnosis of primary epithelial carcinoma of the uterine cervix without a history of other malignancy; (2) bulky primary tumour larger than 4 cm without distant metastases other than the paraaortic LNs; (3) age between 45 and 60 years; (4) scheduled for curative-intent CCRT. The second cohort consisted of patients with pretreatment PET/CT studies in Chang Gung Memorial Hospital (Linkou) from 2006 to 2014 collected retrospectively, with the following selection criteria: diagnosis of primary squamous cell carcinoma of the uterine cervix receiving CCRT without a history of other malignancy and clinical FIGO stage III/IVA or pelvic/paraaortic LN metastases on pretreatment PET/CT imaging without distant metastases outside the paraaortic LNs.
Statistical analysis
The associations between categorical variables and tumour relapse were examined using Fisher’s exact test. The Mann-Whitney U test was used to compare differences in continuous variables between different treatment arms and the significance of the associations with treatment failure and different failure sites. Receiver operating characteristic (ROC) curve analysis of significant parameters was performed to calculate the area under the curve (AUC) values with sensitivities (proportion of failures correctly predicted) and specificities (proportion of non-failures correctly predicted) determined using Youden’s index as the cut-off point. The predictive values of the predictors/cut-off points were determined using two cohorts independent of the study population. Overall survival (OS) was defined from the date of enrolment until the date of death or censored to the last date of follow-up. Univariate Cox regression analysis and stepwise multivariate Cox regression analysis using the forward Wald method for OS were performed. OS curves were generated using the Kaplan-Meier method and compared using the log-rank test between patients grouped according to significant OS predictors at the cut-off points acquired as above. Two-sided P values less than 0.05 were considered statistically significant. Statistical analyses were performed using SPSS statistical software version 19.0 (IBM Corp., Armonk, NY).
Results
Patient characteristics and failure patterns
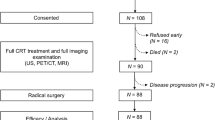
Between March 2009 and March 2013, a total of 74 patients were enrolled for random assignment in the AGOG 09-001 trial. Six patients were excluded before protocol treatment, and in 13 patients a scan was not performed during treatment or was performed at the wrong time (protocol violation). A total of 55 patients completed this parallel study (Fig. 1). The general characteristics of the participants and the associations with treatment failure are summarized in Table 1. Parameters from PET/CT and MRI between arm C and arm CG did not show significant differences and are presented in Table 2. With a median follow-up of 54 months and a minimum follow-up of 25 months in survivors, 20 of the 55 eligible patients (36 %) had treatment failure. Patients with positive paraaortic LNs had a significantly greater predisposition to treatment failure than those without (84.6 vs. 21.4 %, P < .001).
The failure sites are shown in Table 3. The numbers of patients with local, regional and distant failure are 5, 11 and 11, respectively.
Prediction of treatment failure
The parameter values obtained from PET/CT and MRI in patients without and with treatment failure are shown in Table 4. The following seven parameters were found to be significantly associated with treatment failure: pretreatment anatomical cervical tumour volume, pretreatment and during-treatment cervical tumour MTV, pretreatment and during-treatment SUVnode, cervical tumour MTVratio, and maximum LN SUVratio. The associations between imaging parameters and sites of failure are presented in Table 5. Pretreatment anatomical cervical tumour volume (P < .001), pretreatment MTV (P = .001), during-treatment MTV (P < .001), and pretreatment SUVnode (P = .043) were significantly associated with local failure. Pretreatment SUVnode (P = .023), during-treatment SUVnode (P = .001), and maximum LN SUVratio (P = .002) were significantly associated with regional failure. During-treatment MTV (P = .011), during-treatment SUVnode (P = .018), primary tumour MTVratio (P = .014), and maximum LN SUVratio (P = .025) were significantly associated with distant failure.
The AUCs, optimum cut-off values by ROC analysis and resultant sensitivities and specificities of significant parameters for predicting different failure sites are presented in Table 6. Anatomical volume and pretreatment and during-treatment cervical tumour MTV significantly predicted local failure with high AUCs (0.980, 0.964 and 0.976, respectively). Images in a patient with local failure are shown in Fig. 2. Pretreatment SUVnode predicted local and regional failure with lower AUCs. During-treatment SUVnode predicted regional and distant failure with AUCs of 0.840 and 0.756, respectively. Maximum LN SUVratio predicted regional and distant failure with AUCs of 0.811 and 0.743, respectively. During-treatment MTV (AUC 0.750) and cervical tumour MTVratio (AUC 0.742) also predicted distant failure. Images in an example patient with distant failure are shown in Fig. 3.
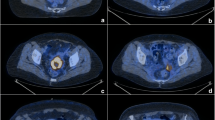
A 60-year-old patient with FIGO stage IIIB cervical cancer and no definite lymph node metastases treated with chemoradiotherapy. The PET/CT image before treatment (a) shows a bulky primary tumour with SUVmax 10.2 and MTV 151 mL. The PET/CT image during treatment (b) shows a primary tumour with SUVmax 5.8 and MTV 93 mL. The PET/CT image after treatment (c) shows residual tumour activity in the uterine cervix. Biopsy findings confirmed the presence of local failure, and surgical salvage was then performed
A 39-year-old patient with FIGO stage IIB cervical cancer treated with chemoradiotherapy. The PET image before treatment (a) shows multiple regional and paraaortic lymph node metastases (SUVnode 11.2). The PET image during treatment (b) shows residual lymph node metastases (SUVnode 8.6). The PET image after treatment (c) shows regression of regional and paraaortic lymph nodes, but also shows a new lesion in a left supraclavicular node. Aspiration of the supraclavicular lymph node confirmed the metastatic nature of the lesion, and salvage chemoradiotherapy was then performed
Validation using independent patient cohorts
The validation set 1 consisted of 31 patients with one PET/CT scan during treatment at the same time point as in the current study. Of the 31 patients, 12 (39 %) had treatment failure with a median follow-up of 73 months for survivors. The failure patterns were as follows: combined local and regional in two patients, regional only in two, combined regional and distant in three, and distant only in five. The validation set 2 consisted of 105 patients with only a pretreatment PET/CT scan . Of the 105 patients, 34 (32 %) had treatment failure with a median follow-up of 42 months for survivors. The failure patterns were as follows: local only in four patients, combined local and regional in four, combined local, regional and distant in four, regional only in four, combined regional and distant in eight, and distant only in ten. The characteristics of the patients in the study cohort and validation sets showed no significant differences (Table 7).
The validation results including AUCs, sensitivities and specificities of predictive factors determined in the current study cohort for different failure sites are shown in Table 8. For local failure prediction, pretreatment and during-treatment MTV had higher AUCs and sensitivities/specificities. For regional failure prediction, during-treatment SUVnode had higher AUC and sensitivity/specificity. For distant failure prediction, during-treatment MTV and during-treatment SUVnode had moderate AUCs and better sensitivities/specificities.
Posttreatment follow-up and overall survival
Overall, of the 55 patients, 15 (27 %) died of disease with a median follow-up of 54 months (range 25 – 73 months). The univariate Cox regression analysis demonstrated that primary tumour grade, positive paraaortic LN, pretreatment anatomical cervical tumour volume, pretreatment and during-treatment MTV, pretreatment and during-treatment SUVnode, cervical tumour SUVratio and MTVratio, and maximum LN SUVratio were significant predictors of OS (Table 9). Stepwise multivariate Cox regression analysis demonstrated that during-treatment SUVnode (P = .001) and cervical tumour MTVratio (P = .004) were independent significant predictors of OS (Table 10). OS curves of patients grouped according to during-treatment SUVnode with a cut-off value of 3.9 and cervical tumour MTVratio with a cut-off value of 38.8 % are shown in Fig. 4.
Discussion
The 5-year OS of cervical cancer patients with persistent or recurrent disease after primary treatment remains dismal (about 10 %) [3]. Curative-intent therapy is indicated only in a minority of patients with isolated relapse [16]. In this scenario, novel strategies for improving primary therapeutic outcome are eagerly sought. Compared with RT alone, primary CCRT has been shown to increase the 5-year OS from 60 % to 66 % [17]. If we can monitor early treatment response and predict failure sites during primary therapy, treatment modifications may be applied early with the hope of improving outcome. Our main goal in the current study was to identify useful pretreatment and in-treatment PET parameters which allow timely modification of therapeutic planning. Although posttreatment PET parameters have been shown to predict survival, they do not allow early and meaningful therapeutic adjustments [5, 18]. Significant parameters predictive of local, regional and distant failure identified in the current study can be incorporated into our new clinical pathway for cervical cancer treatment.
The prognostic importance of primary tumour size and volume has long been recognized [19]. A prospective study in 52 cervical cancer patients with PET/CT and MRI scans before, during and after CCRT revealed significant decreases in tumour volume both on PET/CT and MRI during CCRT [20]. In our study cohort, pretreatment cervical tumour volume was significantly associated with local failure both on MRI and PET/CT. Similar association was noted for during-treatment MTV. More intensive local therapy or post-CCRT surgery may be required in patients with larger cervical tumour volumes. Pretreatment SUVnode was associated with local and regional failure with moderate AUCs. During-treatment SUVnode was associated with regional and distant failure, and was the most significant predictor of OS. Maximum LN SUVratio was also associated with regional and distant failure with moderate AUCs. Higher during-treatment SUVnode or maximum LN SUVratio may reflect the presence of treatment-resistant tumour cells in LNs, ultimately leading to the requirement for increased regional RT doses, the addition of radiosensitizing drugs to platinum compounds, and/or adjuvant therapy. During-treatment cervical tumour MTV and MTVratio were associated with distant failure. Primary and regional tumours with limited response to therapy may show a more aggressive biological nature and higher propensity for haematogenous and distant lymphatic spread, ultimately leading to poor outcome.
Cervical cancer spreads mainly via the lymphatic system and sequentially from the primary tumour to the pelvic, paraaortic and supraclavicular LNs [21]. In the RTOG 0116 study, 26 patients with paraaortic or high common iliac LN metastasis received extended-field RT and BCT with cisplatin. Local failure occurred in 8 % and nodal failure occurred in 38 % [22]. Yoon et al. found that a complete metabolic nodal response after RT predicted better 3-year OS, whereas a complete metabolic response of the primary tumour did not [23]. Taken together, these findings highlight the paramount importance of LN control in patients with advanced cervical cancer. In our institution, the radiation dose to positive LNs is generally between 54 and 57.6 Gy. How to increase the effectiveness of LN control in patients with a high risk of regional and distant LN failure will be the topic of our new trials.
In this study, a fixed SUVmax of 3.0 was used to define the boundaries for the MTV of cervical tumours. We did not utilize a percentage tumour SUVmax threshold because this parameter decreases significantly during treatment, possibly leading to overestimation of during-treatment tumour volume [24]. Kidd et al. have suggested that pretreatment MTV and week-4 cervical tumour SUVmax are predictors of posttreatment PET response [25]. Oh et al. found that cervical tumour SUVratio after 4 weeks of CCRT was associated with progression-free survival [26]. Patient selection bias, different timing of during-treatment imaging and analysis of different parameters make the comparison of these and our studies complicated.
There were several limitations of the current study. Firstly, the numbers of patients with failure at different sites were small, consequently reducing the reliability of the findings. Validation with independent patient cohorts was thus performed to reduce type I errors. Secondly, the timing of pretreatment and during-treatment PET/CT imaging relative to the start of treatment had a range of 1 week among the patients. The heavy clinical loading of the PET/CT facility and difficulties in patient scheduling prevented this period from being reduced further. This may have had some influence on the values of the quantitative PET/CT parameters. Thirdly, the patients in the validation sets were analysed retrospectively and the treatment in these patients was less standardized, possibly reducing the resultant AUCs, sensitivities and specificities. Large-scale validation of our findings is needed.
Nonetheless, the current study was the first prospective trial to predict treatment failure in advanced cervical cancer by classification of failure sites into local, regional and distant categories, and identified the following clinically useful predictors: pretreatment and during-treatment MTV for local failure, during-treatment SUVnode for regional and distant failures, and during-treatment MTV for distant failure. During-treatment SUVnode and cervical tumour MTVratio were independent significant predictors of OS.
In conclusion, we found that pretreatment and during-treatment PET/CT imaging may be clinically useful for predicting failure sites and OS in patients with advanced cervical cancer. Based on the results of serial PET/CT scans, therapy may be tailored to the individual patient at week 3 during treatment, potentially leading to improved outcomes in patients with advanced cervical cancer.
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. Accessed 7 Nov 2015.
Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–80.
Hong JH, Tsai CS, Lai CH, Chang TC, Wang CC, Chou HH, et al. Recurrent squamous cell carcinoma of cervix after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60:249–57.
Lai CH, Lin G, Yen TC, Liu FY. Molecular imaging in the management of gynecologic malignancies. Gynecol Oncol. 2014;135:156–62.
Schwarz JK, Siegel BA, Dehdashti F, Grigsby PW. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA. 2007;298:2289–95.
Gold MA. PET in cervical cancer – implications for staging, treatment planning, assessment of prognosis, and prediction of response. J Natl Compr Canc Netw. 2008;6:37–45.
Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. The standardized uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer. 2007;110:1738–44.
Schwarz JK, Lin LL, Siegel BA, Miller TR, Grigsby PW. 18-F-fluorodeoxyglucose-positron emission tomography evaluation of early metabolic response during radiation therapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2008;72:1502–7.
Dueñas-González A, Cetina-Perez L, Lopez-Graniel C, Gonzalez-Enciso A, Gómez-Gonzalez E, Rivera-Rubi L, et al. Pathologic response and toxicity assessment of chemoradiotherapy with cisplatin versus cisplatin plus gemcitabine in cervical cancer: a randomized phase II study. Int J Radiat Oncol Biol Phys. 2005;61:817–23.
Dueñas-González A, Zarbá JJ, Patel F, Alcedo JC, Beslija S, Casanova L, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011;29:1678–85.
Wang CC, Chou HH, Yang LY, Lin H, Liou WS, Tseng CW, et al. A randomized trial comparing concurrent chemoradiotherapy with single-agent cisplatin versus cisplatin plus gemcitabine in patients with advanced cervical cancer: an Asian Gynecologic Oncology Group study. Gynecol Oncol. 2015;137:462–7.
Tsai CS, Lai CH, Chang TC, Yen TC, Ng KK, Hsueh S, et al. A prospective randomized trial to study the impact of pretreatment FDG-PET for cervical cancer patients with MRI-detected positive pelvic but negative para-aortic lymphadenopathy. Int J Radiat Oncol Biol Phys. 2010;76:477–84.
Hong JH, Tsai CS, Lai CH, Chang TC, Wang CC, Chou HH, et al. Risk stratification of patients with advanced squamous cell carcinoma of cervix treated by radiotherapy alone. Int J Radiat Oncol Biol Phys. 2005;63:492–9.
Chao A, Ho KC, Wang CC, Cheng HH, Lin G, Yen TC, et al. Positron emission tomography in evaluating the feasibility of curative intent in cervical cancer patients with limited distant lymph node metastases. Gynecol Oncol. 2008;110:172–8.
Lin G, Ho KC, Wang JJ, Ng KK, Wai YY, Chen YT, et al. Detection of lymph node metastasis in cervical and uterine cancers by diffusion-weighted magnetic resonance imaging at 3T. J Magn Reson Imaging. 2008;28:128–35.
Chao A, Lin CT, Lai CH. Updates in systemic treatment for metastatic cervical cancer. Curr Treat Option Oncol. 2014;15:1–13.
Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–12.
Schwarz JK, Siegel BA, Dehdashti F, Grigsby PW. Metabolic response on post-therapy FDG-PET predicts patterns of failure after radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2011;83:185–90.
Perez CA, Grigsby PW, Chao KSC, Mutch DG, Lockett MA. Tumor size, irradiation dose, and long-term outcome of carcinoma of uterine cervix. Int J Radiat Oncol Biol Phys. 1998;41:307–17.
Lee JE, Huh SJ, Nam H, Ju SG. Early response of patients undergoing concurrent chemoradiotherapy for cervical cancer: a comparison of PET/CT and MRI. Ann Nucl Med. 2013;27:37–45.
Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. 2001;19:3745–9.
Small W, Winter K, Levenback C, Iyer R, Gaffney D, Asbell S, et al. Extended-field irradiation and intracavitary brachytherapy combined with cisplatin chemotherapy for cervical cancer with positive para-aortic or high common iliac lymph nodes: results of ARM 1 of RTOG 0116. Int J Radiat Oncol Biol Phys. 2007;68:1081–7.
Yoon MS, Ahn SJ, Nah BS, Chung WK, Song HC, Yoo SW, et al. Metabolic response of lymph nodes immediately after RT is related with survival outcome of patients with pelvic node-positive cervical cancer using consecutive [18F]fluorodeoxyglucose-positron emission tomography/computed tomography. Int J Radiat Oncol Biol Phys. 2012;84:e491–7.
Moule RN, Kayani I, Moinuddin SA, Meer K, Lemon C, Goodchild K, et al. The potential advantages of 18FDG PET/CT-based target volume delineation in radiotherapy planning of head and neck cancer. Radiother Oncol. 2010;97:189–93.
Kidd EA, Thomas M, Siegel BA, Dehdashti F, Grigsby PW. Changes in cervical cancer FDG uptake during chemoradiation and association with reponse. Int J Radiat Oncol Biol Phys. 2013;85:116–22.
Oh D, Lee JE, Huh SJ, Park W, Nam H, Choi JY, et al. Prognostic significance of tumor response as assessed by sequential 18F-fluorodeoxyglucose-positron emission tomography/computed tomography during concurrent chemoradiation therapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2013;87:549–54.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Chang Gung Memorial Hospital-Linkou (CMRPG381141 and CMRPG381142) and the Ministry of Health and Welfare-Taiwan (DOH101-TD-B-111-005, DOH102-TD-B-111-005, and DOHW103-TDU-B-212-113003).
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Feng-Yuan Liu, Chyong-Huey Lai and Lan-Yan Yang contributed equally to this work.
A related abstract (OP460) has been previously published in the EANM’15 abstract book from the 28th Annual EANM Congress of the European Association of Nuclear Medicine (Eur J Nucl Med Mol Imaging. 2015;42:S191).
Rights and permissions
About this article
Cite this article
Liu, FY., Lai, CH., Yang, LY. et al. Utility of 18F-FDG PET/CT in patients with advanced squamous cell carcinoma of the uterine cervix receiving concurrent chemoradiotherapy: a parallel study of a prospective randomized trial. Eur J Nucl Med Mol Imaging 43, 1812–1823 (2016). https://doi.org/10.1007/s00259-016-3384-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3384-7