Abstract
Objective
Our purpose is to describe the MRI findings with pathologic correlation, in five patients with groove pancreatitis, a specific form of chronic pancreatitis affecting the groove between the pancreatic head, the common bile duct and duodenum.
Materials and methods
Five patients with pathologically proven (four cases) and clinical and MRI findings (follow-up) consistent with the diagnosis of groove pancreatitis (one case) were reviewed. Three patients underwent cephalic pancreatoduodenectomy (Whipple procedure) due to severe duodenal stenosis; MRI findings were correlated with the histological findings.
Results
In all patients a mass was seen affecting the groove between the pancreatic head and the duodenum. Precontrast images demonstrated hypointense tissue relative to pancreatic parenchyma on T1-weighted images and iso to slightly hyperintense tissue on STIR and T2-weighted images. Postcontrast dynamic Gd-DTPA images, showed peripheral mass enhancement on immediate postgadolinium images and progressive and centripetal mass enhancement on delayed images with good delineation of multiple cysts. Histologically, fibro-inflamatory tissue was demonstrated in the groove and the duodenal wall with obliterative concentric scarring of the distal common bile duct.
Conclusions
MRI findings are demonstrative of the pathologic features characteristic of this entity: the fibrous tissue in the pancreaticoduodenal groove, the duodenal wall inflammation and the groove and/or duodenal wall cyst formation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
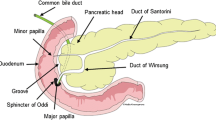
Groove pancreatitis is a special form of chronic pancreatitis affecting the groove between the head of the pancreas, the duodenum, and the common bile duct. The pancreatic parenchyma is spared or only slightly affected; therefore, it may be difficult to recognize this disease as a form of chronic pancreatitis and to differentiate it from pancreatic carcinoma [1].
Cicatrization in this anatomical space following acute pancreatitis in pancreatic heterotopies is discussed as the most probable cause for groove pancreatitis. Several reports [2–4] suggest that disruption of the flow of pancreatic juice in the accessory pancreatic duct and leakage into the groove are associated with the development of groove pancreatitis, at least in a subgroup of patients.
Although described more than 30 years ago, little is known about this entity by most clinicians, and only a few cases have been reported. The clinical and pathologic features of groove pancreatitis have been described in the literature [5, 6]. There have been few reports concerning CT findings [7, 8] and only one report describing findings on MRI [1]. Two reports highlight the differential diagnosis with malignancies [8, 9].
Materials and methods
Five patients (4 men and 1 woman), with a mean age of 47 years (ranges 40–53 years), were included in this retrospective study. The inclusion criteria were: pathologic confirmation of this entity or MRI findings and clinical follow-up consistent with the diagnosis of groove pancreatitis. The main clinical features are summarized in Table 1.
MRI was performed with a 1.5 T superconducting unit (Philips Gyroscan Intera Master; Best; The Netherlands). The standard protocol was: BH-FFE T1 in phase–out phase (sense body coil with SENSE factor 1.5, FOV of 380–410 mm, matrix size of 256–304 × 512r, scan percentage of 70%, 24 slices 7-mm thick with 1-mm intersection gap, TR/TE: 100/2.3–4.6 ms, flip angle of 80°, 1 NSA), Free Breath-TSE-T2 (sense body coil with SENSE factor 2, FOV of 380–410 mm, matrix size of 315 × 512, scan percentage of 60%, 24 slices 7-mm thick with 1-mm intersection gap, TR/TE: 2200/130 ms, respiratory compensation, 1 NSA), Free Breath-STIR (sense body coil with SENSE factor 2, FOV of 380–410 mm, matrix size of 241 × 512r, scan percentage of 75%, 24 slices 7-mm thick with 1-mm intersection gap, TR/TE: 1800/30 ms, IT: 165 ms, respiratory compensation, 2 NSA). Dynamic BH-3D-FFE images were obtained at baseline, 25, 90 and 180 s after a bolus injection of 0.15 mmol/kg of Gd-DTPA. Parameters for the BH-3D-FFE sequence were as follows: sense body coil with SENSE factor 2, FOV of 380–410 mm, matrix size of 256 × 256, scan percentage of 60%, 64 partitions 3-mm thick, TR/TE: 8.4/3.6 ms, Flip angle of 20°, 1 NSA, with fat suppression (PROSET). MRCP was performed using a BH-SS-TSE sequence with the following parameters: sense body coil, FOV of 270 mm, matrix size of 288 × 512r, scan percentage of 80%, nine radial slices 45-mm thick, TR/TE: 3000/800 ms, 1 NSA, with fat suppression (SPIR).
The MR images were reviewed and correlated with the pathologic findings in the resected specimens. None of the patients were submitted to ERCP. In one patient endoscopic ultrasound was performed confirming the MRI findings.
Results
We reviewed MR images in five patients with groove pancreatitis to describe the MR features and compare with those published [1]. All the patients showed an occupying mass lesion in the pancreaticoduodenal groove. The mass was hypointense relative to the pancreatic tissue on T1-weighted images, iso or slightly hyperintense on T2-weighted images and hyperintense on STIR images (Figs. 1, 3, 4, 6). Dynamic 3D-FFE sequence demonstrated minimal and patchy mass enhancement on arterial and portal phases with delayed enhancement (Figs. 1, 3, 4). In four patients the pancreatic parenchyma showed normal high-signal intensity on T1-weighted fat-suppressed images (pure form) (Figs. 1, 3, 4, 6). Only one patient showed hypointense areas on T1-weighted fat-suppressed images in the pancreatic head, with normal enhancement on the dynamic study (segmental form) (Fig. 2). Duodenal-wall thickening was seen in all patients, circumferentially in three (with mild to moderate stenosis in 2 and severe stenosis in 1) and medially in two, with periduodenal fluid in two patients (Figs. 1, 2, 3, 6). Four patients showed cysts in the groove or/and duodenal wall. The cysts ranged from a few mm to 4 cm, and they ranged in number from 4 to 12. Cysts were well demonstrated on T2-weighted, postgadolinium and MRCP images (Figs. 4, 5, 6). Fat-suppressed T1-Weighted images (FFE-PROSET) revealed the best delineation of the pancreatic head (normal hyperintensity) from the hypointense mass in the pancreaticoduodenal groove (Fig. 3). The duodenal wall showed hyperintensity on T2-weighted images with strong gadolinium enhancement in four patients. Peripancreatic vessels, mainly gastroduodenal artery, showed displacement in all five cases without encasement. Small peripancreatic lymphatic nodules were seen in three patients. The main pancreatic duct showed distal stenosis with tapering near the papilla with mild proximal and lateral branch dilatation in two cases and no abnormality in the other three patients. RMCP showed smooth distal common bile duct stenosis in three cases with medial displacement and no abnormality in two (Figs. 1, 4, 5).
Patient 1. Groove pancreatitis (pure form) in a 46-year-old man with history of recurrent upper abdominal pain. A–C A mass, band-like lesion, between duodenal wall and pancreatic head is well demonstrated (arrows). It shows hypointensity relative to pancreatic parenchyma on T1-weighted (A), slightly hypointensity with hyperintensity focus on FS-T2-weighted (B) and isointensity on STIR images (C). Note the duodenal wall swelling, with periduodenopancreatic ill-defined fluid, best visualized on T2-FS weighted and STIR images (arrow head in B, C). SS-TSE-T2 CPRM image shows distended gallbladder with slightly dilated bile ducts and main pancreatic duct with a relative hypointense mass with inner tiny hyperintense foci with duodenal lumen stenosis (arrows, D). Arterial phase of dynamic study shows mild mass enhancement (arrows) with strong enhancement of the duodenal wall and normal pancreatic head enhancement (E). In the equilibrium phase, groove mass enhancement (arrows) is similar to that of duodenal wall and pancreatic head (F).
Patient 4. Groove pancreatitis (segmental form). A FFE-T1-FS weighted image reveals a hypointense band-like mass in the pancreatoduodenal groove (black arrow). The pancreatic head shows hypointense areas (white arrow). B FS-FFE-T1 gadolinium dynamic portal phase shows normal pancreatic head enhancement (double arrow) and a mildly enhanced band-like mass in the pancreatoduodenal groove (white arrow ).
Patient 2. Groove pancreatitis (pure form) in a 47-year-old man with history of ethanol abuse with recurrent upper abdominal pain. A FFE-T1-FS weighted image reveals normal hyperintense pancreatic head (thin arrow) and a hypointense band-like mass in the pancreatoduodenal groove (thick arrows). B In the FS-TSE-T2 WI the band-like mass shows moderate hyperintensity (arrow). C On gadolinium dynamic study the mass showed progressive enhancement (not shown) with maximum contrast enhancement in the equilibrium phase (arrow). Note small cystic areas within the fibrous tissue. Control 15 months later, NORMAL FS-FFE-T1 (D), FS-TSE-T2 (E) and FS-FFE-T1 gadolinium dynamic equilibrium phase (F) shows normal pancreatic head (arrow) and normal pancreatic groove (double arrow ).
Patient 3. (A) A hypointense mass, sheet-like lesion, relative to pancreatic parenchyma is seen between duodenal wall and pancreatic head on T1-weighted image (arrow). (B) SS-TSE CPRM image shows small cysts surrounding the distal CBD, mainly between the duodenal wall and the bile duct (double arrow). (C) The fibrous tissue shows maximum gadolinium enhancement on equilibrium phase (arrow). (D) The resected specimen reveals a fibrous tissue mass between the pancreatic head and medial duodenal wall. (E) Microscopic view demonstrates a dense fibrous area with muscular fibers surrounding atrophic pancreatic parenchyma.
Patient 1. Four months later the upper abdominal pain worsened. The patient developed nausea and vomiting with loss of weight, due to duodenal stenosis. A–C A new MRI reveals developing of multiple cysts (thin arrows) in the duodenopancreatic groove and inside the duodenal wall, with secondary severe duodenal stenosis and biliopancreatic ductal dilatation (thick arrows B, C). Note the dilated main pancreatic duct and lateral branches with irregular distal stenosis; the common bile duct is also mildly dilated (thick arrows B, C). Normal T1-weighted fat-suppressed hyperintensity pancreatic head is well demonstrated (A). Hypointense T1 and T2 weighted tissue surrounds the cysts. The dynamic study reveals slow and progressive enhancement of this tissue, mild on arterial phase and maximum on delayed phase (double arrow in A, D, E). F The resected specimen demonstrated a fibrous tissue mass between duodenal wall and pancreatic head (thin arrows) and several cysts within the fibrous mass (thick arrows). Pancreatic head (p). Duodenum (d). G Histologic examination reveals atrophic pancreatic acini embedded in concentric fibrosis (thin arrows); muscular fibers of the duodenal muscularis propia layer can be seen in the peripheria (thick arrows). H Brunner glands hyperplasia in the duodenal wall submucosal layer (thin arrows) with adjacent abscesificant inflammation (thick arrow ).
Patient 5. A A hypointense mass relative to normal pancreatic parenchyma is seen between duodenal wall and pancreatic head on T1-weighted image (thin arrow). Normal pancreatic head (thick arrow). B The FS-TSE-T2 image shows a small cyst between the hyperintense mass (thin arrow) and the normal pancreatic head (thick arrow) C The resected specimen demonstrated fibrous tissue in duodenal wall and pancreatic groove (thin arrows). D Microscopic examination shows duodenal wall fibrosis with embebed pancreatic tissue, pancreatic ducts surrounded by muscular fibers (myoadenomatosis) and Brunner glands hyperplasia.
None of the patients were submitted to ERCP. In one patient endoscopic ultrasound was performed confirming the MRI findings. Four patients underwent surgery. High-grade duodenal stenosis was the major indication.
Three patients underwent cephalic duodenopancreatectomy (Whipple procedure). Histological examination demonstrated fibrous tissue proliferation between the pancreatic head and duodenal wall with thickening and fibrosis of duodenal wall, Brunner glands hyperplasia and clustering of microcysts inside the layers of duodenal wall and the groove. No heterotopic pancreas was found. The common bile duct showed obliterative concentrical fibrosis in its pancreatic segment. The resected pancreatic head showed normal pancreatic parenchyma in two cases and segmental chronic pancreatitis in the other one (Figs. 4, 5).
In one patient several biopsies were obtained from pancreatic head and groove. None revealed malignancy. Fibroinflamatory changes were found.
The diagnosis of the fifth patient was based on a combination of consistent imaging findings and clinical follow-up. No surgery was needed. The patient underwent a control CT and a new MRI study (for investigational purpose) 15 months later showing complete resolution of his process (Fig. 3D–F).
Discussion
Groove pancreatitis is a special form of chronic pancreatitis affecting the groove between the head of the pancreas, the duodenum, and common bile duct. Becker first described a segmental type of chronic pancreatitis, which involves the anatomic space (“groove”) between pancreatic head, common bile duct, and duodenum [10]. Stolte et al. [11] reported 30 cases of groove pancreatitis among 123 surgical pancreatoduodenectomy specimens in patients with chronic pancreatitis. They distinguished between a genuine and a segmental forms, using the term “pure groove pancreatitis” in cases where scarring was only found in the groove, and “segmental groove pancreatitis” if dorsocranial parts of the pancreatic head were also involved. The main morphologic features included replacement of pancreatic parenchyma by scar tissue in the segmental form and minimal dilatation of the bile duct. Cicatrization and stenosis of the duodenal wall, and hyperplasia of Brunner’s glands have often been identified. Encasement of the common bile duct was present in almost all specimens, while duct stenosis was seen in 67% and 27% of pure and segmental forms, respectively. The authors concluded that these features might help to distinguish the disease from pancreatic carcinoma, where hyperplasia of Brunner’s glands is usually absent, duodenal stenosis less frequent, and tubular bile duct stenosis rare. Pancreatic carcinoma usually shows irregular stenosis of biliary and pancreatic duct instead. Gabata et al. [8] think that groove pancreatitis and cystic dystrophy of the duodenal wall are one and the same entity. Imaging findings, clinical evolution and outcome are very similar in both diseases. Focus of heterotopic pancreas in the groove and duodenal wall is the landmark of cystic dystrophy of the duodenal wall. However, heterotopic pancreas is found not always in groove pancreatitis; other factors have also been proposed. Outflow of pancreatic juice in the accessory pancreatic duct and leakage into the groove are associated with the development of groove pancreatitis, at least in a subgroup of patients.
Adsay et al. [12] suggested the term “paraduodenal pancreatitis” to refer to a distinct clinico-pathological entity unifying “cystic dystrophy of heterotopic pancreas”, “para-duodenal wall cyst” and “groove pancreatitis”. These lesions have the following common characteristics: (a) the duodenal wall contains dilated ducts, some with inspissated secretions, and pseudocystic changes as well as adjacent stromal reactions including hypercellular granulation tissue, foreign-body type giant cell reaction engulfing mucoprotein material, and myofibroblastic proliferation. (b) Brunner’s gland hyperplasia is typically present. (c) Dense myoid stromal proliferation, with intervening rounded lobules of pancreatic acinar tissue, creates a histologic picture reminiscent of “myoadenomatosis”, “pancreatic hamartoma”, or even leiomyoma in some cases. (d) Spill over of fibrosis into the adjacent pancreas and soft tissue occurs, especially in the “groove” area (between the pancreas, common bile duct and duodenum), including the region around the common bile duct. (e) Clinically, these lesions often mimic “pancreatic cancer” or pariampullary tumors, because of marked scarring as well as ill-defined borders of the process.
Itoh et al. [7] first report a CT-pathological correlation in four patients with this entity.
Gabata et al. [8] reported the radiological (CT and MRI) and pathological findings in nine patients with groove pancreatitis. Irie et al. [1] reported the MRI findings in five patients with groove pancreatitis. The most characteristic finding on MRI was a sheet-like mass between the pancreatic head and the duodenum associated with duodenal-wall thickening. The mass was hypointense to pancreatic parenchyma on T1-weighted images and iso-to slightly hyperintense on T2-weighted images, and dynamic imaging showed delayed enhancement. In our series the mass was also hypointense relative to the pancreatic tissue on T1-weighted images and iso or slightly hyperintense on T2-weighted images and hyperintense on STIR images. Fat-suppressed T1-Weighted images (FFE-PROSET) reveal the best delineation of the pancreatic head (normal hyperintensity) from the hypointense mass in the pancreaticoduodenal groove. Dynamic study demonstrated minimal and patchy enhancement on arterial and portal phases with enhancement of the fibrous tissue on delayed phase. None of our patients were submitted to ERCP because MRCP images were considered of high quality depicting the bile duct and MPD, the relationship between the duct system and cysts within the groove and duodenal wall with high conspicuity.
The differential diagnosis of groove pancreatitis includes duodenal adenocarcinoma, common bile duct cholangiocarcinoma, acute pancreatitis and pancreatic adenocarcinoma. The distinction between fibrous scar in groove pancreatitis and scirrhous adenocarcinoma of pancreas is difficult on CT and MRI [7, 10]. Both entities show similar findings on baseline and postgadolinium images: hypointense mass on T1-weighted images and iso-hiperintensity on T2-weighted images, with hypovascularity in the arterial phase and delayed enhancement in the late phase of dynamic CT and MR imaging [7, 10, 13, 14]. Pancreatic carcinomas arises from the epithelium of the main pancreatic duct or a side branch. When the ductal carcinoma increases in size, it usually invades and obstructs the MPD with proximal dilatation; however, in the case of pancreatic carcinoma arising in the groove region, the main pancreatic duct is occasionally spared tumor invasion. Groove pancreatitis usually does not obstruct the MPD. In our study RMCP showed smooth distal common bile duct stenosis in three cases with medial displacement and no abnormality in two. An important differentiating point is the presence of peripancreatic vessel encasement. In groove pancreatitis, periarterial fibrosis is observed histologically; however, the affected vessels are small arterioles in the fibrous scar tissue. No major vessel encasement has been reported. Pancreatic carcinoma extending to the groove or duodenal wall invades along peripancreatic vessels [10]. In our study peripancreatic vessels, mainly gastroduodenal artery, showed displacement in all five cases without encasement. Thickening of the duodenal wall and luminal stenosis are very common in groove pancreatitis [7]; however, these findings are also common in the pancreatic cancer arising in the pancreatic groove. Mucosal biopsy of erosive duodenal lesions showed by endoscopy demonstrated high positive predictive value in diagnosing adenocarcinoma preoperatively. Cystic lesions in the groove region (mass or duodenal wall) are more common in groove pancreatitis than in pancreatic cancer [8]. A band-like hypovascular mass lesion with delayed enhancement with cysts in the groove region, vascular (gastroduodenal artery) displacement without obstruction and pathological examination of mucosal biopsy specimens of duodenal lesions, are effective in differentiating groove pancreatitis and groove carcinomas; if carcinoma is small and does not invade the duodenal mucosa, distinction may be difficult [8].
In conclusion, the MRI and MRCP findings of groove pancreatitis although not pathognomonic, are suggestive of this entity and so MRI is an important tool in the diagnosis and management of this rare entity.
References
Irie H,Honda H, Kuroiwa T, et al. (1998) MRI of groove pancreatitis. J Comput Ass Tomogr 22:651–655
Shudo R, Obara T, Tanno S, et al. (1998) Segmental groove pancreatitis accompanied by protein plugs in Santorini´s duct. J Gastroenterol 33:289–294
Taya N, Okamoto M, Shirota K, et al. (1993) A case of groove pancreatitis accompanied by minute carcinoma of Santorini’s duct (Japanese with English abstract). J Jpn Panc Soc 8:449–55
Isayama H, Kawabe T, Komatsu Y, et al. (2005) Successful treatment for groove pancreatitis by endoscopic drainage via the minor papilla. Gastroint Endosc 61:175–178
Yamaguchi K, Tanaka M (1992) Groove pancreatitis masquerading as pancreatic carcinoma. Am J Surg 163:312–316
Becker V, Mischke U (1991) Groove pancreatitis. Int J Pancreatol 10:173–182
Itoh S, Yamakawa K, Shimamoto K, et al. (1994) CT findings in groove pancreatiitis: correlation with histopathological findings. J Comput Assist Tomogr 18:911–915
Gabata T, Kadoya M, Terayama N (2003) Groove pancreatic carcinomas: radiological and pathological findings. Eur Radiol 13:1679–1684
Mohl W, Hero-Gross R, Feifel G, et al. (2001) Groove pancreatitis: an important differential diagnosis to malignant stenosis of the duodenum. Dig Dis Sci 46(5):1034–1038
Becker V (1973) Bauchspeicheldrüse (inselapperat augsgenommen). In: Doerr W, Seiffert G, Ühlinger E, (eds). Spezielle Pathologische Anatomie. Berlin: Springer
Stolte M, Weiss W, Volkholz H, et al. (1982) A special form of segmental pancreatitis: “groove pancreatitis”. Hepatogastroenterology 29:198–208
Adsay N, Zamboni G (2004) Paraduodenal pancreatitis: a clinico-pathologically distinct entity unifying “cystic dystrophy of heterotopic pancreas”, “para-duodenal wall cysts”, and “groove pancreatitis”. Sem Diagn Pathol 21:247–254
Jhonson PT, Outwater EK (1999) Pancreatic adenocarcinoma versus chronic pancreatitis: dynamic MR imaging. Radiology 212:213–218
Fischer U, Vosshenrich R, Horstmann O, et al. (2002) Preoperative local MRI-staging of patients with a suspected pancreatic mass. Eur Radiol 12:267–269
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castell-Monsalve, F.J., Sousa-Martin, J.M. & Carranza-Carranza, A. Groove pancreatitis: MRI and pathologic findings. Abdom Imaging 33, 342–348 (2008). https://doi.org/10.1007/s00261-007-9245-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-007-9245-x