Abstract
Purpose
The purpose of this study is to evaluate the correlation between KRAS mutation, 18F-FDG uptake, and metastatic pattern in advanced stage colorectal cancer (CRC) patients.
Methods
Medical records of stage IV CRC patients who underwent 18F-FDG PET/CT for staging and KRAS mutation analysis were selected. On PET scans, a volume of interest (VOI) was drawn on the primary lesion. 18F-FDG indices (SUVmax, SUVmean, MTV, TLG) of the primary lesions were obtained and correlated with KRAS mutation of the primary lesion. Also, metastatic sites were recorded. Association between metastatic pattern and KRAS expression and FDG indices were analyzed.
Results
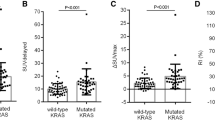
KRAS mutation was positive in 40 (43%) patients. Evaluation of FDG indices showed that higher SUVmax (14.0 vs. 11.2, p = 0.004), higher SUVmean (5.3 vs. 4.7, p = 0.005), and higher TLG (301.4 vs. 205.5, p = 0.023) were predictive of KRAS mutation compared to wild-type (WT) KRAS. Lung metastasis was more frequently involved in patients with KRAS mutation (50.0% vs. 22.6%, p = 0.006), and liver metastasis was more frequently involved in patients with WT KRAS (81.1% vs. 55.0%, p = 0.007). Multivariate analysis showed that primary tumor location (OR 3.92, p = 0.07) and KRAS mutation (OR 2.45, p = 0.09) were significant factors in lung metastasis model.
Conclusion
KRAS mutation patients had more frequent lung metastasis and had higher 18F-FDG uptake compared to WT KRAS in stage IV CRC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Colorectal cancer (CRC) is the third most frequently diagnosed cancer in the world [1]. In many Asian countries, colorectal cancer incidence and mortality levels have also increased due to the trends and availability of Western diet. However, advances in target therapy have significantly increased patient survival, and recently, Kirsten rat sarcoma (KRAS) mutation status has become an important factor in colorectal cancer treatment. Studies have shown that colorectal cancer patients with KRAS mutation have poorer therapeutic response to anti-epidermal growth factor receptor (EGFR) monoclonal therapy [2–5]. As approximately 40% of CRC has KRAS mutation, KRAS gene mutation evaluation has become an important step in planning therapeutic strategies.
18F-FDG PET-CT scan is established modality in many cancer types and has been shown to be useful in cancer staging, therapy response, and prognosis. 18F-FDG, a radionuclide glucose analogue, is accumulated in cancer cells with increased GLUT1 and hexokinase II expression. In colorectal cancer cell lines, in vitro studies suggest that low-glucose environment drives KRAS and BRAF mutations during tumorigenesis, which in turn, increases of GLUT1 expression and increases glucose uptake [6]. Only a few clinical studies have evaluated 18F-FDG uptake and KRAS mutation in CRC. Most studies suggest a positive correlation between increased 18F-FDG with KRAS mutations [7, 8]. One study has reported the lack of correlation between colorectal cancer with KRAS mutation and 18F-FDG PET-CT in Caucasian patients [9].
The most clinically useful role of 18F-FDG PET/CT in CRC diagnosis is the detection of additional metastasis in potentially surgically curable M1 disease [10, 11]. Additionally, a previous meta-analysis suggests that 18F-FDG PET/CT shows similar sensitivity as MRI in detection of liver metastasis [12]. Clinical treatment of metastatic CRC with wild-type (WT) KRAS is recommended as first-line treatment with doublet therapy in the ESMO practice guidelines, and second or third line therapy in NCCN guidelines [11, 13]. Therefore, correlation analysis of KRAS mutation with 18F-FDG uptake in advanced stage CRC may be more clinically relevant.
Due to the rising importance of KRAS mutation profiling in patient treatment and prognosis, previous studies have evaluated the clinical factors correlating with KRAS mutation. Preclinical studies have shown that KRAS mutation promotes tumor invasion and metastasis, a basis to which a few clinical studies have shown a correlation between metastatic dissemination patterns with KRAS mutation [14], as CRC patients with lung metastasis have more incidences of KRAS mutation compared to patients without lung metastasis [15]. Because FDG PET/CT is routinely used in metastasis evaluation, we undertook this study to evaluate metastatic patterns in advanced stage CRC as well as evaluate FDG uptake in the primary lesion and to evaluate for correlation with KRAS mutation status.
Materials and methods
Patients
Between 2009 and 2014, medical charts were reviewed for patients who underwent 18F-FDG PET/CT for colorectal cancer staging. A total of 184 patients were selected, and of those, 113 patients (61.4%) underwent KRAS mutation analysis in the primary lesion. Of these 113 patients, 93 patients (82.3%) had synchronous metastasis detected on MRI, CT, or PET/CT. The diagnosis of colorectal cancer was confirmed by pathologic examination at the primary colorectal lesion.
18F-FDG PET/CT protocol and imaging analysis
All patients underwent routine 18F-FDG PET/CT either with DSTe PET/CT (GE Healthcare) or with Biograph TruePoint 40 PET/CT (Siemens Medical Systems, CTI, Knoxville, TN, USA). All patients fasted for at least 6 h, and glucose levels in the peripheral blood in all patients were confirmed to be 140 mg/dL or less before the 18F-FDG injection. 18F-FDG (approximately 5.5 MBq/kg (0.149 mCi/kg) of body weight) was administered intravenously 1 h before image acquisition. After the initial low-dose CT (DSTe: 30 mAs, 130 kVp, Biograph TruePoint: 36 mAs, 120 kVp), a standard PET imaging protocol from the neck to the proximal thighs with an acquisition time of 3 min/bed in a three-dimensional mode was undertaken. Images were then reconstructed using the ordered subset expectation maximization (2 iterations, 20 subsets).
Images were separately reviewed by two experienced nuclear medicine specialists on a GE AW 4.0 workstation (GE Healthcare, Milwaukee, WI, USA). Identification of the primary lesion was done by reviewing contrast-enhanced CT or MR images. On PET scans, a volume of interest (VOI) was drawn on the primary lesion. An absolute SUV of 2.5 was used as a cut-off threshold for VOI measurements. The maximum standard uptake value (SUVmax), mean SUV (SUVmean), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) of the primary lesion were then obtained and recorded. Maximum SUV of the VOI was calculated as (decayed corrected activity/tissue volume)/(injected dose/body weight). TLG was calculated by the multiplication of MTV with the SUVmean within the VOI.
KRAS mutation analysis
KRAS mutations were determined by the following methods. DNA was extracted from the primary tumor tissue paraffin sections using the PNAClamp™ KRAS Mutation Detection kits (Panagene, Inc., Daejeon, Korea). KRAS codon 12 and 13 was amplified using polymerase chain reaction and KRAS mutation was analyzed.
Statistical analysis
All values are presented as mean ± SD. The statistical significance of differences in Table 2 was determined by the χ 2 test or Mann–Whitney U test. All analyses were 2-sided, and a p value of less than 0.05 was considered statistically significant. Receiver operation characteristic (ROC) curve analysis was performed to evaluate SUV cut-off values that best predicted KRAS mutation with diagnostic accuracy. Differences in PET indices between mutated and WT KRAS were tested by a Mann–Whitney U test. Univariate logistic regression was performed to evaluate clinically relevant factors associated with KRAS mutation: age, gender, CEA level, histologic grade, and PET-derived metabolic indices. Metastatic pattern was also analyzed and correlated with KRAS mutation. To determine the factors associated with specific organ metastasis for liver and lung and KRAS mutational status in Table 3, multivariate logistic regression analysis was performed, and factors with a p value of less than 0.10 was included in the model. All statistical computations were performed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA).
Results
Patient population
A total of 93 patients (46 female, mean age 60.0 ± 12.6, range 32–86 year old) were included in this study. Primary lesion was distributed to rectum (n = 25, 26.9%), sigmoid colon (50, 53.8%), and other colon site (n = 18, 19.4%). KRAS mutations were identified in the primary lesion in 40 patients (43.0%). CEA was performed in 84 patients (90.3%), with an overall median value of 34.9 ng/dl (range 0.6–20,000 ng/dl). The T-stage of patients were mostly either T-stage III (n = 67, 72%) or T-stage IV (n = 24, 26%), with one T-stage I and one T-stage II patients. The majority of pathologic subtype was moderately differentiated (MD) (78%), well differentiated (WD) (17%), and poorly differentiated (PD) (4%). Twenty-three patients had no LN metastasis (24%); 35 patients had regional LN metastasis (37.6%); and 35 patients had distant LN metastasis (37.6%). All the patients had synchronous metastasis at time of PET/CT; 41 patients (44.1%) had single-organ metastasis (AJCC 7th edition M1a); and 52 patients (55.9%) had multiple organ metastasis (AJCC 7th edition M1b). Table 1 shows the demographics of patients.
Analysis of metastatic site patterns showed that the liver was the most frequently involved organ (n = 65, 69.9%), and besides distant LN metastasis, the lung was the next most frequently involved organ (n = 32, 34.4%). Other frequent metastatic sites were peritoneum (n = 23, 24.7%), bone (n = 13, 14.0%), and ovary (n = 6, 6.5%). Operation was performed on 45 patients (48%); of those, 17 patients underwent neoadjuvant chemotherapy.
Factors associated with 18F-FDG parameters and KRAS mutation
A cut-off level was determined by ROC curve analysis for SUVmax, SUVmean, MTV, and TLG; the best discriminative values between those tumors with WT and mutated KRAS were calculated. Correlation with KRAS with 18F-FDG uptake showed that mutated KRAS CRC was significantly more higher compared to WT KRAS (Table 2). ROC analysis showed that SUVmax cut-off of 12.3 (area under the curve (AUC) 0.668 ± 0.06, p = 0.004) showed sensitivity of 62.5% (25/40), specificity of 69.8% (37/53), and accuracy of 66.7% (62/93) in predicting KRAS mutation. Using a cut-off of 4.5 (AUC = 0.647 ± 0.06, p = 0.011), SUVmean showed higher sensitivity of 80% (32/40), but lower specificity of 50.9% (27/53), and accuracy of 63.4% (59/93). A TLG cut-off of 190.2 (AUC = 0.629 ± 0.06, p = 0.03) showed a sensitivity of 65% (26/40), specificity of 64.2% (34/53), and accuracy of 64.5% (70/93) in predicting KRAS mutation. MTV was not significantly different between KRAS mutation and WT (55.6 ± 42.4 vs. 41.9 ± 28.1, p = 0.064).
Correlation between KRAS mutation with clinical factors
Chi-squared analysis was performed to evaluate KRAS mutation with clinical findings. Univariate analysis showed that age (<65, ≥65), gender, histologic grade (WD, MD, and PD), T-stage (I-III, IV) and LN metastasis (no LN metastasis, regional, and distant metastasis) were not significant. CEA was not significant, but more patients with KRAS mutations tended to have higher CEA levels (32/38, 84.2%) than patients with WT KRAS (33/46, 71.7%, p = 0.161).
Metastatic patterns were categorized according to metastatic organ and number to evaluate for predilection of metastatic site in KRAS-mutated CRC. There was no significant difference in single or multi-organ metastasis in KRAS mutation compared to WT KRAS (p = 0.07). Patients with lung metastasis were more likely to have mutated KRAS compared to WT KRAS (50% vs. 22.6%, p = 0.006). Liver metastasis were more likely to harbor WT KRAS compared to mutated KRAS (81.1% vs. 55.0%, respectively, p = 0.006).
Multivariate logistic regression was performed to evaluate significant clinical and PET indices in predilection distant metastatic organ, lung/liver metastasis (Table 3). Due to multicollinearity of metastasis involved organ, two models were used to evaluate factors that were correlated with KRAS mutation. In both models, primary tumor location, multi-organ metastasis, and KRAS mutation were significantly correlated with lung/liver metastasis. The primary tumor location in sigmoid colon had highest odds ratio (OR) in lung metastasis (3.924, p < 0.07). The KRAS mutation had higher OR in lung metastatic model (2.446, p = 0.094). The SUVmax was not significantly correlated with predilection for lung/liver metastasis. Considering distant metastatic pattern, patients harboring mutant KRAS tended to metastasis to lung rather than liver. In contrast, WT KRAS tended to metastasis to the liver than to lung (Figs. 1, 2).
Representative cases of metastatic patterns according to KRAS status. A Multiple intensity projection (MIP) of 61-year-old female with CRC with liver metastasis. B FDG uptake is intense in the primary mass (SUVmax: 16.1; SUVmean: 6.1, TLG: 682.5). C Patient had multiple huge liver metastasis. Biopsy results showed adenocarcinoma, moderately differentiated, and wild-type KRAS. D MIP of 47-year-old female with primary sigmoid colon cancer with lung metastasis. E Intense FDG uptake in the primary sigmoid colon mass (SUVmax: 15.5, SUVmean: 5.4; TLG 356.0). F Multiple lung metastasis is seen. Biopsy resulted in adenocarcinoma, well differentiated, and KRAS mutation
Discussion
Determination of KRAS mutation has become an important gene mutation in colorectal cancer treatment determination. Previous studies have shown that patients with WT KRAS showed good response to anti-EGFR therapy in colorectal cancer. NCCN guidelines suggest that EGFR monoclonal antibody is recommended only in KRAS WT colorectal cancer patients [13]. Therefore, KRAS mutation analysis will be most clinically useful in patients with stage IV CRC.
Accordingly, we have evaluated the correlation between KRAS mutation and FDG uptake in stage IV CRC patients and shown that patients with KRAS mutation has higher 18F-FDG uptake compared to WT KRAS. Our results were concordant with previous studies that suggest KRAS mutation is associated with increased 18F-FDG uptake. Chen et al. also reported that patients with mutated KRAS had 1.23-fold higher SUVmax compared to WT KRAS, with an accuracy of 71.4% [8]. Kawada et al. also reported significantly higher FDG uptake in mutated KRAS, with an accuracy of 75% when using SUVmax cut-off of 13 or 14 [7]. In vitro and in vivo studies have shown that CRC with KRAS mutation increased 18F-FDG uptake by GLUT1 upregulation, and by partially up-regulating HIF1a. [16]. Higher 18F-FDG uptake in the primary lesion suggests more aggressive phenotype, with increased propensity for LN or distant metastasis. We selected CRC metastasis group to include a more homogenous group of aggressive CRC, to evaluate whether KRAS mutation is associated with increased 18F-FDG uptake in the similar clinical setting.
We have also shown that metastatic pattern differs according to KRAS mutation. All of our patients had synchronous metastasis during PET/CT, and in our patient population, KRAS mutation patients had more frequent lung metastasis, and WT KRAS had more frequent liver metastasis. Our findings are in concordance with two previous studies on CRC with KRAS mutation. These studies have also shown that patients with KRAS mutation have more frequent lung metastasis compared to WT KRAS [14, 15]. Although the mechanisms for organ predilection according to KRAS mutation were not elucidated in these studies or in our studies, these findings suggest that 18F-FDG PET/CT will be useful in CRC staging and follow-up, as the whole body is evaluated during 18F-FDG PET/CT.
Our findings suggest that 18F-FDG PET/CT may be especially useful in KRAS-mutated stage IV CRC, as higher FDG uptake in KRAS mutation tumors will help in better visibility of metastatic lesions. This may potentially help in visualization of smaller lung metastasis, as KRAS mutation is suggested to have more frequent lung metastasis. Knowledge of KRAS mutation and associated potential metastatic spread pattern may help nuclear medicine physicians and radiologists during reading PET/CT scans.
Our study has several limitations. First, although SUVmax was significantly higher in KRAS mutation group, the SUV overlap between the two groups is too large for SUVmax to be used as clinical index for KRAS mutation evaluation. Second, the clinical impact of KRAS mutation and FDG uptake in EGFR therapy response was not evaluated in our study. Future studies evaluating the association between SUV and KRAS in evaluation of therapy response to EGFR treatment may show a relationship between 18F-FDG avidity and EGRF response within KRAS mutation positive CRCs. Third, Evaluation of KRAS mutation in metastatic sites was not performed, which may influence FDG uptake. Further studies evaluating KRAS mutation in CRC metastatic sites, correlated with 18F-FDG uptake, is needed to fully evaluate to potential of 18F-FDG PET/CT in evaluation for KRAS mutation.
In conclusion, in stage IV CRC patients, patients with KRAS mutation has higher 18F-FDG uptake compared to WT KRAS and had more frequent lung metastasis.
References
Torre LA, Bray F, Siegel RL, et al. (2015) Global cancer statistics 2012. CA Cancer J Clin 65(2):87–108
Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357(20):2040–2048
Ku GY, Haaland BA, de Lima LopesGJr (2012) Cetuximab in the first-line treatment of K-ras wild-type metastatic colorectal cancer: the choice and schedule of fluoropyrimidine matters. Cancer Chemother Pharmacol 70(2):231–238
Lievre A, Bachet JB, Boige V, et al. (2008) KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26(3):374–379
Karapetis CS, Khambata-Ford S, Jonker DJ, et al. (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359(17):1757–1765
Yun J, Rago C, Cheong I, et al. (2009) Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 325(5947):1555–1559
Kawada K, Nakamoto Y, Kawada M, et al. (2012) Relationship between 18F-fluorodeoxyglucose accumulation and KRAS/BRAF mutations in colorectal cancer. Clin Cancer Res 18(6):1696–1703
Chen SW, Chiang HC, Chen WT, et al. (2014) Correlation between PET/CT parameters and KRAS expression in colorectal cancer. Clin Nucl Med 39(8):685–689
Krikelis D, Skoura E, Kotoula V, et al. (2014) Lack of association between KRAS mutations and 18F-FDG PET/CT in Caucasian metastatic colorectal cancer patients. Anticancer Res 34(5):2571–2579
Culverwell AD, Chowdhury FU, Scarsbrook AF (2012) Optimizing the role of FDG PET-CT for potentially operable metastatic colorectal cancer. Abdom Imaging 37(6):1021–1031
Van Cutsem E, Nordlinger B, Cervantes A, et al. (2010) Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann Oncol 21(Suppl 5):v93–v97
Niekel MC, Bipat S, Stoker J (2010) Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 257(3):674–684
Network NCC Clinical practice Guidelines in Oncology, Colon cancer, version 3.2015. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf Accessed August 23, 2014
Kim MJ, Lee HS, Kim JH, et al. (2012) Different metastatic pattern according to the KRAS mutational status and site-specific discordance of KRAS status in patients with colorectal cancer. BMC Cancer 12:347
Cejas P, Lopez-Gomez M, Aguayo C, et al. (2009) KRAS mutations in primary colorectal cancer tumors and related metastases: a potential role in prediction of lung metastasis. PLoS ONE 4(12):e8199
Iwamoto M, Kawada K, Nakamoto Y, et al. (2014) Regulation of 18F-FDG accumulation in colorectal cancer cells with mutated KRAS. J Nucl Med 55(12):2038–2044
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by the Grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A110853).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
The institutional review board approved this retrospective study. Informed consent was waived.
Rights and permissions
About this article
Cite this article
Cho, A., Jo, K., Hwang, S.H. et al. Correlation between KRAS mutation and 18F-FDG uptake in stage IV colorectal cancer. Abdom Radiol 42, 1621–1626 (2017). https://doi.org/10.1007/s00261-017-1054-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1054-2