Abstract.
The aim of this study was to clarify the characteristics of pancreatic head carcinomas mainly invading the groove between the duodenum and the pancreatic head. Nine patients with pathologically proven pancreatic head carcinomas underwent thin-slice dynamic CT, MR imaging, duodenal endoscopy, and angiography (seven patients). Plate-like masses within the groove region were seen in all cases, which showed hypointensity on T1-weighted images and slight hyperintensity on T2-weighted MR images. The masses appeared hypovascular in the early phase and delayed enhancement in the late phase of dynamic CT and MR imaging. On MR cholangiopancreatography, stenosis of intrapancreatic common bile duct was seen in all patients, whereas stenosis of the main pancreatic duct was seen in only three cases. Endoscopy revealed luminal narrowing of the duodenum in all patients, and duodenal mucosal biopsy demonstrated adenocarcinoma in seven patients. Abdominal arteriography showed serrated encasement of peripancreatic arteries in seven patients who received angiographic examinations. The CT and MR imaging findings of groove pancreatic carcinomas resemble those of groove pancreatitis. Differential diagnosis may be achieved by the pathological diagnosis of a biopsy specimen of the duodenal mucosa and arterial encasement on arteriography.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
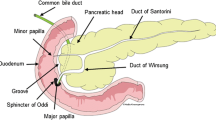
Groove pancreatitis is a special form of segmental chronic pancreatitis affecting the "groove" area (Fig. 1) between the pancreatic head, duodenum, and common bile duct [1, 2, 3, 4, 5]. Patients with groove pancreatitis frequently complain of abdominal pain and vomiting due to duodenal stenosis and obstructive jaundice due to common bile duct stenosis. Radiologically and pathologically, sheet-like scarred mass formation is seen between the duodenum and pancreatic head (Fig. 2). In the pure form of groove pancreatitis the main pancreatic duct is not involved; however, if the pancreatic head is involved by scar tissue, the main pancreatic duct shows stenosis and upstream dilatation. Groove pancreatitis and cystic dystrophy of the duodenal wall are thought to be the same entity [6, 7]. Distinction between this special form of pancreatitis and pancreatic head carcinoma is often difficult [8, 9, 10]. Several radiological findings of groove pancreatitis have been described on upper gastrointestinal series and endoscopic retrograde cholangiopancreatography (ERCP) [5]. Recently, dynamic CT findings and MR imaging, including MR cholangiopancreatography (MRCP), have also been reported [11, 12]. We experienced nine cases of pathologically proven pancreatic head carcinoma existing mainly in the groove area whose imaging findings resembled those of groove pancreatitis.
A 46-year-old man with groove pancreatitis. a Precontrast T1-weighted image shows a plate-like hypointense mass in the groove area (arrow). b The mass shows slight enhancement on early phase of dynamic MRI (arrow) and c prolonged enhancement on late phase (arrow). Duodenum shows enhancement with mural thickening (b, arrowhead)
The purpose of this study was to retrospectively review the clinical, radiological, and pathological characteristics of groove pancreatic carcinomas, and to clarify the points of differentiation between carcinoma and pancreatitis in the groove area.
Materials and methods
Nine patients (four men and five women) were included in the study, with a mean age of 72 years (age range 56–87 years). The study was based on 46 pancreatic head carcinomas diagnosed radiologically or pathologically from April 1998 to June 2001 in our institution. Nine cases included in this study were initially suspected as groove pancreatitis on CT examinations because of the existence of the scar-like masses within the groove area. Initial complaints were upper abdominal pain and vomiting in three patients and obstructive jaundice in six. Serum amylase was elevated in three patients. Pancreas divisum was suspected in one patient at endoscopic cholangiopancreatography (ERCP), which was performed in eight patients. Endoscopic examinations of the duodenum and mucosal biopsy of stenotic portions were done in all patients. Seven patients underwent pancreatoduodenectomy and two patients a bypass operation with biopsy of the pancreatic head tumor. The diagnosis was confirmed pathologically in all patients.
CT examinations
Helical dynamic CT was performed with a HiSpeed Advantage CT scanner, Remage CT scanner (GE Medical Systems, Milwaukee, Wis.). Precontrast CT and dynamic contrast-enhanced CT were done in all patients. Dynamic CT was scanned 35 s after iodized contrast medium injection (early phase) and 90 s (late phase). The 100 ml of contrast medium was injected at a rate of 3 ml/s with a power injector (Nemoto Kyorindo, Tokyo, Japan). The CT images were obtained with 3-mm collimation, pitch of 1.4, 3-mm reconstruction, and 200 mA.
MR examinations
The MR imaging was performed with a superconducting 1.5-T MR imager (Signa Horizon, GE Medical Systems, Milwaukee, Wis.). Spin-echo (SE) T1-weighted images (TR 500 ms, TE 9 ms, two acquisitions, 256×192 matrix), respiratory-triggered fast SE (FSE) T2-weighted images with frequency selective fat-suppression technique (TReff 3333–6666 ms/TEeff 80–90 ms), echo train length of 8–12, 256×224 matrix, three acquisitions). The slice thickness was 6 mm with a 2-mm intersection gap for T1- and T2-weighted images. Axial or oblique coronal dynamic MR imaging (SPGR; TR 160 ms/TE 1.6 ms/flip angle 90°, 256×128 matrix, one acquisition, fat suppression) with breath holding were performed. Dynamic MR imaging with SPGR sequence was acquired before and after intravenous administration of gadopentetate dimeglumine (Magnevist, Schering, Berlin, Germany). The slice thickness was 4–6 mm with 2-mm gap. Magnetic resonance cholangiopancreatography (MRCP) was also performed using a single-shot first spin echo (SSFSE) sequence with a phased-array coil. Both coronal thick slice MRCP (5 cm thick) using long TE (900 ms) and multislice MRCP images using medium TE (90 ms) in multiple oblique planes (4 mm thick, gapless) were obtained.
Angiographic examinations
In 7 patients, preoperative angiography was done focused on the pancreatic head and duodenal regions. Celiac and superior mesenteric arteriography were performed.
The CT, MR, and angiographic images were retrospectively reviewed by three experienced radiologists (T.G., M.K., N.T.) and correlated radiologic and pathologic findings.
Results
In all 9 patients a solid sheet-like mass was demonstrated between the duodenum and pancreatic head (Figs. 3, 4, 5). The masses were hypovascular in the early phase of dynamic CT (Figs. 3a, 4a, 5a). and revealed delayed enhancement (Figs. 3b, 4b). The duodenum adjacent to the mass showed concentric wall thickening with luminal narrowing (Figs. 3, 5). On MR imaging, the masses showed hypointensity on T1-weighted or fat-suppressed T1-weighted images (Figs. 3c) and slight hyperintensity or isointensity on fat-suppressed T2-weighted images (Fig. 3d), and hypovascularity in the early phase of dynamic MR imaging (Figs. 3e, 5b) and delayed enhancement in its late phase (Fig. 3f). Main pancreatic duct dilatation was depicted on MRCP in three of seven patients. One patient showed pancreas divisum, and the dorsal pancreatic duct was occluded at the minor papilla. In the other four patients, the main pancreatic duct was normal on MRCP (Fig. 4c). On CT and MR imaging, the pancreatic head seemed to be normal (Figs. 3, 4, 5) in five patients and was slightly invaded by the mass in four patients. Common bile duct dilatation due to stenosis was seen in all patients. From CT and MR imagings, we initially suspected groove pancreatitis rather than pancreatic head carcinomas because of specific location of the mass lesions.
A 56-year-old woman with groove pancreatic carcinoma presented with obstructive jaundice and duodenal stenosis. a Early-phase dynamic contrast-enhanced CT shows the poorly enhancing plate-like mass (arrow) in the groove area between the pancreas head and duodenum. b Late phase of dynamic CT shows heterogeneous enhancement (arrow). The duodenum shows circumferential thickening with luminal narrowing. Common bile duct and main pancreatic duct are dilated. c A sheet-like mass shows hypointensity on fat suppressed T1-weighted MR image (arrow) and d slight hyperintensity on fat-suppressed T2-weighted MR image (arrow). Pancreas head seems normal on T1- and T2-weighted images. e The mass is hypovascular in early phase of dynamic MR image (arrow) and f shows inhomogeneous enhancement in late phase (arrow). g Endoscopy of the duodenum shows a swollen mucosa with ulceration (arrow) of the minor papilla. Pathological diagnosis of the biopsy specimen of the ulcer demonstrated adenocarcinoma. h Macroscopically, the tumor (arrow) exists between the pancreatic head and duodenum. Common bile duct shows occlusion due to tumor invasion
A 63-year-old man with groove pancreatic carcinoma presented with obstructive jaundice. a Early phase of dynamic CT shows hypovascular plate-like mass in the groove region (arrow). b Late phase of dynamic CT shows delayed enhancement of the mass which is isodense to the pancreas head (arrow). c Thick-slice MR cholangiopancreatography (MRCP) shows irregular stenosis of common bile duct (arrowhead), whereas main pancreatic duct is normal. d Macroscopically, the tumor (open arrow, solid arrow) between the pancreatic head and the duodenum is seen. Ulcer of the duodenal mucosa (arrowhead) caused by tumor invasion is also visible
A 75-year-old man with groove pancreatic carcinoma presented with obstructive jaundice and duodenal stenosis. a Dynamic contrast-enhanced CT shows poorly enhancing mass (arrow) in the groove area with duodenal wall thickening. Biliary drainage tube is already introduced (small arrow). Peripancreatic lymphadenopathy (arrowheads) is seen. b Oblique coronal dynamic MR imaging shows poorly enhancing mass (arrowhead) in the groove region (arrowhead). Circumferential duodenal wall thickening (arrow) is also seen
However, endoscopy of the duodenum showed luminal stenosis due to mucosal edema with erosions in all patients. Mucosal biopsy of the duodenal lesions could demonstrate adenocarcinoma in seven of nine patients (Fig. 3g). Celiac and superior mesenteric arteriography showed serrated encasement of gastroduodenal arteries and/or pancreaticoduodenal arteries arcade branches in seven patients who received angiographic examinations. So preoperatively all nine patients were suspected carcinomas of the duodenum or the pancreatic head.
Pancreatoduodenectomy was done in seven patients and bypass operation (gastrojejunostomy and anastomosis of the common bile duct and jejunum) with biopsy of the mass in two. In all cases, plate-like scarred masses were seen extending into the groove between the duodenum and the pancreatic head. Pathological diagnosis was pancreatic adenocarcinoma of the pancreatic head extending to the groove and duodenal wall (Figs. 3h, 4d). Pathologically, main pancreatic duct (MPD) stenosis due to tumor invasion was seen only three patients. Intrapancreatic common bile duct was invaded in all seven cases who received pancreatoduodenectomy. A cystic lesion noted adjacent to the tumor in one patient was a retention cyst pathologically.
Discussion
Groove pancreatitis is a variant form of chronic pancreatitis affecting mainly the groove between the head of the pancreas, duodenum, and common bile duct. Becker et al. [2] classified it into a pure form and segmental form. The pure form of groove pancreatitis involves the groove only, with the pancreatic parenchyma and main pancreatic duct preserved. The segmental form of groove pancreatitis involves both the groove and the pancreatic head with main pancreatic duct stenosis. Groove pancreatitis and cystic dystrophy of the duodenal wall are the same entity. In both cases there is inflammation of the pancreatic tissue in the groove, which leads to chronic obstructive pancreatitis; the former may be a solid aspect which poses problems in differential diagnosis with pancreatic carcinoma [8], and the latter a macrocystic aspect [6, 7, 11, 12].
In previous studies the radiological findings of groove pancreatitis revealed smooth stenosis of bile duct and main pancreatic duct at ERCP, duodenal luminal narrowing at upper gastrointestinal series, and a plate-like mass in this region at ultrasonography, CT, and MRI [3, 4, 5, 8, 9, 10, 11, 12]. The distinction between fibrous scar in groove pancreatits and scirrhous adenocarcinoma of pancreatic cancer is difficult on CT and MR imagings [11, 12]. Both lesions show hypointensity on T1-weighted and fat-suppressed T1-weighted images and hyper- or isointensity on T2-weighted images. Both lesions also showed hypovascularity in the early phase and delayed enhancement in the late phase of dynamic CT and dynamic MR imaging [11, 12, 13, 14, 15, 16].
Pancreatic head carcinoma arises from the epithelium of the main pancreatic duct or a side branch. When the ductal carcinoma increases in size, it usually invades the main pancreatic duct with dilatation of its distal portion; however, in the case of pancreatic carcinoma arising in the groove region, the main pancreatic duct is occasionally spared tumor invasion. Scarring of the duodenal wall and stenosis of the duodenal lumen are very common in groove pancreatitis [1, 11]; however, duodenal wall thickening and luminal stenosis were seen in all of the groove pancreatic cancers in our series. Cystic lesions, either true cysts or pseudocysts in the groove area (mass or duodenal wall), are frequently noted in groove pancreatitis [1, 11, 12]. In cases of groove carcinomas, only one patient had a relatively large retention cyst adjacent to the tumor. Endoscopy of the duodenum showed luminal narrowing with mucosal edema and erosions. Mucosal biopsy of erosive lesions demonstrated adenocarcinoma in seven of nine patients, with pancreatic carcinoma diagnosed preoperatively (Fig. 3g). From the results of our examinations, hypovascular mass lesions with delayed enhancement and without cysts in the groove region, and pathological examination of mucosal biopsy specimens of duodenal lesions, are effective in differentiating groove pancreatitis and groove carcinoma; however, if the pancreatic carcinoma in the groove area is small and does not invade the duodenal mucosa, distinction between inflammatory mass and carcinoma may be quite difficult.
While arteriography showed encasement of gastroduodenal arteries and/or pancreaticoduodenal arcade branches in our study, thin-slice dynamic CT especially using multislice CT may evaluate the relationship of groove lesions with the vessels, particularly the gastroduodenal artery, which appears to be displaced to the left with "groove pancreatitis" and infiltrated when it is a tumor.
The differential diagnosis of groove pancreatic carcinoma other than groove pancreatitis includes duodenal carcinoma, common bile duct carcinoma, and peripancreatic lymph node metastases from other gastrointestinal malignant tumors.
In conclusion, pancreatic carcinomas arising in the groove area cannot be reliably differentiated from groove pancreatitis with CT and MR imagings when there are no cystic lesions within the mass and/or the thickened duodenal wall. Pathological diagnosis of a biopsy specimen of the duodenal mucosa invaded by the tumors and angiographic examination may provide the clue to the correct diagnosis.
References
Stolte M, Weiss W, Volkholz H et al. (1982) A special form of segmental pancreatitis: "groove pancreatitis". Hepatogastroenterology 29:198–208
Becker V, Mischke U (1991) Groove pancreatitis. Int J Pancreatol 10:173–182
Tio TL, Luiken GJ, Tytgat GN (1991) Endosonography of groove pancreatitis. Endoscopy 23:291–293
Shudo R, Obara T, Tanno S et al. (1998) Segmental groove pancreatitis accompanied by protein plugs in Santorini's duct. J Gastroenterol 33:289–294
Fujita N, Shirai Y, Tsukada K et al. (1997) Groove pancreatitis with recurrent duodenal obstruction. Report of a case successfully treated with pylorus-preserving pancreaticoduodenectomy. Int J Pancreatol 21:185–188
Procaccci C, Graziani R, Zamboni G et al. (1997) Cystic dystrophy of the duodenal wall: radiologic findings. Radiology 205:741–747
Vullierme MP, Vilgrain V, Flejou JF et al. (2000) Cystic dystrophy of the duodenal wall in the heterotopic pancreas: radiopathological correlations. J Comput Assist Tomogr 24:635–643
Yamaguchi K, Tanaka M (1992) Groove pancreatitis masquerading as pancreatic carcinoma. Am J Surg 163:312–316
Scapa E, Broide E, Halevy A et al. (1994) Groove pancreatitis and adenocarcinoma of the pancreatic head. Harefuah 127:161–162
Wapnick S, Hadas N, Purow E et al. (1979) Mass in the head of the pancreas in cholestatic jaundice: carcinoma or pancreatits? Ann Surg 190:587–591
Itoh S, Yamakawa K, Shimamoto K et al. (1994) CT findings in groove pancreatitis: correlation with histopathological findings. J Comput Assist Tomogr 18:911–915
Irie H, Honda H, Kuroiwa T et al. (1998) MRI of groove pancreatitis. J Comput Assist Tomogr 22:651–655
Gabata T, Matsui O, Kadoya M et al. (1994) Small pancreatic adenocarcinomas: efficacy of MR imaging with fat suppression and gadolinium enhancement. Radiology 193:683–688
Johnson PT, Outwater EK (1999) Pancreatic carcinoma versus chronic pancreatitis: dynamic MR imaging. Radiology 212:213–218
Furukawa H, Takayasu K, Mukai K et al. (1996) Late contrast-enhanced CT for small pancreatic carcinoma: delayed enhanced area on CT with histopathological correlation. Hepatogastroenterology 43:1230–1237
Fischer U, Vosshenrich R, Horstmann O et al. (2002) Preoperative local MRI-staging of patients with a suspected pancreatic mass. Eur Radiol 12:267–269
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gabata, T., Kadoya, M., Terayama, N. et al. Groove pancreatic carcinomas: radiological and pathological findings. Eur Radiol 13, 1679–1684 (2003). https://doi.org/10.1007/s00330-002-1743-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-002-1743-1