Abstract
Pancreatic ductal adenocarcinomas (PDACs) occasionally have atypical and uncommon imaging presentations that can present a diagnostic dilemma and result in false interpretation. This article aimed to illustrate these CT and MR imaging findings, including isoattenuating PDAC, coexisting acute pancreatitis, PDAC with a cystic feature, groove PDAC, diffuse PDAC, hypointensity on diffusion-weighted imaging (DWI), multifocal PDAC, intratumoral calcification, and extrapancreatic invasion with a barely discernable mass. A subset of PDACs with atypical features are occasionally encountered during routine clinical practice. Knowledge of and attention to these atypical and uncommon variable imaging features may allow radiologists to avoid misinterpretation and a delayed diagnosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer and accounts for over 90% of cases. Vague or even absence of symptoms at the early stage often contributes to late detection of the tumor. A large proportion of patients in the locally advanced stage are not eligible for curative surgical resection. The timely detection and diagnosis of PDAC are particularly important. CT and MR imaging play an important role in the detection, staging, and resectability assessment of PDAC. The CT and MR imaging protocols recommended by the consensus and guidelines are summarized in Tables 1 and 2 [1, 2]. It is a great challenge for radiologists to detect subclinical PDAC as well as to distinguish it from its mimickers in routine clinical practice. Extensive literature has documented the typical imaging appearance of PDAC. However, PDAC may occasionally have atypical CT and MR imaging features that cause a diagnostic dilemma, including isoattenuating PDAC, coexisting acute pancreatitis, PDAC with a cystic feature, groove PDAC, diffuse PDAC, hypointensity on diffusion-weighted imaging (DWI), multifocal PDAC, intratumoral calcification, and extrapancreatic invasion with a barely discernable mass. Table 3 summarizes the atypical and uncommon pancreatic ductal carcinomas listed in this article and their incidences [3,4,5,6,7,8,9,10]. Although endoscopic ultrasound (EUS) may provide complementary diagnostic information and allows tissue acquisition for pathology diagnosis via EUS-guided fine-needle aspiration, which is particularly helpful when lesions are equivocal or questionable on CT and MR imaging [2], from the perspective of the radiologist, awareness of these atypical or uncommon presentations may avoid a delayed diagnosis of PDAC considering its highly aggressive nature and poor prognosis. In this article, we thus illustrate the various atypical and uncommon CT and MR imaging features of PDAC.
Isoattenuating PDAC
Isoattenuating PDAC is defined as a tumor that is visually indistinguishable from the surrounding pancreatic parenchyma by attenuation on dynamic CT imaging. The attenuation difference between isoattenuating PDAC and the surrounding parenchyma is typically less than 10 HU, which makes the lesion generally imperceivable and easily overlooked. In one large retrospective study by Kim et al. [3], isoattenuating PDAC was found in 5.4% of 644 patients. Other studies with smaller populations reported higher frequencies, ranging from 11 to 14% [4, 5]. The tumor histologic grade, T category, and size of isoattenuating PDACs in Kim et al.’s study [3] did not differ significantly from those of “usual” hypoattenuating PDAC, however, patients with isoattenuating tumors also tended to have prolonged median survival after curative surgery.
Multiple factors, including tumor stroma, degree of interstitial fibrosis, residual pancreatic tissue, and microvessel density, will affect the enhancement degree and pattern of PDAC on CT. Compared with usual hypoattenuating PDACs, isoattenuating tumors are histopathologically characterized by lower tumor cellularity with well-differentiated tumor cells, a higher prevalence of residual pancreatic acini, higher microvessel density, a more predominant proportion of fibrous stroma with loose fibrosis, and less prominent tumor necrosis [3, 11, 12].
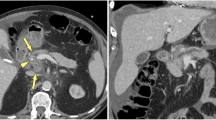
Isoattenuation is a major cause of delay in the diagnosis of PDAC. In Yoon's study [12], 33 missed PDAC cases on initial prediagnostic CT were analyzed. The mean interval between prediagnostic CT and the final histopathological diagnosis was 6.4 months. Among those 33 cases, 11 missed tumors (33.3%) were isoattenuating. Several secondary findings may indicate the presence of a tumor. Both Kim et al. [3] and Prokesch et al. [4] found an interrupted and dilated pancreatic duct as the most common indirect finding (Fig. 1). Other signs, including biliary duct dilatation and interruption, upstream parenchymal atrophy, focal contour deformity, small retention cysts and perivascular cuffing, also indicate the presence of a tumor. Given the difficulty in visualizing the tumor, it is crucial that the radiologist raise the suspicion for possible isoattenuating PDAC in the presence of unexplained secondary findings to avoid a missed diagnosis. MR imaging is helpful for improving tumor conspicuity owing to its superior soft tissue contrast. It has been reported that nearly 80% of isoattenuating PDACs can be observed as focal hypointense lesions on nonenhanced or contrast-enhanced T1-weighted images and/or as mild hyperintense lesions on T2-weighted images [3]. Adding DWI to conventional MR imaging may further help improve the sensitivity of isointense PDAC detection. In Park et al.’s study [13], 15 out of 83 PDACs with isointensity on contrast-enhanced T1-weighted images and T2-weighted images were only discerned on DW images.
Pathologically proven missed isoattenuating PDAC in a 68-year-old man. The initial contrast-enhanced CT image a shows mild dilation of the pancreatic duct without obvious lesion (arrow). b Follow-up contrast-enhanced CT imaging 8 months later reveals typical hypovascular PDAC (arrow) with pancreatic duct cutoff and upstream parenchymal atrophy (arrowhead)
Coexisting acute pancreatitis
PDAC often accompanies chronic obstructive pancreatitis due to duct obstructions, and chronic inflammation in patients with chronic pancreatitis may also cause a marked increase in the risk of cancer. However, there is a less distinct association between acute pancreatitis and pancreatic carcinoma. Approximately 6.8% to 13.8% of PDAC cases may manifest as acute pancreatitis [6, 7]. Conversely, 0.9% to 3.6% of patients with acute pancreatitis are ultimately diagnosed with pancreatic cancer [6, 14]. Due to the overlapping clinical manifestations and equivocal imaging features, the tumors in such cases are easily overlooked and diagnosed in an advanced stage. A study of 313 patients who were finally diagnosed with PDAC found that 98 patients were initially misdiagnosed, and the median time of delay was 4.2 months. Among these 98 patients, 28 (28.6%) patients had at least one episode of pancreatitis [15].
Typical diagnostic features of PDAC, such as a focal hypoattenuated mass, distorting pancreatic contour, or infiltrating peripancreatic fat, might be obscured by the presence of parenchymal swelling, edematous fat, and heterogeneous peripancreatic collections observed in acute pancreatitis (Fig. 2). The mechanism of cancer-related acute pancreatitis remains unclear. Ductal obstruction is generally hypothesized, while some studies speculate that the enzymes secreted by the tumor may activate the process of pancreatic self-digestion [16]. The possibility of acute pancreatitis with pancreatic cancer should be routinely considered, and a careful search for potential masses on contrast-enhanced CT images should be conducted, especially for high-risk patients ≥ 50 years old with idiopathic recurrence but mild episodes of pancreatitis and elevated CA19-9 levels [6, 17]. Local lymphadenopathy, the detection of a dilated pancreatic duct with an interrupting or cutoff configuration, and unexplained distal pancreatic atrophy may help identify the underlying tumor [16].
Pathologically proven PDAC in a 52-year-old man who presented with epigastric pain. a Contrast-enhanced CT image at the level of the uncinate process shows a small hypovascular lesion (arrow) in the pancreatic head and thickening of the renal fascia (arrowhead). b Image at a more cranial level shows swelling of the pancreatic parenchyma, as well as encapsulated fluid collection in the lesser sac (black arrow). Laboratory test results: serum amylase level, 1090 U/L; lipase level, 676.6 U/L
Cystic feature
Approximately 8% of PDAC cases may have a cystic feature [8], which can mimic cystic pancreatic tumors and lead to misinterpretation. Cystic features can range in diameter from a few millimeters to several centimeters, sometimes even exceeding the tumor itself. The cystic feature can be found as a neoplastic cystic region within the tumor (Fig. 3) or as a nonneoplastic cyst surrounding the tumor (Fig. 4) [18].
a Axial and b coronal contrast-enhanced CT images show pathologically proven PDAC with degenerative cystic change (arrows) originating from the uncinate process infiltrating the third portion of the duodenum in a 51-year-old man. The patient had no history of neoadjuvant chemotherapy or radiation therapy. Ill-defined intratumoral necrosis of cystic features with scattered gas was observed, indicating perforation of the duodenal wall. c The endoscopic image shows an ulceroinfiltrative lesion (arrows) with dirty yellow exudate and hemorrhage. d Photomicrograph (original magnification, × 200; H-E stain) shows extensive necrosis within the tumor. The thumbnail of the slide (original magnification, × 3; H-E stain) is displayed in the upper right corner.
The neoplastic cystic features that occur within the tumor represent large-gland type cysts, neoplastic mucin cysts, or degenerative cystic changes. Large-gland-type cysts are microcysts (< 1 cm) that are scattered within the tumor. Histologically, these cysts represent dilated neoplastic duct-like glands. The neoplastic mucin cysts are usually macrocysts with smooth margins that are distributed eccentrically within the tumor at imaging. They are lined by mucin-producing neoplastic epithelial cells microscopically. The degenerative cystic change represents tumor necrosis. Radiologically, it usually shows a solitary nonenhancing cystic area with irregular margins and centric locations. Pathologically, degenerative cystic changes are formed by tumor necrosis containing necrotic and hemorrhagic tissue [8, 18, 19]. Degenerative cysts may also be a component of large pancreatic adenocarcinoma after preoperative neoadjuvant chemotherapy and radiation therapy.
The nonneoplastic cysts surrounding the tumors are mostly retention cysts or pseudocysts. Retention cysts may be the result of tumor obstruction of the pancreatic duct, whereas pseudocysts are thought to be related to secondary pancreatitis. Due to their mechanisms of formation, retention cysts and pseudocysts are located at the edge of the tumor rather than inside the tumor [8, 18, 19].
PDACs with cystic features should be distinguished from intraductal papillary mucinous neoplasms (IPMNs) with associated invasive carcinoma of the pancreas (IPMC), which may have similar imaging manifestations, such as mixed solid-cystic appearance, ductal dilation, and neurovascular invasion. IPMC indicates progression from its precursor IPMN to invasive adenocarcinoma, which is similar to the usually seen PDAC not arising in association with an IPMN. Imaging features favoring IPMC include complicated tubular cystic mass with predominantly intraductal enhancing mural nodules, dilation of the upstream and downstream pancreatic duct, and communication between cystic mass and the main pancreatic duct [20, 21].
Groove PDAC
The pancreatic groove is a space surrounded by the pancreatic head, the duodenum, and the common bile duct. Pancreatic ductal adenocarcinoma arising in the groove area may not show typical signs, such as pancreatic cutoff, ductal obstruction, and upstream atrophy [22]. The exact histopathologic origin of groove PDACs is unclear. Theory suggests that groove PDACs may arise from pancreatic tissue around the accessory pancreatic duct penetrating the groove and duodenum [23]. Although the pancreatic tissue at this location is generally considered ectopic, in approximately 40% of cases it is continuous and/or closely related to the proper pancreas with the same morphologies and functions, suggesting that it may be a portion of the dorsal pancreas rather than an ectopic pancreas [24]. Groove PDAC may resemble groove pancreatitis on both CT and MR images because scirrhous adenocarcinoma of the pancreas and sheet-like fibrous scar in groove pancreatitis show similar findings on both pre- and post-enhanced dynamic CT and MR images [25]. Both lesions may show hypointensity on T1-weighted and fat-suppressed T1-weighted images and hyperintensity or isointensity on T2-weighted images, with hypoenhancement in the early phase and delayed enhancement in the late phase on dynamic CT and dynamic MR images [26]. According to previous studies, the radiological findings, including a thickened duodenal wall (> 3 mm), the presence of a cystic lesion in or near the pancreatic groove, no dilatation or dilatation of the common bile duct with distal tapering, focal and patchy enhancement rather than peripheral enhancement in the portal venous phase, and increased duodenal wall enhancement on MR imaging (relative to the proximal jejunal loops), favor the diagnosis of groove pancreatitis [27,28,29]. Moreover, adenocarcinoma is much more likely to infiltrate posteriorly into the retroperitoneum and encase the vasculature [22]. Kalb et al. found that using all three features (i.e., focal thickening of the second portion of the duodenum wall, abnormal enhancement of the second portion of the duodenum, and cystic focus in the expected region of the accessory pancreatic duct) achieved an accuracy of 87.2% in distinguishing groove pancreatitis from carcinoma [27]. However, there is still considerable overlap in the imaging manifestations of the two entities (Fig. 5). Distinction can be extremely difficult when there are no cystic lesions within the mass and in the presence of a thickened duodenal wall [26].
Pathologically proven groove PDAC in a 74-year-old woman. Gadolinium-enhanced T1-weighted MR images at the a arterial and b venous phases show the predominant hypoenhancing soft tissue mass (arrows) in the pancreatic groove with persistent enhancement. c Magnetic resonance cholangiopancreatography (MRCP) image shows a focal cystic lesion within the mass. The presence of cystic lesions in the groove area is, however, frequently noted in groove pancreatitis. d Specimen photograph shows the cutting surface of the yellow-colored tumor tissue (*)
Diffuse PDAC
Diffuse PDAC refers to a tumor that involves the pancreas continuously along more than half of the longest pancreatic axis. It is a rare morphologic pattern, accounting for approximately 2–5% of all cases of PDAC [9]. This rare imaging manifestation can be misdiagnosed by radiologists as other diseases, such as acute pancreatitis, diffuse-form IgG4-related autoimmune pancreatitis, lymphoma, and pancreatic metastasis [30]. Most diffuse PDACs involve the body and tail, whereas the others involve the entire pancreas. A peripheral hyperenhancing capsule-like structure relative to the tumor is the hallmark that may help differentiate this lesion from other diffuse lesions. This capsule-like structure is pathologically correlated with dense fibrous changes on the tumor periphery. In addition, the direct invasion of neighboring organs and vascular infiltration are common in diffuse PDAC, as is typical in cancer. However, pancreatic parenchyma atrophy is usually absent, and dilatation of the upstream pancreatic duct is not remarkable (Fig. 6).
Diffuse PDAC needs to be differentiated from lymphoma and autoimmune pancreatitis (AIP). Compared with the homogeneous enhancement of lymphoma, capsule-like structures are more common in diffuse PDAC, whereas bulky lymphadenopathy involving the infrarenal spaces and less neurovascular invasion favor lymphoma against diffuse PDAC [9]. Diffuse form AIP may also show similar diffuse enlargement of the pancreas. In contrast to the thin capsule-like structure that is hyperattenuating on nonenhanced CT or hyperenhancing on the arterial or portal venous phase seen in diffuse PDAC, a peripancreatic low attenuation band-like halo with delayed enhancement is a typical finding on AIP. Besides, the duct-penetrating sign, the presence of extrapancreatic autoimmune processes, and elevated serum immunoglobulin G fraction 4 levels may also be helpful to distinguish diffuse form AIP from diffuse PDAC [9].
Hypointensity on diffusion-weighted imaging (DWI)
The use of DWI is recommended for the detection of pancreatic cancer and for the characterization of pancreatic masses [31]. PDAC usually manifests as hyperintense on high-b-value DWI. However, in a few cases, it can exhibit isointensity or even hypointensity compared to the surrounding parenchyma (Fig. 7). In a study of 80 patients using a b value of 1000 s/mm2on a 3.0-T system with a six-channel body coil, Fukukura et al. [10] found that twelve (15%) pancreatic adenocarcinomas were isointense, and four (5%) were hypointense. The mean apparent diffusion coefficients (ADCs) of all pancreatic adenocarcinomas in their study, on the other hand, were significantly lower than those of the surrounding parenchyma. There are two hypotheses regarding the hypointensity of PDAC on DWI: (a) The restricted diffusion of the distal gland caused by obstructive pancreatitis may result in the relative hypointensity of the tumor [10]; (b) intratumoral fibrosis and cellularity can increase diffusion signals, whereas necrosis within the tumor may decrease diffusion signals. The combined effect of fibrosis, cell density and necrosis leads to signal variability on DWI [31]. The possibility of PDAC hypointensity on DWI reminds radiologists to be aware when using DWI to screen patients at high risk for cancer.
Pathologically proved PDAC in a 60-year-old woman. a Contrast-enhanced CT and b gadolinium-enhanced T1-weighted fat-suppressed MR images show a hypovascular mass (arrows) in the head of the pancreas. c The mass (arrow) appears hypointense compared with the surrounding pancreatic parenchyma on DWI (b = 600)
Multifocal PDAC
Multifocal PDAC is a very rare condition observed on CT or MR imaging and refers to the presence of two or more foci in the pancreas. The cause of multifocality is not well understood; however, it could be related to a family history of pancreatic cancer and pancreatic intraepithelial neoplasias [32, 33]. Reports of multifocal adenocarcinomas of the pancreas are often made based on histological examinations of total pancreatectomy specimens. In these cases, truly multifocal lesions are found occurring with many islands of carcinoma in situ and invasive carcinoma separated by regions of the normal pancreas, although some multifocal lesions are actually continuous tumors spread along either the common bile duct or the main pancreatic duct [34, 35]. According to our observations of individual cases, each single lesion of multifocal PDAC still retains typical imaging features of classical PDAC, including a hypovascular mass, pancreatic duct cutoff and obstruction, and vascular infiltration (Fig. 8).
Multifocal PDAC should be distinguished from hypovascular pancreatic metastases and multifocal pancreatic neuroendocrine neoplasms (PanNETs). Hypovascular pancreatic metastases are relatively uncommon, and the majority of primary sites are the lung, breast, and colorectum. A known history of primary malignancy, discrete well-defined margins, absence of or relatively mild duct obstruction, and lack of peripheral vascular invasion can help in distinguishing hypovascular metastasis from multifocal PDAC [36]. Multifocal PanNETs are usually seen in familial syndromes, most commonly multiple endocrine neoplasia type 1, von Hippel-Lindau syndrome, neurofibromatosis type 1, and tuberous sclerosis. Although PanNETs could be either hypervascular or nonhypervascular, rarely, multifocal PanNETs appear solely as nonhypervascular tumors. Compared to PDACs, well-defined tumor margins, intratumoral cystic components, calcifications, and blood vessels and the absence of pancreatic duct dilatation and peripancreatic infiltration are favorable features for nonhypervascular PanNETs [37].
Intratumoral calcification
A variety of pancreatic neoplasms may contain calcifications; however, intratumoral calcification is a very rare finding in PDACs (Fig. 9). According to Eelkema's study [38], calcification is present in 2% of PDACs. In another study by Campisi et al. [39], in all cases of pancreatic calcification, intratumoral calcification of PDAC accounted for only 1%. Two different etiologies of calcification have been proposed. The first possible cause is that PDAC develops from underlying chronic pancreatitis, which is often prone to calcification and is an important risk factor for PDAC [40]. Barthet et al. found that the calcification of these pancreatic cancers is often located in the peripheral region of the tumor [41]. The second possible cause is the dystrophic calcification resulting from ischemic and necrotic areas within the tumors [42].
Extrapancreatic invasion with a barely discernable mass
Some exophytic ductal carcinomas are initially found with extrapancreatic invasion, but the tumor itself is barely discernible. As the tumor occurs in the periphery of the pancreas, it is likely to be missed because of the lack of typical duct obstruction and distal gland atrophy. One of the most representative examples is an exophytic tumor arising at the most medial edge of the uncinate process, and the initial imaging manifestation may be the perivascular soft tissue cuff with encasement of the superior mesentery artery or celiac trunk rather than a clear low-density mass (Fig. 10). Although the perivascular cuff is not specific, this finding may occasionally be the sole radiologic sign of PDAC [43, 44]. Another example is an exophytic tumor from the most inferior pancreatic head with early infiltration of the duodenum that presents the initial manifestation of duodenal obstruction in the absence of obstructive jaundice. This may easily be mistaken as a duodenal tumor [45].
Pathologically proved PDAC in a 48-year-old man. a–c Consecutive contrast-enhanced CT images from the cranial to caudal levels with a 5 mm slice thickness show easily overlooked exophytic tumors arising from the medial border of the uncinate process (arrowheads). A circumferential tumor contacting the superior mesenteric artery (approximately 180°) is observed as the perivascular soft tissue cuff
Conclusion
A subset of PDACs with atypical features are occasionally encountered during routine clinical practice. Knowledge of and attention to these atypical and uncommon imaging features may allow radiologists to avoid misinterpretation and a delayed diagnosis.
Data availability
The material analyzed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- PDAC:
-
Pancreatic ductal adenocarcinoma
- DWI:
-
Diffusion weighted imaging
- EUS:
-
Endoscopic ultrasound
- H-E:
-
Hematoxylin–eosin
- IPMC:
-
Intraductal papillary mucinous neoplasms with associated invasive carcinoma of the pancreas
- AIP:
-
Autoimmune pancreatitis
- PanNETs:
-
Pancreatic neuroendocrine tumors
References
Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, et al. Pancreatic Ductal Adenocarcinoma Radiology Reporting Template: Consensus Statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology. 2014;270(1):248-60. https://doi.org/10.1148/radiol.13131184.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma. Version 2.2021. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (2021). Accessed Mar 23, 2021.
Kim JH, Park SH, Yu ES, Kim MH, Kim J, Byun JH, et al. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology. 2010;257(1):87-96. https://doi.org/10.1148/radiol.10100015.
Prokesch RW, Chow LC, Beaulieu CF, Bammer R, Jeffrey RB, Jr. Isoattenuating pancreatic adenocarcinoma at multi-detector row CT: secondary signs. Radiology. 2002;224(3):764-8. https://doi.org/10.1148/radiol.2243011284.
Ishigami K, Yoshimitsu K, Irie H, Tajima T, Asayama Y, Nishie A, et al. Diagnostic value of the delayed phase image for iso-attenuating pancreatic carcinomas in the pancreatic parenchymal phase on multidetector computed tomography. European journal of radiology. 2009;69(1):139-46. https://doi.org/10.1016/j.ejrad.2007.09.012.
Munigala S, Kanwal F, Xian H, Scherrer JF, Agarwal B. Increased risk of pancreatic adenocarcinoma after acute pancreatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(7):1143–50 e1. https://doi.org/10.1016/j.cgh.2013.12.033.
Li S, Tian B. Acute pancreatitis in patients with pancreatic cancer: Timing of surgery and survival duration. Medicine (Baltimore). 2017;96(3):e5908. https://doi.org/10.1097/md.0000000000005908.
Kosmahl M, Pauser U, Anlauf M, Kloppel G. Pancreatic ductal adenocarcinomas with cystic features: neither rare nor uniform. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2005;18(9):1157–64. https://doi.org/10.1038/modpathol.3800446.
Choi YJ, Byun JH, Kim JY, Kim MH, Jang SJ, Ha HK, et al. Diffuse pancreatic ductal adenocarcinoma: characteristic imaging features. European journal of radiology. 2008;67(2):321-8. https://doi.org/10.1016/j.ejrad.2007.07.010.
Fukukura Y, Takumi K, Kamimura K, Shindo T, Kumagae Y, Tateyama A, et al. Pancreatic adenocarcinoma: variability of diffusion-weighted MR imaging findings. Radiology. 2012;263(3):732-40. https://doi.org/10.1148/radiol.12111222.
Hata H, Mori H, Matsumoto S, Yamada Y, Kiyosue H, Tanoue S, et al. Fibrous stroma and vascularity of pancreatic carcinoma: correlation with enhancement patterns on CT. Abdominal imaging. 2010;35(2):172-80. https://doi.org/10.1007/s00261-008-9460-0.
Yoon SH, Lee JM, Cho JY, Lee KB, Kim JE, Moon SK, et al. Small (</= 20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology. 2011;259(2):442-52. https://doi.org/10.1148/radiol.11101133.
Park MJ, Kim YK, Choi SY, Rhim H, Lee WJ, Choi D. Preoperative detection of small pancreatic carcinoma: value of adding diffusion-weighted imaging to conventional MR imaging for improving confidence level. Radiology. 2014;273(2):433-43. https://doi.org/10.1148/radiol.14132563.
Tummala P, Tariq SH, Chibnall JT, Agarwal B. Clinical predictors of pancreatic carcinoma causing acute pancreatitis. Pancreas. 2013;42(1):108-13. https://doi.org/10.1097/MPA.0b013e318254f473.
Swords DS, Mone MC, Zhang C, Presson AP, Mulvihill SJ, Scaife CL. Initial Misdiagnosis of Proximal Pancreatic Adenocarcinoma Is Associated with Delay in Diagnosis and Advanced Stage at Presentation. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2015;19(10):1813-21. https://doi.org/10.1007/s11605-015-2923-z.
Balthazar EJ. Pancreatitis associated with pancreatic carcinoma: Preoperative diagnosis: Role of CT imaging in detection and evaluation. Pancreatology : official journal of the International Association of Pancreatology. 2005;5(4):330–44. https://doi.org/10.1159/000086868.
Mujica VR, Barkin JS, Go VL. Acute pancreatitis secondary to pancreatic carcinoma. Study Group Participants. Pancreas. 2000;21(4):329-32.
Youn SY, Rha SE, Jung ES, Lee IS. Pancreas ductal adenocarcinoma with cystic features on cross-sectional imaging: radiologic-pathologic correlation. Diagnostic and interventional radiology (Ankara, Turkey). 2018;24(1):5-11. https://doi.org/10.5152/dir.2018.17250.
Yoon SE, Byun JH, Kim KA, Kim HJ, Lee SS, Jang SJ, et al. Pancreatic ductal adenocarcinoma with intratumoral cystic lesions on MRI: correlation with histopathological findings. The British Journal of Radiology. 2010;83(988):318-26. https://doi.org/10.1259/bjr/69770140.
Kawamoto S, Horton KM, Lawler LP, Hruban RH, Fishman EK. Intraductal Papillary Mucinous Neoplasm of the Pancreas: Can Benign Lesions Be Differentiated from Malignant Lesions with Multidetector CT? Radiographics : a review publication of the Radiological Society of North America, Inc. 2005;25(6):1451–68. https://doi.org/10.1148/rg.256055036.
Kim JH, Eun HW, Kim KW, Lee JY, Lee JM, Han JK, et al. Intraductal papillary mucinous neoplasms with associated invasive carcinoma of the pancreas: imaging findings and diagnostic performance of MDCT for prediction of prognostic factors. AJR American journal of roentgenology. 2013;201(3):565-72. https://doi.org/10.2214/AJR.12.9511.
Raman SP, Salaria SN, Hruban RH, Fishman EK. Groove pancreatitis: spectrum of imaging findings and radiology-pathology correlation. AJR American journal of roentgenology. 2013;201(1):W29-39. https://doi.org/10.2214/AJR.12.9956.
Izumi S, Nakamura S, Mano S, Akaki S. Well differentiation and intact Smad4 expression are specific features of groove pancreatic ductal adenocarcinomas. Pancreas. 2015;44(3):394-400. https://doi.org/10.1097/MPA.0000000000000260.
Suda K. Histopathology of the minor duodenal papilla. Digestive surgery. 2010;27(2):137-9. https://doi.org/10.1159/000286920.
Castell-Monsalve FJ, Sousa-Martin JM, Carranza-Carranza A. Groove pancreatitis: MRI and pathologic findings. Abdominal imaging. 2008;33(3):342-8. https://doi.org/10.1007/s00261-007-9245-x.
Gabata T, Kadoya M, Terayama N, Sanada J, Kobayashi S, Matsui O. Groove pancreatic carcinomas: radiological and pathological findings. European radiology. 2003;13(7):1679-84. https://doi.org/10.1007/s00330-002-1743-1.
Kalb B, Martin DR, Sarmiento JM, Erickson SH, Gober D, Tapper EB, et al. Paraduodenal pancreatitis: clinical performance of MR imaging in distinguishing from carcinoma. Radiology. 2013;269(2):475-81. https://doi.org/10.1148/radiol.13112056.
Shin LK, Jeffrey RB, Pai RK, Raman SP, Fishman EK, Olcott EW. Multidetector CT imaging of the pancreatic groove: differentiating carcinomas from paraduodenal pancreatitis. Clinical imaging. 2016;40(6):1246-52. https://doi.org/10.1016/j.clinimag.2016.08.004.
Ishigami K, Tajima T, Nishie A, Kakihara D, Fujita N, Asayama Y, et al. Differential diagnosis of groove pancreatic carcinomas vs. groove pancreatitis: usefulness of the portal venous phase. European journal of radiology. 2010;74(3):e95-e100. https://doi.org/10.1016/j.ejrad.2009.04.026.
Choi EK, Park SH, Kim DY, Kim KW, Byun JH, Lee MG, et al. Unusual manifestations of primary pancreatic neoplasia: Radiologic-pathologic correlation. Journal of computer assisted tomography. 2006;30(4):610-7.
Barral M, Taouli B, Guiu B, Koh DM, Luciani A, Manfredi R, et al. Diffusion-weighted MR imaging of the pancreas: current status and recommendations. Radiology. 2015;274(1):45-63. https://doi.org/10.1148/radiol.14130778.
Goong HJ, Moon JH, Choi HJ, Lee YN, Choi MH, Kim HK, et al. Synchronous Pancreatic Ductal Adenocarcinomas Diagnosed by Endoscopic Ultrasound-Guided Fine Needle Biopsy. Gut and liver. 2015;9(5):685-8. https://doi.org/10.5009/gnl14215.
Aimoto T, Uchida E, Nakamura Y, Matsushita A, Katsuno A, Chou K, et al. Multicentric pancreatic intraepithelial neoplasias (PanINs) presenting with the clinical features of chronic pancreatitis. Journal of hepato-biliary-pancreatic surgery. 2008;15(5):549-53. https://doi.org/10.1007/s00534-007-1269-7.
Siassi M, Klein P, Hohenberger W. Organ-preserving surgery for multicentric carcinoma of the pancreas. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 1999;25(5):548-50. https://doi.org/10.1053/ejso.1999.0696.
Tryka AF, Brooks JR. Histopathology in the evaluation of total pancreatectomy for ductal carcinoma. Annals of surgery. 1979;190(3):373-81. https://doi.org/10.1097/00000658-197909000-00013.
Manning MA, Paal EE, Srivastava A, Mortele KJ. Nonepithelial Neoplasms of the Pancreas, Part 2: Malignant Tumors and Tumors of Uncertain Malignant Potential From the Radiologic Pathology Archives. Radiographics : a review publication of the Radiological Society of North America, Inc. 2018;38(4):1047–72. https://doi.org/10.1148/rg.2018170201.
Karmazanovsky G, Belousova E, Schima W, Glotov A, Kalinin D, Kriger A. Nonhypervascular pancreatic neuroendocrine tumors: Spectrum of MDCT imaging findings and differentiation from pancreatic ductal adenocarcinoma. European journal of radiology. 2019;110:66-73. https://doi.org/10.1016/j.ejrad.2018.04.006.
Eelkema EA, Stephens DH, Ward EM, Sheedy PF, 2nd. CT features of nonfunctioning islet cell carcinoma. AJR American journal of roentgenology. 1984;143(5):943-8. https://doi.org/10.2214/ajr.143.5.943.
Campisi A, Brancatelli G, Vullierme MP, Levy P, Ruszniewski P, Vilgrain V. Are pancreatic calcifications specific for the diagnosis of chronic pancreatitis? A multidetector-row CT analysis. Clinical radiology. 2009;64(9):903-11. https://doi.org/10.1016/j.crad.2009.05.005.
Lesniak RJ, Hohenwalter MD, Taylor AJ. Spectrum of causes of pancreatic calcifications. AJR American journal of roentgenology. 2002;178(1):79-86. https://doi.org/10.2214/ajr.178.1.1780079.
Barthet M, Portal I, Boujaoude J, Bernard JP, Sahel J. Endoscopic ultrasonographic diagnosis of pancreatic cancer complicating chronic pancreatitis. Endoscopy. 1996;28(6):487-91. https://doi.org/10.1055/s-2007-1005528.
Ohike N, Sato M, Kawahara M, Ohyama S, Morohoshi T. Ductal adenocarcinoma of the pancreas with psammomatous calcification. Report of a case. JOP : Journal of the pancreas. 2008;9(3):335-8.
Baker ME, Cohan RH, Nadel SN, Leder RA, Dunnick NR. Obliteration of the fat surrounding the celiac axis and superior mesenteric artery is not a specific CT finding of carcinoma of the pancreas. AJR American journal of roentgenology. 1990;155(5):991-4. https://doi.org/10.2214/ajr.155.5.2120970.
Brennan DD, Zamboni GA, Raptopoulos VD, Kruskal JB. Comprehensive preoperative assessment of pancreatic adenocarcinoma with 64-section volumetric CT. Radiographics : a review publication of the Radiological Society of North America, Inc. 2007;27(6):1653–66. https://doi.org/10.1148/rg.276075034.
Calabrese PR, Frank HD, Bartolomeo RS, Taubin HL. An unusual clinical presentation of pancreatic carcinoma: Duodenal obstruction in the absence of jaundice. The American journal of gastroenterology. 1976;66(5):480-2.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Material preparation: XHG and LJQ, idea for the article: LJQ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Formal approvals are not required for this type of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gong, X.H., Xu, J.R. & Qian, L.J. Atypical and uncommon CT and MR imaging presentations of pancreatic ductal adenocarcinoma. Abdom Radiol 46, 4226–4237 (2021). https://doi.org/10.1007/s00261-021-03089-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03089-6