Abstract
The present study aimed to investigate the prognostic value of intra-tumor metabolic heterogeneity on 2-[18F] Fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) for patients with gastric cancer. Fifty-five patients with advanced gastric cancer that had received neoadjuvant chemotherapy and radical surgery were included. Clinicopathological information, 18F-FDG PET/CT before chemotherapy, pathological response, recurrence or metastasis, progression-free survival (PFS), and overall survival (OS) of the patients were collected. The maximum, peak, and mean standardized uptake values (SUVmax, SUVpeak, and SUVmean), tumor-to-liver ratio (TLR), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) on PET/CT were measured. Heterogeneity index-1 (HI-1) was calculated as SUVmean divided by the standard deviation, and heterogeneity index-2 (HI-2) was evaluated through linear regressions of MTVs according to different SUV thresholds. Associations between these parameters and patient survival outcomes were analyzed. None of the parameters on PET were associated with tumor recurrence. Pathological responders had significantly smaller TLR, MTV and HI-2 values than non-responders (P = 0.017, 0.017 and 0.013, respectively). In multivariate analysis of PFS, only HI-2 was an independent factor (hazard ratio [HR] = 2.693, P = 0.005) after adjusting for clinical tumor-node-metastasis (TNM) stage. In multivariate analysis of OS, HI-2 was also an independent predictive factor (HR = 2.281, P = 0.009) after adjusting for tumor recurrence. Thus, HI-2 generated from baseline 18F-FDG PET/CT is significantly associated with survival of patients with gastric cancer. Preoperative assessment of HI-2 by 18F-FDG PET/CT might be promising to identify patients with poor prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is one of the most aggressive tumors and has a dismal prognosis. The 5-year survival rate has been less than < 30% [1]. Preoperative neoadjuvant chemotherapy has demonstrated significant value for patient survival [2,3,4]. However, there is no practical method that can precisely identify patients with unfavorable treatment responses for intensified treatment, such as adjuvant chemotherapy or target therapy. The current prognostic model, which relies mainly on the tumor-node-metastasis (TNM) staging system, seems insufficient.
In recent years, 2-[18F] Fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) has demonstrated efficiency in staging, detecting recurrence, assessing treatment response, and predicting prognosis in patients with gastric cancer [5,6,7,8,9]. In particular, many image-derived biomarkers in PET/CT have been reported to be effective to predict survival outcomes, such as the maximum standardized uptake value (SUVmax), peak SUV (SUVpeak), and the tumor-to-liver ratio (TLR) of SUV [7, 10]; as well as volumetric parameters, including the metabolic tumor volume (MTV) [7, 8, 11] and the total lesion glycolysis (TLG) [8, 11]. Furthermore, some studies have demonstrated positive associations between treatment response and SUVmax in patients with gastric cancer [5, 12]. However, none of these parameters is able to reflect the intra-tumor metabolic heterogeneity.
Malignant tumors commonly demonstrate a heterogeneous growth pattern, even those of the same pathological type and stage. Intra-tumor heterogeneity has been demonstrated to correlate with treatment failure and worse patient outcome [13,14,15,16]. Several heterogeneity indices from 18F-FDG PET/CT, such as the coefficient of variance [17] and the slope of linear regression [18, 19], have been reported in previous studies to show prognostic value in various cancers, such as pancreatic, breast, cervical and oral cavity cancers [18, 20,21,22]. However, the prognostic value of these heterogeneity indices has not been studied in gastric cancer.
In the present study, we aimed to investigate the prognostic value of heterogeneity parameters from 18F-FDG PET/CT for patients with gastric cancer in comparison with conventional 18F-FDG PET/CT prognostic parameters.
Methods
This study was approved by the Ethics Committee of Zhongshan Hospital of Fudan University (approval number: IRB2015-098). Written informed consent was obtained from all patients.
Study subjects
In this study, 55 patients with gastric cancer, who had received neoadjuvant chemotherapy followed by radical surgery, were included. The chemotherapy regimen was one of the following: Oxaliplatin and 5-FU; oxaliplatin and capecitabine; epirubicin, oxaliplatin and capecitabine; docetaxel, oxaliplatin and 5-FU; or docetaxel and oxaliplatin. The inclusion criteria are as follows: (1) Pathologically confirmed gastric adenocarcinoma; (2) clinical staging was cT2N3M0 or cT3-4N + M0; (3) the patient underwent 18F-FDG PET/CT before chemotherapy; (4) the tumor in situ was FDG avid with an SUVmax > 3.5. The exclusion criteria are as follows: (1) A second primary malignancy; (2) another life threatening illness.
Clinicopathologic information
The clinicopathological information of the patients was recorded. According to the Japanese classification of gastric cancer [23], all tumors were classified into well differentiated adenocarcinoma (WDA, including papillary adenocarcinoma and tubular adenocarcinoma), moderately differentiated adenocarcinoma (MDA), poorly differentiated adenocarcinoma (PDA), signet-ring cell carcinoma (SRC), and mucinous adenocarcinoma (MAC). In addition, the tumors were classified into two types by the Lauren classification: Intestinal and non-intestinal (including diffuse, mixed, and unclassifiable types).
18F-FDG PET/CT scanning
After fasting for at least 6 h, patients were injected with 18F-FDG (3.7 MBq/kg; range, 240.5–488.4 MBq). PET/CT scanning was performed approximately 1 h (63.1 ± 12.5 min) later using a hybrid GE Discovery VCT 64 PET/CT scanner (General Electric, Milwaukee, WI, USA). Helical CT acquisition was performed initially from the skull base to the proximal thigh in a supine position (200 mAs; 120 kV; collimation, 64 × 0.6 mm; matrix size, 512 × 512; scanning time, 0.8 s per rotation; slice thickness, 1.5 mm; increments, 1.25 mm). PET acquisition of the same area was performed for 2 min every bed position in three-dimensional mode, and images were reconstructed using ordered-subsets expectation maximization iterative reconstruction.
Analysis of PET/CT images
Metabolic and volumetric parameters were measured by a reviewer who had more than 5 years of working experience on a uWS-MI R001 workstation (United Imaging, Shanghai, China), by creating a volume of interest (VOI) using an isocontour threshold of SUV ≥ 2.5. The SUV was calculated as: SUV = (activity in the volume of interest [Bq/g])/(injected dose [Bq]/body weight [g]). The metabolic parameters included SUVmax, SUVmean, and SUVpeak. SUVpeak was the mean SUV from a fixed 1-cm3 spherical VOI centered over the highest metabolic part of the tumor. In addition, the tumor-to-liver ratio (TLR) was calculated as the SUVmax of gastric cancer divided by the SUVmean of the liver. The volumetric parameters included MTV (cm3) and TLG (g). MTV was the sum of the metabolic volume, while TLG was the product of MTV and SUVmean.
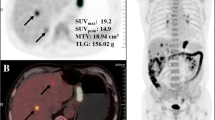
In addition, two heterogeneity indices were calculated: 1) Heterogeneity index-1 (HI-1), namely the coefficient of variance, which was the ratio of the standard deviation (SD) of SUV to SUVmean as described previously [22, 24]; and 2) heterogeneity index-2 (HI-2), which was the negative form of the slope of the linear regression of MTV according to various SUV thresholds (2.5, 3.0 and 3.5), calculated by a slight modification of previous methods [18, 20, 21], as shown in Fig. 1.
Process of measuring metabolic heterogeneity indices on 18F-FDG PET/CT. a Fused PET/CT image showing an 18F-FDG avid tumor in the cardiac area of the stomach. A rectangle (blue) was drawn to include the whole tumor and an isocontour volume of interest (VOI; red) was automatically generated by using a SUV cutoff of 2.5. Heterogeneity index-1 was defined as the coefficient of variance, which was calculated as standard deviation of the SUV divided by SUVmean. b Metabolic tumor volumes (MTVs) were calculated at three different SUV cutoffs (2.5, 3.0, and 3.5) and general linear regression was performed to determine the slope of MTVs. The slop was − 16.6 in this case and heterogeneity index-2 was its negative form, namely, 16.6
Treatment response assessment
Immediately after neoadjuvant chemotherapy, tumor resectability was confirmed in all patients, and radical surgery was performed within 4–6 weeks. Postoperatively, pathological response was defined if the percentage of the residual tumor volume was roughly less than two thirds of the tumor bed [23].
Patient follow-up
Patients were followed up every 3–4 months in the first 3 years and every 4–6 months thereafter, according to the National Comprehensive Cancer Network guidelines [25]. Recurrence and metastasis were diagnosed based on either a positive biopsy or unequivocal clinical/radiographical evidence. The overall survival (OS) and progression-free survival (PFS) times were recorded, which were defined as the time (in months) from the date of baseline PET/CT to the date when patients died of any cause and to the date when progression was confirmed, respectively. Progression was defined as at least a 20% increase in the sum of the diameters of the lesions, according to the Response Evaluation Criteria in Solid Tumor (RECIST 1.1) criteria [26].
Statistical analysis
Statistical analyses were performed using SPSS 20 (IBM Corp., Armonk, NY, USA) and all hypothesis tests were two sided with a significance level of 0.05. Comparisons of categorical variables were performed using the Chi square test or Fisher’s exact test. Comparisons of continuous variables were conducted using Student’s t test when normal distributions were obtained, otherwise the Mann–Whitney U test was used. The cutoff value for classifying high and low PET parameter groups was the median value of each variable. Kaplan–Meier survival analysis with the log-rank test was performed to compare PFS and OS between patient groups. Univariate and multivariate Cox regression were conducted and hazard ratios (HR) were calculated to compare the predictive values of clinicopathological factors and PET parameters with patient clinical outcomes.
Results
Patient basic information
Basic information about the patients is shown in Table 1. None of these clinical and pathological characteristics were significantly associated with tumor recurrence (Table 2).
18F-FDG PET/CT and quantitative parameters
The metabolic parameters on PET were 9.8 ± 5.74 (mean ± SD) for SUVmax, 7.9 ± 4.72 for SUVpeak, 4.2 ± 1.17 for SUVmean and 5.4 ± 3.20 for TLR. For the volumetric parameters, the MTV and TLG were 61.9 ± 55.12 mL and 335.4 ± 396.63 g, respectively. The heterogeneity indices were calculated as 0.79 ± 0.20 for HI-1 and 36.5 ± 27.09 for HI-2 (Table 1). None of these quantitative parameters were significantly associated with tumor recurrence (Table 2). Restricted by the retrospective nature of this study, information regarding pathological response was only available for 36 patients. Pathological responders had significantly smaller TLR, MTV, and HI-2 than non-responders (P = 0.017, 0.017, and 0.013, respectively), while the other parameters were not significant factors (Table 3).
Univariate and multivariate analyses for PFS
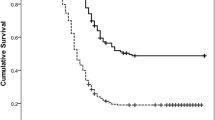
Univariate and multivariate analyses for associations with PFS are summarized in Table 4. Baseline carbohydrate antigen 19-9 (CA19-9) (HR = 1.947, P = 0.036), clinical TNM stage (P = 0.002), and HI-2 (HR = 1.967, P = 0.026; Fig. 2) were significantly correlated with PFS. Multivariate analysis showed that HI-2 (HR = 2.639, P = 0.005) was an independent variable that was significantly associated with PFS after correction for clinical TNM stage.
Univariate and multivariate analyses for OS
The results of univariate and multivariate analyses for significant variables with OS are summarized in Table 5. In univariate analysis, recurrence (HR = 2.285, P = 0.020; Fig. 3a) and HI-2 on PET (HR = 2.447, P = 0.005; Fig. 3b) were significant variables. On multivariate analysis, both of these variables independently correlated with patient OS, with HRs being 2.054 and 2.281, and P values of 0.043 and 0.009 for distant metastasis and HI-2, respectively.
Discussion
In the present study, the heterogeneity index-2 (HI-2), calculated by the slope of linear regression of serial MTVs under different SUV cutoffs, was significantly associated with the response to neoadjuvant chemotherapy. Furthermore, this index independently predicted PFS and OS. When compared with the conventional PET quantitative parameters, HI-2 showed superiority in predicting patient clinical outcomes. To the best of our knowledge, this is the first study to investigate the prognostic value of metabolic heterogeneity on 18F-FDG PET/CT to survival outcomes of patients with gastric cancer.
Malignant tumors always grow in a heterogeneous pattern. The differences in properties, such as gene expression, cellular proliferation, growth rate, vascularity, necrosis, and hypoxia in tumor cells contribute to tumor heterogeneity [19, 27,28,29]. Tumor heterogeneity is a major challenge to personalized medicine and always results in treatment failure [28]. The distribution of 18F-FDG PET activity correlates highly with several physiological processes, including glucose metabolism, necrosis, vascularization, and angiogenesis [30]. Analyzing metabolic heterogeneity on PET/CT has the potential to assess the heterogeneous characteristics of tumors. In the current study, patients that did not respond to neoadjuvant chemotherapy presented significantly larger metabolic heterogeneity of their primary tumors. Besides, the risk of disease progression and death increased with increasing metabolic heterogeneity index. These findings highlighted the importance of tumor heterogeneity and the possibility that it might be a powerful predicator of patient clinical outcomes. In addition, this imaging parameter has the potential to improve risk stratification and optimize patient selection for more aggressive treatment.
Several methods have been proposed to investigate the heterogeneity of tumors on 18F-FDG PET/CT, among which texture analysis is used widely. Recent studies have shown that the heterogeneity index on 18F-FDG PET/CT from texture analysis was useful to predict therapy response and prognosis in many patients with cancer [31,32,33]. However, texture analysis is not clinically available, because a standard method has not been established, the software remains inaccessible in most PET viewing workstations, and it is a complicated and time-consuming process. In addition, most studies using texture analysis to characterize tumor heterogeneity considered volumes greater than 3–5 cm3, indicating that texture analysis might not be suitable to assess the heterogeneity of tumors with smaller volumes [33]. Adequate MTV is necessary for the efficient use of texture features in texture analysis. By contrast, HI-2 can be easily achieved on the commonly used workstation, and thus is suitable for clinical practice.
The method for generating HI-2 in this study was slightly different from that of previous studies. A percentage threshold method (usually 40%, 50%, 60% and 80% of SUVmax) had been proposed to generate an MTV-based heterogeneity index, and had demonstrated significant prognostic value in breast cancer [20] and oral cavity cancer [21]. However, the percentage threshold method relies strongly on the SUVmax of the tumor, leading to a large variance between cancer lesions with high FDG uptake. In addition, the physiological uptake by the adjacent normal gastric wall might hamper accurate evaluation of the gastric-cancer margin, especially in tumors with low FDG uptake, such as MAC/SRC. In contrast, Kim et al. [18] chose the absolute SUV threshold method (2.0, 2.5, and 3.0) to obtain the slope of linear regression in patients with pancreatic cancer. However, physical uptake by the gastric wall commonly has an SUVmax higher than 2.0. The MTV generated from an SUV cutoff of 2.0 commonly cannot accurately reflect the real metabolic volume of the tumor. Therefore, we altered the method to use SUV cutoffs of 2.5, 3.0, and 3.5 to generate the slope of linear regression and a significant result was achieved.
Another easily acquired parameter to characterize metabolic tumor heterogeneity is the coefficient of variance, namely HI-1. Previous studies have demonstrated the prognostic values of this index in many cancers, such as uterine cervical cancer [22] and ovarian cancer [24]. However, this index failed to correlate with the clinical outcomes of patients with gastric cancers in the current study. Negative result was also identified in pancreatic ductal carcinoma [18]. Thus, the prognostic value of HI-1 is inconstant across different types of cancer, and should not be recommended for clinical practice. The underlying reason might be related to its threshold-dependent property, because the VOI under different SUV thresholds might generate different SDs and different SUVmean values, while different types of cancer have different optimal thresholds for VOI delineation.
The metabolic parameters, including SUVmax, SUVmean, SUVpeak, and TLR, are commonly used in the clinic. Some studies have advocated the prognostic values of these parameters in patients with cancer [34,35,36]. However, the prognostic value of these parameters was negative in patients with gastric cancer in the current study, although TLR was associated significantly with treatment response. This might be related to the heterogeneity of the tumor, a partial volume effect, the time of SUV evaluation, and body size [37]. In recent years, the volumetric parameters, including the metabolic tumor volume (MTV) and total lesion glycolysis (TLG), have demonstrated excellent performance in predicting clinical outcomes of patients with gastric cancer [10, 11]. One problem is that these volumetric parameters cannot escape from influences of volume effects from adjacent lesions with high radioactivity, for example, metastatic lymph nodes.
Several limitations of this study should be mentioned. Firstly, this study was a retrospective review of a small patient cohort who had been treated with various regimens of adjuvant chemotherapy. The heterogeneity of treatment modality could have confounded the prognostication. Secondly, restricted by the method for calculating HI-2, we only included patients with SUVmax higher than 3.5; therefore, the bias in patient selection might have influenced the results of the survival analysis. Thirdly, the relationship between intra-tumor heterogeneity and the underlying biological mechanisms remains unclear.
Conclusions
The HI-2 value generated by the MTV-based linear regression from baseline 18F-FDG PET/CT was significantly associated with survival outcomes of patients with gastric cancer. The method for determining this index is simple and convenient. Preoperative assessment of HI-2 by 18F-FDG PET/CT might be a promising method to identify patients with poor prognosis.
Availability of data and materials
The dataset used and/or analyzed in current study are available from the corresponding author on reasonable request.
Abbreviations
- 18F-FDG:
-
2-[18F] Fluoro-2-deoxy-d-glucose
- PET/CT:
-
Positron emission tomography/computed tomography
- SUVmax:
-
Maximum standard uptake value
- SUVpeak :
-
Peak standard uptake value
- MTV:
-
Metabolic tumor volume
- TLG:
-
Total lesion glycolysis
- TLR:
-
Tumor-to-liver ratio
- HI:
-
Heterogeneity index
- VOI:
-
Volume of interest
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- HR:
-
Hazard ratio
- WDA:
-
Well differentiated adenocarcinoma
- MDA:
-
Moderate differentiated adenocarcinoma
- PDA:
-
Poorly differentiated adenocarcinoma
- SRC:
-
Signet-ring cell carcinoma
- MAC:
-
Mucinous adenocarcinoma
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Park I, Ryu M, Choi Y, et al. A phase II study of neoadjuvant docetaxel, oxaliplatin, and S-1 (DOS) chemotherapy followed by surgery and adjuvant S-1 chemotherapy in potentially resectable gastric or gastroesophageal junction adenocarcinoma. Cancer Chemother Pharmacol. 2013;72:815–23.
Boige V, Pignon J, Saint-Aubert B, et al. Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. J Clin Oncol. 2007;25:4510.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Wang C, Guo W, Zhou M, et al. The predictive and prognostic value of early metabolic response assessed by positron emission tomography in advanced gastric cancer treated with chemotherapy. Clin Cancer Res. 2016;22:1603–10.
Lee JW, Lee SM, Son MW, Lee MS. Diagnostic performance of FDG PET/CT for surveillance in asymptomatic gastric cancer patients after curative surgical resection. Eur J Nucl Med Mol Imaging. 2016;43:881–8.
Kim J, Lim ST, Na CJ, et al. Pretreatment F-18 FDG PET/CT parameters to evaluate progression-free survival in gastric cancer. Nucl Med Mol Imaging. 2014;48:33–40.
Sun G, Cheng C, Li X, Wang T, Yang J, Li D. Metabolic tumor burden on postsurgical PET/CT predicts survival of patients with gastric cancer. Cancer Imaging. 2019;19:18.
Tang L, Wang XJ, Baba H, Giganti F. Gastric cancer and image-derived quantitative parameters: part 2-a critical review of DCE-MRI and (18)F-FDG PET/CT findings. Eur Radiol. 2020;30:247–60.
Na SJ, O JH, Park JM, et al. Prognostic value of metabolic parameters on preoperative 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with stage III gastric cancer. Oncotarget. 2016; 7:63968–80.
Park JS, Lee N, Beom SH, et al. The prognostic value of volume-based parameters using (18)F-FDG PET/CT in gastric cancer according to HER2 status. Gastric Cancer. 2018;21:213–24.
Park S, Ha S, Kwon HW, et al. Prospective evaluation of changes in tumor size and tumor metabolism in patients with advanced gastric cancer undergoing chemotherapy: association and clinical implication. J Nucl Med. 2017;58:899–904.
Razzak M. Genetics: new molecular classification of gastric adenocarcinoma proposed by The Cancer Genome Atlas. Nat Rev Clin Oncol. 2014;11:499.
Giganti F, Antunes S, Salerno A, et al. Gastric cancer: texture analysis from multidetector computed tomography as a potential preoperative prognostic biomarker. Eur Radiol. 2017;27:1831–9.
Li M, Ke J, Wang Q, et al. Upregulation of ROCK2 in gastric cancer cell promotes tumor cell proliferation, metastasis and invasion. Clin Exp Med. 2017;17:519–29.
Ohbatake Y, Fushida S, Tsukada T, et al. Elevated alpha1-acid glycoprotein in gastric cancer patients inhibits the anticancer effects of paclitaxel, effects restored by co-administration of erythromycin. Clin Exp Med. 2016;16:585–92.
Asselin MC, O’Connor JP, Boellaard R, Thacker NA, Jackson A. Quantifying heterogeneity in human tumours using MRI and PET. Eur J Cancer. 2012;48:447–55.
Kim YI, Kim YJ, Paeng JC, et al. Heterogeneity index evaluated by slope of linear regression on (18)F-FDG PET/CT as a prognostic marker for predicting tumor recurrence in pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging. 2017;44:1995–2003.
Huang B, Chan T, Kwong DL, Chan WK, Khong PL. Nasopharyngeal carcinoma: investigation of intratumoral heterogeneity with FDG PET/CT. AJR Am J Roentgenol. 2012;199:169–74.
Kim TH, Yoon JK, Kang DK, et al. Correlation between F-18 fluorodeoxyglucose positron emission tomography metabolic parameters and dynamic contrast-enhanced MRI-derived perfusion data in patients with invasive ductal breast carcinoma. Ann Surg Oncol. 2015;22:3866–72.
Kwon SH, Yoon JK, An YS, et al. Prognostic significance of the intratumoral heterogeneity of F-18-FDG uptake in oral cavity cancer. J Surg Oncol. 2014;110:702–6.
Chung HH, Kang SY, Ha S, et al. Prognostic value of preoperative intratumoral FDG uptake heterogeneity in early stage uterine cervical cancer. J Gynecol Oncol. 2016;27:e15.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14:101–12.
Lee M, Lee H, Cheon GJ, et al. Prognostic value of preoperative intratumoral FDG uptake heterogeneity in patients with epithelial ovarian cancer. Eur Radiol. 2017;27:16–23.
Ajani JA, Barthel JS, Bekaii-Saab T, et al. Gastric cancer. J Natl Compr Canc Netw. 2010;8:378–409.
Schwartz LH, Litiere S, de Vries E, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer. 2016; 62:132–7.
Sorensen M, Horsman MR, Cumming P, Munk OL, Keiding S. Effect of intratumoral heterogeneity in oxygenation status on FMISO PET, autoradiography, and electrode Po2 measurements in murine tumors. Int J Radiat Oncol Biol Phys. 2005;62:854–61.
Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92.
Tateishi U, Nishihara H, Tsukamoto E, Morikawa T, Tamaki N, Miyasaka K. Lung tumors evaluated with FDG-PET and dynamic CT: the relationship between vascular density and glucose metabolism. J Comput Assist Tomogr. 2002;26:185–90.
Basu S, Kwee T, Gatenby R, Saboury B, Torigian D, Alavi A. Evolving role of molecular imaging with PET in detecting and characterizing heterogeneity of cancer tissue at the primary and metastatic sites, a plausible explanation for failed attempts to cure malignant disorders. Eur J Nucl Med Mol Imaging. 2011;38:987–91.
Hyun SH, Kim HS, Choi SH, et al. Intratumoral heterogeneity of 18F-FDG uptake predicts survival in patients with pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol I. 2016;43:1461–8.
Chicklore S, Goh V, Siddique M, Roy A, Marsden PK, Cook GJR. Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol I. 2013;40:133–40.
Hatt M, Majdoub M, Vallières M, et al. 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi–cancer site patient cohort. J Nucl Med. 2015;56:38–44.
Wang SL, Cao S, Sun YN, et al. Standardized uptake value on positron emission tomography/computed tomography predicts prognosis in patients with locally advanced pancreatic cancer. Abdom Imaging. 2015;40:3117–21.
Chirindel A, Alluri KC, Chaudhry MA, et al. Prognostic value of FDG PET/CT-derived parameters in pancreatic adenocarcinoma at initial PET/CT staging. AJR Am J Roentgenol. 2015;204:1093–9.
Choi HJ, Kang CM, Jo K, et al. Prognostic significance of standardized uptake value on preoperative (1)(8)F-FDG PET/CT in patients with ampullary adenocarcinoma. Eur J Nucl Med Mol Imaging. 2015;42:841–7.
Thie JA. Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med. 2004;45:1431–4.
Acknowledgements
The authors would like to express their gratitude to the staff of the Department of Nulcear Medicine of Zhongshan Hospital, Fudan University, for the support of this study.
Funding
This study was funded by the National Natural Science Foundation of China (Grant Number: 81701730), the training program for excellent young medical talents of Zhongshan Hospital of Fudan University (Grant Number: 2019ZSYQ28), and the Shanghai “Rising Stars of Medical Talent”—–Youth Development Program (Grant Number: HWJRS2019-72). The funding body had no role in the design, data collection, analysis, interpretation or manuscript writing of this study.
Author information
Authors and Affiliations
Contributions
GL and YH conceived and wrote this article. GL, HY, and XC collected the study materials or patients. XC, YW and YH performed the data analysis and interpretation. TL and HS controlled the quality of the study. All authors have read and approved the manuscript for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that there is no conflict of interest regarding this article.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Zhongshan Hospital of Fudan University (Approval Number: IRB2015-098). Written informed consents were obtained from all patients.
Consent for publication
Written informed consents were obtained from included patients for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, G., Yin, H., Cheng, X. et al. Intra-tumor metabolic heterogeneity of gastric cancer on 18F-FDG PETCT indicates patient survival outcomes. Clin Exp Med 21, 129–138 (2021). https://doi.org/10.1007/s10238-020-00659-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-020-00659-8