Abstract
Purpose
The study aimed to evaluate the relationship between intra-tumor metabolic heterogeneity parameters of 18F-FDG and KRAS mutation status in colorectal cancer (CRC) patients and which threshold heterogeneity parameters could better reflect the heterogeneity characteristics of colorectal cancer.
Methods
Medical data of 101 CRC patients who underwent 18F-FDG PET/CT and KRAS mutation analysis were selected. On PET scans, 18F-FDG traditional indices maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), total lesion glycolysis (TLG), and heterogeneity parameters coefficient of variation with a threshold of 2.5 (CV2.5), CV40%, heterogeneity index-1 (HI-1), and HI-2 of the primary lesions were obtained. We inferred correlations between these 18F-FDG parameters and KRAS mutation status.
Results
41 patients (40.6%) had KRAS gene mutation. Assessment of FDG parameters showed that SUVmax (19.00 vs. 13.16, p < 0.001), MTV (11.64 vs. 8.83, p = 0.001), and TLG (102.85 vs. 69.76, p < 0.001), CV2.5 (0.55 vs. 0.46, p = 0.006), and HI-2 (14.03 vs. 7.59, p < 0.001) of KRAS mutation were higher compared to wild-type (WT) KRAS. CV40% (0.22 vs. 0.24, p = 0.001) was lower in the KRAS mutation group, while HI-1 had no significant difference between the two groups. Multivariate analysis showed that MTV (OR = 4.97, 1.04–23.83, p = 0.045) was the only significant predictor in KRAS mutation, using a cut-off of 7.62 (AUC = 0.695), and MTV showed a sensitivity of 90.2% and specificity of 45.0%. However, the PET parameters were not independent predictors in KRAS mutation.
Conclusion
KRAS gene mutant CRC patients had more 18F-FDG uptake (SUVmax, MTV, TLG) and heterogeneity (CV2.5, HI-2) than WT KRAS. MTV was the only independent predictor of KRAS gene mutation in colorectal cancer patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is one of the worlds within the scope of the third common malignant tumor in both men and women [1]. The disease has high morbidity and mortality in developed countries, mainly Europe, while the incidence is rising in developing countries [2]. The China Cancer Statistics Report 2018 showed that the incidence and mortality of colorectal cancer in our country ranked third and fifth among all malignant tumors, with 376,000 new cases and 191,000 deaths [3]. Radical operation is currently the most valid way for colorectal cancer patients (T1-4N0-2M0) without distant metastasis. For locally advanced middle or lower rectal cancer, preoperative imaging studies suggest cT3—4 and/or N + , advice before radical surgery for neoadjuvant chemoradiation or neoadjuvant chemotherapy. However, nearly 20% of CRC patients are already in the advanced stage of the disease at the time of diagnosis, and radical surgery cannot be performed due to extensive metastasis [4]. In recent years, anti-epidermal growth factor receptor (EGFR) monoclonal therapy has made breakthroughs in colorectal treatment. Still, studies have shown that 40% of colorectal cancer patients have v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations. KRAS gene mutation predicts a lack of response to EGFR-targeted therapy in patients with metastatic CRC [5]. KRAS protein is an important signal molecule in the EGFR signal transduction pathway. The abnormal protein encoded by KRAS gene mutation is not regulated by the upstream EGFR signal. It can initiate and activate downstream signal transduction to stimulate tumor cell growth, proliferation, and metastasis. Therefore, KRAS gene status, as one of the heterogeneity characteristics of tumors is crucial for selecting treatment options for CRC patients. Prediction of KRAS mutation status by non-invasive imaging is necessary and valuable, especially for patients who cannot afford invasive testing.
18F-2-fluoro-2-deoxy-D-glucose (18F-FDG) positron emission tomography-computed tomography (PET-CT), as a whole-body non-invasive examination, is increasingly widely used in tumors. The 18F-FDG PET/CT has been proven effective for diagnosing, staging, prognosis prediction, and treatment response assessment in numerous cancers [6,7,8]. At the same time, 18F-FDG PET/CT metabolic heterogeneity evaluations can assess the heterogeneity characteristics of tumors. Regarding CRC patients, although PET/CT is not a routine recommended inspection, it is an effective assistant examination method for patients whose condition is intricated, and regular tests cannot confirm the diagnosis. The preoperative assessment suggests that colorectal cancer is stage III or higher. It can be recommended to understand whether there is distant metastasis and clarify the location and number of distant metastases. The traditional metabolic parameters of 18F-FDG PET/CT, such as standardized uptake value (SUV), metabolic tumor volume (MTV), total lesion glycolysis (TLG), etc., have been proven efficient in predicting gene mutations in colorectal cancer patients [9,10,11]. In recent years, the concepts of 18F-FDG PET/CT intra-tumor metabolic heterogeneity parameters, such as variance coefficient and linear regression slope, have been proven to reflect the characteristics of intra-tumor heterogeneity to a certain extent and play a role in predicting prognosis in some solid tumors [12,13,14]. However, a relationship between intra-tumor metabolic heterogeneity parameters and KRAS gene status in patients with colorectal cancer has not been studied yet. In addition, PET/CT can use different thresholds to measure intra-tumor metabolic heterogeneity indices, and which point can more truly reflect intra-tumor heterogeneity of CRC has not been studied.
We carried out retrospective research to evaluate the correlation between intra-tumor metabolic heterogeneity parameters of 18F-FDG and KRAS mutations in CRC and heterogeneity parameters under which threshold could reflect the heterogeneity qualities of colorectal cancer more appropriate.
Materials and methods
Study subjects
We reviewed the preoperation 18F-FDG PET/CT of 297 consecutive CRC patients in the First Affiliated Hospital of USTC from January, 2015 to December, 2021. KRAS gene mutation detection was performed in 113 of these patients. Moreover, we excluded six patients who received other treatment before PET/CT, four patients with the second primary tumor, one patient with squamous cell carcinoma; partial image data were missing in one patient. Ultimately, this study enrolled 101 patients. Inclusion criteria:(1) newly diagnosed patients who had not received radiotherapy or chemotherapy before PET/CT examination; (2) adenocarcinoma or adenocarcinoma with partial mucinous adenocarcinoma confirmed by colonoscopy or surgical pathology, and KRAS gene detection was performed; (3) patient detected serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19-9) levels within one week of PET/CT examination; (4) FDG uptake in the primary lesion was higher than the standard background. Exclusion criteria :(1) multiple primary cancers; (2) other pathological types (such as a neuroendocrine tumor, squamous cell carcinoma); and (3) incomplete case data. The staging was performed based on the Tumor-Node-Metastasis (TNM) staging system for colorectal cancer following the American Joint Committee on Cancer Guidelines (AJCC). The study was approved by the Medical Ethics Committee of the First Affiliated Hospital of USTC.(2021-RE-125).
18F‑FDG PET/CT examination
PET/CT scan has proceeded on the Siemens Biography Sensation 16 PET/CT imager (Knoxville, Tennessee, USA). 18F-FDG was automatically synthesized by Siemens Eclipse RD cyclotron and positron radiopharmaceutical synthesis module FDG 1 or ALL IN ONE, with radiochemical purity > 95%. Patients should be fasting for more than 6 h before the examination. Until the blood glucose level reached the normal range (< 11.1 mmol/L), 18F-FDG (3.7–7.4 MBq/kg) was injected intravenously into the patients. First, a low-dose CT scan was performed (DST: 100 mAs, 120 kV, pitch 0.75, slice thickness 5 mm, automatic reconstruction 3 mm, interval 5 mm, and matrix size 512 × 512), ranging from the middle part of the eye to above the upper femur. According to the CT scanning field, the PET scan was conducted in a three-dimensional model, 6–7 beds were generally collected, and collection time was 2 min per bed. Attenuation correction of PET data was performed using CT data, and the ordered subset maximum expected iteration method was used for reconstruction. Finally, PET and CT pictures were automatically generated on the workstation.
Semi-quantitative analysis of tumor PET/CT graphics
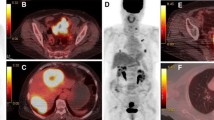
The PET/CT graphics of the primary lesion of colorectal cancer were measured by the Siemens Syngo Via workstation (Knoxville, Tennessee, USA), which measured the maximum diameter of the tumor along the intestinal tract. The 40% SUVmax was used as a cut-off threshold to establish the volume of interest (VOI) to measure the lesion's traditional metabolic parameters and volume parameters, including maximum standardized uptake value (SUVmax), MTV, and TLG (Fig. 1a). In addition, coefficient of variation (CV) and heterogeneity index (HI) were calculated. CV is the ratio of the standard deviation of SUV to SUVmean [13]. We measured CV2.5 with 2.5 of SUV as the threshold and CV40% with 40% of the SUVmax as the threshold. HI is the negative form of the linear regression slope of MTV calculated in accordance with different SUV points. 30%–70% SUVmax thresholds were used to generate MTV-based HI-1 [15]. A slight improvement of previous methods counted HI-2, different SUV points (2.5, 3.0, 3.5) were used to create MTV-based HI-2 (Fig. 1b) [16]. Two experienced nuclear medicine specialists separately reviewed images.
Measured process of 18F-FDG PET/CT metabolic parameters. Fusion PET/CT image demonstrates an 18F-FDG-active tumor in the left colon. We drew a circle (pink) to include the whole tumor, and a volume of interest (VOI; pink) was automatically generated by using a 40% SUVmax cut-off (A). CV was defined as the coefficient of variance, calculated as the SD of the SUV divided by SUVmean. Metabolic tumor volume (MTV) was assessed according to different thresholds (30%–70% of SUVmax or SUV 2.5, 3.0, and 3.5, respectively), and linear regression analysis was performed to find the slope, and heterogeneity index was the negative form of the hill (B)
KRAS mutation analysis
The samples were paraffin-embedded tissue. We used amplification transformation systems (ARMS) to analyze the mutation of the KRAS gene. The detection kit for human KRAS gene seven mutations (Wuhan Youzhiyou Medical Technology Co., LTD, China, YZYMT-001-A) was used to analysis KRAS mutation status, using the polymerase chain reaction (PCR) amplification KRAS codon 12 and 13, and the sensitivity was 1%. PCR was performed by PRISM 7500 (Applied Biosystems, Inc. American) real-time fluorescence quantitative PCR, and experienced pathologists analyzed the final chromatograms.
Statistical analysis
Each variable used The Shapiro Wilk test to test the normal distribution. Continuous data were represented by medians (interquartile ranges) (Qr) or mean ± standard deviation (SD), and categorical data were expressed as proportions. Chi-square test, t test, and Mann–Whitney U test were used to compare clinicopathological and PET indicators between KRAS mutant and KRAS wild type (WT). The optimal threshold of PET parameters that best predicted KRAS mutation status was obtained using the receiver operating characteristic (ROC) and the area under the curve (AUC) was calculated. The cut-off value for differentiating PET parameter groups was the optimal threshold of each variable. Univariate and multivariate logistic regression was conducted to analyze the predictors of gene mutation. Data analysis was executed on SPSS (version 19.0).
Result
Demographics
Basic patients’ information are summarized in Table 1. Among 101 patients, there were 69 (68.3%) males and 32 (31.7%) females. Median age was 60 years (quartile range, 49–86 years). Patients’ primary lesion locations were rectum (n = 44, 43.6%), sigmoid colon (n = 29, 28.7%), and other colon site (n = 28, 27.7%). Tumor stages were mostly III (n = 21, 20.8%) or IV (n = 71, 70.3%), with three stages I and six stages II patients. Of these 101 colorectal cancers, KRAS mutations were detected in 41 (40.6%) patients.
Comparison between KRAS mutant and KRAS WT
There was no statistical significance in clinicopathological features in gender, age, primary tumor site, tumor length, pathological grade, clinical tumor stage, lymph node metastasis, distant metastasis site, CEA, and CA-199 between the KRAS mutant patients and the KRAS wild-type patients. In the PET traditional parameters, SUVmax (19.00 vs. 13.16, p < 0.001) (Fig. 2a), MTV (11.64 vs. 8.83, p = 0.001) (Fig. 2b), and TLG (102.85 vs. 69.76, p < 0.001) (Fig. 2c) in the KRAS mutant group were significantly higher than those in the KRAS wild-type group. Heterogeneity parameters CV2.5 (0.55 vs. 0.46, p = 0.006) (Fig. 2d) and HI-2 (14.03 vs. 7.59, p < 0.001) (Fig. 2e) in the KRAS mutant group were significantly higher than those in the wild group, while CV40% (0.22 vs. 0.24, p = 0.001) (Fig. 2f) was significantly lower than that in the wild group. There was no significant difference in HI-1(p = 0.177) (Fig. 2g) between the two groups (detail in Table 2).
Predictive value
The ROC curve and area under the curve (AUC) were used to infer the potential predictive value of PET parameters on KRAS mutation status. The optimum threshold of SUVmax was 17.24 (AUC = 0.722), with sensitivity and specificity of 63.4% and 76.7% in predicting KRAS mutant (Fig. 3a). With a point of 7.62, the AUC of MTV was 0.695. MTV showed higher sensitivity of 90.2% but lower specificity of 45.0% (Fig. 3b). Using a cut-off of 68.66, the AUC of TLG was 0.749 with a sensitivity of 94.9% and specificity of 50.0% (Fig. 3c). The optimum cut-off value of CV2.5 was 0.51 (AUC = 0.661), the sensitivity was 63.4%, and the specificity was 73.3% (Fig. 3d). The AUC of HI-2 was 0.746, the sensitivity was 73.2%, and the specificity was 66.7% when using a threshold of 9.95 in predicting KRAS mutant (Fig. 3e).
With a cut-off of 0.24, the AUC of CV40% was 0.694 with a sensitivity of 73.2% and specificity of 60.0% in predicting KRAS WT (Fig. 3f).
Univariate and multivariate analysis of the predictors of KRAS mutations
Since HI-1 was meaningless in inter-group comparisons and was the same type parameter as HI-2, while CV40% was shown to predict KRAS wild type and was the same type parameter as CV2.5, these two factors were not included in the univariate and multivariate analysis for predicting KRAS mutations.
Univariate analysis demonstrated that SUVmax (OR = 5.20, 2.20–12.33, p < 0.001), MTV (OR = 7.57, 2.40–23.91, p = 0.001), TLG (OR = 19.50, 4.31–88.13, p < 0.001), CV2.5 (OR = 4.38, 1.88–10.24, p = 0.001), and HI-2 (OR = 5.46, 2.27–13.08, p < 0.001) were significantly related to KRAS mutation. On multivariate analysis, significant independent predictors of KRAS mutation were only MTV (OR = 4.97, 1.04–23.83, p = 0.045) (Table 3).
Discussion
Detection of KRAS gene expression status in CRC patients is crucial for selecting treatment regimens [17]. Molecular detection is commonly used in clinical practice for tumor surgical specimens or biopsy specimens. However, it is not suitable for every CRC patient to obtain tumor tissue specimens. A non-invasive indicator is needed to predict KRAS gene expression status in colorectal cancer to assist clinical decision making.
The current study found that MTV was an independent predictor of KRAS mutation in CRC. At the same time, PET/CT intra-tumor heterogeneity parameters failed to become independent predictors of KRAS gene mutation in multivariate analysis. We compared clinicopathological characteristics and several traditional and heterogeneity metabolic parameters of 18F-FDG PET/CT. We found that the probability of KRAS mutation in colorectal cancer was positively correlated with SUVmax, MTV, TLG, and CV2.5, HI-2, inversely correlated with CV40%, and had no significant correlation with clinicopathological parameters and HI-1. These findings suggest that KRAS mutant colorectal cancers are biologically more active and heterogeneous, with higher potential glucose metabolism and intra-tumor heterogeneity than wild-type colorectal cancers. In addition, the heterogeneity parameters CV2.5 and HI-2 are better than CV40% and HI-1 to reflect the intra-tumor heterogeneity of KRAS mutant colorectal cancer.
As far as we know, this is the first research to assess the relationship between 18F-FDG intra-tumor heterogeneity parameters and KRAS mutation status in colorectal cancer. This is also the first study to explore which PET intratumoral heterogeneity parameters at the different thresholds are more suitable to reflect colorectal cancer heterogeneity.
Our results are consistent with numerous previous studies that suggested KRAS mutation had enhanced 18F-FDG uptake and the higher the MTV, the higher the TLG. Arslan et al. [18] showed that SUVmax of patients with KRAS mutation (24.0 ± 9.0) was significantly higher than that of patients with KRAS wild type (17.7 ± 8.2) (p = 0.001). LV Y et al. [19] reached the same conclusion in their study: both MTV and SUVmax were higher in KRAS mutant patients. Arthur Cho et al. [11] pointed out that KRAS mutant had higher SUVmax (14.0 vs. 11.2, p = 0.004), SUVmean (5.3 vs. 4.7, p = 0.005), and TLG (301.4 vs. 205.5, p = 0.023) than KRAS WT. Glucose transport Protein 1 (GLUT-1) and some Hypoxia-inducible factor-1α (HIF1a) expressions are increased in KRAS mutant cells. Therefore, the increased uptake of FDG leads to an increase in SUV value [20]. MTV represents the volume of metabolically active tumors. The MTV in the KRAS mutant group is significantly higher than that in the KRAS WT group, which may be because the more prominent the tumor size, the more times of division and proliferation, and the greater the probability of random gene mutations in progeny cells [21]. TLG is the product of the entire tumor SUV and MTV, which can better reflect the burden of the whole tumor.
18F-FDG PET/CT intra-tumor metabolic heterogeneity parameters have been demonstrated to predict tumor prognosis in some solid tumors such as pancreatic cancer, gastric cancer, epithelial ovarian cancer, etc. [12,13,14,15,16], which mean that they can reflect tumor heterogeneity to a certain extent. However, no study has compared the correlation between metabolic heterogeneity parameters and KRAS mutation status so far. In our study, we found CV2.5 (0.55 vs. 0.46, p = 0.006) and HI-2 (14.03 vs. 7.59, p < 0.001) were significantly higher in the KRAS mutant patients, CV40% (0.22 vs. 0.24, p = 0.001) was significantly lower in the KRAS mutant patients, while HI-1 had no significant difference between the two groups. HI-1 use the percent threshold method. The percentage threshold method is forcefully dependent on the SUVmax, resulting in significant differences between tumor lesions with high FDG uptake [16]. This method cannot judge the heterogeneity of the lower part of the tumor's internal metabolism for tumors with higher SUVmax. HI-2 just solves this problem. With 2.5, 3.0, and 3.5 as the threshold, it can reflect the heterogeneity of the real metabolic part of the tumor with high FDG uptake. The metabolism of the masses in this group of CRC patients was high, so the percentage threshold method may not reflect the true heterogeneity. Our results are compatible with this theory. HI-2 can reflect tumor heterogeneity better than HI-1 in colorectal cancer. Early research reports mostly used the SUV value of 2.5 as a fixed threshold or a certain proportion of SUVmax, such as 40%, as a standard to outline VOI [22, 23]. We used the same thresholds to measure CV. The results of CV2.5 and CV40% in comparing the KRAS mutant group and the wild-type group were opposite. At the same time, the ROC curve found that CV2.5 had a certain value for predicting KRAS gene mutation while CV40% had a certain value for predicting KRAS WT. The underlying cause may be related to the threshold. VOI under different SUV thresholds may produce different SDs and SUVmeans, and different types of cancer have different optimal depiction thresholds for VOI. The SD of CV2.5 was larger, and the SUVmean was smaller, so the value obtained was more significant, while the SD, SUVmean, and the obtained value of CV40% were opposite. From the comparison results between groups and the ROC curve, we know that CV2.5 can better reflect tumor heterogeneity than CV40% in colorectal cancer.
Univariate analysis for predicting KRAS gene mutation showed that all PET traditional parameters and heterogeneity parameters were correlated with KRAS gene mutation. However, in multivariate analysis, MTV was the only independent predictor of KRAS gene mutation. With 7.62 as the cut-off value, the sensitivity, specificity, and accuracy were 90.2%, 45.0%, and 63.3%, respectively. The results are partially consistent with the outcomes reported in the literature [9, 19]. We failed to conclude that SUVmax and metabolic heterogeneity parameters CV2.5 and HI-2, which could better reflect intra-tumor heterogeneity in colorectal cancer, could be used as independent predictors. The reason may be the small sample size, sample distribution differences, and statistical grouping differences.
Tumor heterogeneity refers to the fact that during the growth process of tumor tissue, after multiple divisions and proliferation, its progeny cells change in molecular biology or genetic, leading to differences in the tumor's increment percent, invasiveness, and migration capability, susceptibility to medications, and the prognosis. As one of the features of malignancy, which may be related to the tumor's aggressiveness, rapid progress, ineffectiveness to treatment, and poor prognosis [24, 25]. Intratumoral heterogeneity can be characterized by spatial heterogeneity. It describes the genetic diversity of tumor subsets in different disease sites or the individual site or uneven distribution within the tumor [26]. Tumor heterogeneity research improves people's comprehension of tumor biology and promotes the development of tumor therapeutics. At the same time, quantification of tumor heterogeneity can add valuable information to imaging studies.
18F-FDG PET imaging mainly uses image-omics texture parameters (i.e., a series of parameters based on texture analysis) and traditional indicators such as SUV, MTV, and TLG to quantify intratumoral heterogeneity. Imaging omics utilize advanced image processing techniques to extract a large number of quantitative features from high-quality imaging data and construct a complete tumor marker for disease prediction and analysis through high-throughput analysis and feature selection [27]. The texture analysis method on PET images is based on the theory of radiomics, which believes that the quantitative parameters extract from medical images are related to tumor molecular phenotypes and genotypes, which can be found in more biological information. Nevertheless, texture analysis is not clinically available due to the software remaining inaccessible in most imaging viewing workstations, the lack of established evaluation criteria, and the characteristics of time consuming and complex.
Some studies pointed out that the traditional PET indicators SUV, MTV, TLG, etc. had a particular predictive value for colorectal cancer gene mutations [9,10,11]. However, there are certain limitations. SUV reflects the metabolic activity of a part or point of the tumor tissue, but it cannot recall the overall metabolism of the tumor. Although MTV and TLG make up for this shortcoming, MTV reflect many tumor cells with abnormal metabolism. TLG can reflect both tumor metabolic activity and tumor metabolic volume. However, MTV and TLG cannot distinguish the heterogeneity of different regions within the tumor. Therefore, we hope to find a more convenient and clinically feasible way to reflect the intratumoral heterogeneity through PET parameters. Several heterogeneity indices are suggested using 18F-FDG PET/CT, such as coefficient of variance or linear regression slope. These heterogeneity parameters are easy to calculate and clinically feasible and demonstrate a prognostic value in various cancer types.
Some limitations of our study should be mentioned. First, due to the small number of cases, it is impossible to conduct a more detailed analysis of the relationship between the specific mutation sites of the KRAS gene and metabolic parameters. Second, our study does not evaluate the relationship between KRAS mutations, FDG uptake, and the clinical response to EGFR treatment effects. Finally, due to the short follow-up time of some patients, it is impossible to predict further the survival period of patients whose KRAS gene mutations are predicted by 18F-FDG PET/CT metabolic parameters.
Conclusions
In summary, CRC patients with KRAS mutation had higher 18F-FDG uptake (SUVmax, MTV, TLG) and heterogeneity (CV2.5, HI-2) than WT KRAS. CV2.5, HI-2 could reflect intratumoral heterogeneity better than CV40%, HI-1 in CRC patients. MTV was the only independent predictor of KRAS gene mutation in colorectal cancer patients.
References
Sung H, Ferlay J, Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209-249. https://doi.org/10.3322/caac.21660
Stewart BC, Wild CP (2019) World cancer report 2014. Available at: http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014. Accessed 8 June 2019
National Health Commission Of The People’s Republic Of China (2020) National guidelines for diagnosis and treatment of colorectal cancer 2020 in China (English version). Chin J Cancer Res 32(4):415-445. https://doi.org/10.21147/j.issn.1000-9604.2020.04.01
Cook AD, Single R, McCahill LE (2005) Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol 12(8):637-645. https://doi.org/10.1245/ASO.2005.06.012
Karapetis CS, Khambata-Ford S, Jonker DJ et al (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359(17):1757-1765. https://doi.org/10.1056/NEJMoa0804385
Send T, Kreppel B, Gaertner FC, et al (2017) PET-CT bei Karzinomen im Kopf-Hals-Bereich [PET-CT in head and neck cancer]. HNO 65(6):504-513. https://doi.org/10.1007/s00106-017-0355-7
Zaucha JM, Chauvie S, Zaucha R, et al (2019) The role of PET/CT in the modern treatment of Hodgkin lymphoma. Cancer Treat Rev 77:44-56. https://doi.org/10.1016/j.ctrv.2019.06.002
Kitajima K, Nakajo M, Kaida H, et al (2017) Present and future roles of FDG-PET/CT imaging in the management of gastrointestinal cancer: an update. Nagoya J Med Sci 79(4):527-543. https://doi.org/10.18999/nagjms.79.4.527
Chen SW, Lin CY, Ho CM, et al (2015) Genetic alterations in colorectal cancer have different patterns on 18F-FDG PET/CT. Clin Nucl Med 40(8):621-626. https://doi.org/10.1097/RLU.0000000000000830
Lovinfosse P, Koopmansch B, Lambert F, et al (2016) (18)F-FDG PET/CT imaging in rectal cancer: relationship with the RAS mutational status. Br J Radiol 89(1063):20160212. https://doi.org/10.1259/bjr.20160212
Cho A, Jo K, Hwang SH, et al (2017) Correlation between KRAS mutation and 18F-FDG uptake in stage IV colorectal cancer. Abdom Radiol (NY) 42(6):1621-1626. https://doi.org/10.1007/s00261-017-1054-2
Kim YI, Kim YJ, Paeng JC, et al (2017) Heterogeneity index evaluated by slope of linear regression on 18F-FDG PET/CT as a prognostic marker for predicting tumor recurrence in pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging 44(12):1995-2003. https://doi.org/10.1007/s00259-017-3755-8
Lee M, Lee H, Cheon GJ, et al (2017) Prognostic value of preoperative intratumoral FDG uptake heterogeneity in patients with epithelial ovarian cancer. Eur Radiol 27(1):16-23. https://doi.org/10.1007/s00330-016-4368-5
Chung HH, Kang SY, Ha S, et al (2016) Prognostic value of preoperative intratumoral FDG uptake heterogeneity in early stage uterine cervical cancer. J Gynecol Oncol 27(2):e15. https://doi.org/10.3802/jgo.2016.27.e15
Kimura M, Kato I, Ishibashi K, et al (2019) The prognostic significance of intratumoral heterogeneity of 18F-FDG uptake in patients with oral cavity squamous cell carcinoma. Eur J Radiol 114:99-104. https://doi.org/10.1016/j.ejrad.2019.03.004
Liu G, Yin H, Cheng X, et al (2021) Intra-tumor metabolic heterogeneity of gastric cancer on 18F-FDG PETCT indicates patient survival outcomes. Clin Exp Med 21(1):129-138. https://doi.org/10.1007/s10238-020-00659-8
Lièvre A, Bachet JB, Boige V, et al (2008) KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26(3):374-379. https://doi.org/10.1200/JCO.2007.12.5906
Arslan E, Aksoy T, Gürsu RU, et al (2020) The prognostic value of 18F-FDG PET/CT and KRAS mutation in colorectal cancers. Mol Imaging Radionucl Ther 29(1):17-24. https://doi.org/10.4274/mirt.galenos.2019.33866
Lv Y, Wang X, Liang L, et al (2019) SUVmax and metabolic tumor volume: surrogate image biomarkers of KRAS mutation status in colorectal cancer. Onco Targets Ther 12:2115-2121. https://doi.org/10.2147/OTT.S196725
Iwamoto M, Kawada K, Nakamoto Y, et al (2014) Regulation of 18F-FDG accumulation in colorectal cancer cells with mutated KRAS. J Nucl Med 55(12):2038-2044. https://doi.org/10.2967/jnumed.114.142927
Marusyk A, Polyak K (2010) Tumor heterogeneity: causes and consequences. Biochim Biophys Acta 1805(1):105-117. https://doi.org/10.1016/j.bbcan.2009.11.002
Paulino AC, Koshy M, Howell R, et al (2005) Comparison of CT- and FDG-PET-defined gross tumor volume in intensity-modulated radiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys 61(5):1385-1392. https://doi.org/10.1016/j.ijrobp.2004.08.037
Devic S, Tomic N, Faria S, et al (2010) Defining radiotherapy target volumes using 18F-fluoro-deoxy-glucose positron emission tomography/computed tomography: still a Pandora’s box?. Int J Radiat Oncol Biol Phys 78(5):1555-1562. https://doi.org/10.1016/j.ijrobp.2010.02.015
Asselin MC, O’Connor JP, Boellaard R, et al (2012) Quantifying heterogeneity in human tumours using MRI and PET. Eur J Cancer 48(4):447-455. https://doi.org/10.1016/j.ejca.2011.12.025
Gerlinger M, Rowan AJ, Horswell S, et al (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing [published correction appears in N Engl J Med. 2012 Sep 6;367(10):976]. N Engl J Med 366(10):883-892. https://doi.org/10.1056/NEJMoa1113205
Dagogo-Jack I, Shaw AT (2018) Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 15(2):81-94. https://doi.org/10.1038/nrclinonc.2017.166
Lambin P, Rios-Velazquez E, Leijenaar R, et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48(4):441-446. https://doi.org/10.1016/j.ejca.2011.11.036
Funding
This study was supported by the National and provincial key specialty construction plan (Grant number: Z155080000004), Anhui Provincial Key Research and Development Program Project, China (1804h08020294), and Anhui Provincial Natural Science Foundation for Youths, China (1908085QH364).
Author information
Authors and Affiliations
Contributions
GYG and WFL conceived of the presented idea. XL wrote the original draft. SCW performed the computations. MN and YFZ collected data. MN and QX performed manuscript editing. All authors discussed the results and contributed to the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The article does not contain any studies with human participants or animals performed by any of the authors. The research protocol was approved by the Medical Ethics Committee of the First Affiliated Hospital of USTC (2021-RE-125).
Appendix publication statement
Our manuscript has not been published elsewhere and that it has not been submitted simultaneously for publication elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Wang, SC., Ni, M. et al. Correlation between 18F-FDG PET/CT intra-tumor metabolic heterogeneity parameters and KRAS mutation in colorectal cancer. Abdom Radiol 47, 1255–1264 (2022). https://doi.org/10.1007/s00261-022-03432-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03432-5