Abstract
Purpose
This study was to investigate the effect of mesorectal fat tissue volume (MRV) on the pathological response to neoadjuvant chemoradiotherapy (nCRT) in patients with locally advanced rectal cancer.
Methods
88 patients who had been diagnosed with locally advanced rectal cancer between January 2017 and June 2020 were reviewed retrospectively. The total abdominal, subcutaneous, visceral, and mesorectal fatty tissue components were measured semiquantitatively by two radiologists using computed tomography (CT)-based findings. The patients were divided into two groups as those with and without a pathological response to nCRT. The relationship of MRV with the other fat tissue components of the body was also evaluated.
Results
We performed a retrospective analysis of 88 patients (mean age 62.7 years [range, 33–90 years]; 31 males and 57 females). A positive response to nCRT was present in 47 patients. There were 59 patients with stage 3 disease. 46 patients demonstrated lymph node involvement. The mean MRV was 69.6 ± 31.0 ml in no-response group and 105.8 ± 47.5 ml in response-positive patients (p < 0.05). MRV showed the highest correlation with visceral fat volume (VFV). There was a negative correlation between the MRV and the N stage. A cut-off value of ≥ 69.4 for MRV predicted the repsonse to nCRT, with 82.9% sensitivity and 58.5% specificity [AUC: 0.757 (0.653–0.842), p < 0.001] in receiver operating characteristic (ROC) curve analysis
Conclusions
MRV can be used as a novel parameter in predicting of pathological response to nCRT in locally advanced rectal cancer patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is a major health issue, with approximately 1,400,000 new cases and 700,000 deaths originating globally every year. In addition to being the third most common malignancy, it is also the fourth most frequent cause of cancer-related deaths [1]. Almost a third of all colorectal cancers are rectal cancers [2]. The frequency of rectal cancer varies from country to country [2]. Although the curative treatment of early-stage rectal cancer is surgery, the prognosis differs in patients with advanced tumor stage and/or positive lymph nodes, which is defined as locally advanced rectal cancer. In these patients, neoadjuvant chemoradiotherapy (nCRT) followed by total mesorectal excision is the standard choice of therapy for the local control and cure of the disease [3, 4]. Tumor downsizing has been reported in patients undergoing nCRT, and a complete pathological response was noted in 15–27% of these patients [5, 6]. Magnetic resonance imaging (MRI), endoscopic ultrasonography, and digital examination are the most suitable methods to evaluate tumor response and morphology following nCRT [7,8,9]. Assessment of response to neoadjuvant chemoradiotherapy (nCRT) is another crucial issue in rectal cancer. Although the RECIST system provides great help for this purpose, there is still no specific evaluation system that has gained wide acceptance and falls within the guidelines. Numerous systems are available for assessing of the pathological response to nCRT, such as those labeled, The American Joint Committee on Cancer (AJCC), the Mandard and Dworak Systems, and the RyanTumor Regression Grading (TRD) System [10, 11]. Recently, radiomics studies have been performed to radiologically predict pathological response [12,13,14].
It has been reported that the presence of lymph node involvement and mesorectal tumor invasion affect the response to nCRT [15]. However, the number of detailed studies on other influencing factors is rather limited. The purpose of this study was to evaluate the effect of mesorectal fat tissue volume (MRV) on the pathological response to nCRT in patients with locally advanced rectal cancer or rectal cancer with metastatic lymph nodes. Another objective of the study was to investigate the relationship of MRV with body mass index (BMI) and abdominal fat tissue components.
Materials and methods
This study was approved by the Ethical Committee. It was conducted in full accordance with the guidelines of the Declaration of Helsinki. 170 patients who had been diagnosed with rectal cancer in our hospital between January 1, 2017 and June 30, 2020 were retrospectively analyzed. 88 patients who had undergone a pre-treatment colonoscopic biopsy procedure and received a pathological diagnosis of rectal cancer were included in the study. The staging of the patients was done by the utilization of the T and N evaluation criteria, by means of MRI. Lymph nodes with an unfavorable morphology and a diameter above 5 mm were evaluated as pathologic, in terms of lymph node involvement. N0 was evaluated as no lymph nodes, whereas N1 was evaluated as 1–3 suspicious nodes, and N2 as 4 or more suspicious nodes. Thoracoabdominal computed tomographic (CT) examinations were performed in order to evaluate distant organ metastases.
These patients participating in the present study had middle and inferior rectal involvement and were operated within 4–8 weeks following nCRT. The demographic data of the patients, such as age, gender, height, weight, BMI, and medical history, were obtained from the hospital information service (HIS) database. Those with inadequate CT image quality, as well as with a history of pelvic surgery and/or pelvic CRT or radiotherapy, and a pelvic mass and fluid collection which might impair evaluation, were excluded from the study. The postoperative pathological response was evaluated by a single pathologist, to maintain an evaluation standard. The modified Ryan scoring system was utilized for pathological evaluation [16]. This system renders the pathological response in the following terms: complete response, if no vital cancer cells are present; near-complete response, if a single or few cancer cells are present; minimal response, if there is a mild regression in tumor cells; and finally no response, if there is no regression of the tumor. Because the distribution of our patients was not homogenous, we defined the complete and partial response groups as response-positive, and the minimal-response and no-response groups as response-negative, categories.
Capecitabine (2 × 825 mg/m2) was administered to the patients, as the chemotherapeutic agent. The dose required for radiotherapy in rectal cancer to treat microscopic disease with conventional fractionation is 45–50.4 Gy. The radiation dose received by the patient varies depending on the presence of T3 and T4 or lymph nodes. Mesorectal adipose tissue volume does not change the radiotherapy dose. However, irradiation is done in 3-dimensions to cover the entire mesorectum.
Volumetric measurements of abdominal and mesorectal adipoid tissue
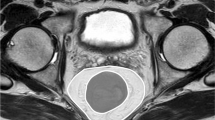
Imaging was performed by a 128-detector MDCT system (Philips Ingenuity 128, Eindhoven, The Netherlands). The technical parameters utilized were as follows: 120 kvP, 200–400 mAs automatic tube current modulation, rotation time 0.42 s, pitch 0.6, slice thickness: 1 mm, for all phases. Contrast-enhanced scanning was performed by the injections of saline, non-ionic iodinated contrast media, and finally 20 ml saline, in sequential order, all via the antecubital vein, and by means of an automatic infusion pump. Oral contrast was administered. All measurements were performed at the portal venous phase. During the measurements and evaluations, all CT images were examined at the soft-tissue window. These images were evaluated at a workstation (Intelli SpacePhilips [IPS], The Netherlands); and abdominal and mesorectal adipose tissue volumes were calculated (Fig. 1a). − 130 to − 30 HU values were utilized for adipose tissue voxels. The abdominal adipose tissue compartment is the space between the diaphragmatic esophageal hiatus and the symphysis pubis. This visceral fatty tissue contains the mesenteric, subperitoneal, and retroperitoneal fatty compartments [17]. The subcutaneous fatty tissue is defined as the region localized between the muscle layers of the abdominal wall, together with the paraspinal subcutaneous fatty compartments [17]. In accordance with the protocol of our study, the total adipose tissue volume was calculated first, followed by the measurement of the visceral and subcutaneous fat tissue volumes (Fig. 1b–d). The mesorectal adipose tissue compartment was defined as the space between the rectosigmoid junction proximally, and the most distal axial sections devoid of perirectal fat density, distally. The adipose tissue volume surrounding the rectum was evaluated. Special attention was paid to the interfaces of the mesorectal fascia and other visceral organs. These structures were the intestines, female genitalia, male bladder, seminal vesicles, and the prostate. During the measurement process, all images created by the software were checked by two radiologists, against the possibility of the erring; and corrections were made by these radiologists, if necessary. Manually performed corrections were made by the utilization of the “editing tool”, in order to prevent the inclusion of solid organs, intestines, vessels, and fat-free tissues like the skeleton, into the measurement areas. The visceral, subcutaneous, and mesorectal fat tissue volumes were measured in millimeters and automatically, by the software program. All measurements were performed by two radiologists, in consensus. For the measurements, all images obtained from the software were used. No extrapolation procedures such as addition and multiplication were utilized. This made possible the calculation of the real adipose tissue volumes.
Statistics
Statistical methods
For the descriptive statistics of the data, the mean value, standard deviation, median, lowest and highest values, frequency, and ratio values were obtained. The distribution of the variables was measured by the utilization of the Kolmogorov–Smirnov test. The independent variable t test, the Kruskal–Wallis, and the Mann–Whitney U tests were used for the analysis of the quantitative independent data. The Chi-square test was utilized for the analysis of the qualitative independent data. Correlation analysis was performed by the Spearman correlation analysis method. Receiver operating characteristic curve analysis was used to determine the cut-off value of MRV in predicting the response to nCRT based on the Youden index. The p values under 0.05 were appointed as statistically significant. The SPSS 26.0 statistical software package was utilized for the statistical analyses.
Results
88 patients, 57 of whom were female, were enrolled in the study. The overall mean age of the patients was 62.7 ± 10.8 years (min–max 33–90 years). The mean BMI was 26.6 ± 3.6 kg/m2 (min–max 19.1–34.9 kg / m2). 59 patients had stage T3 disease, while 29 had stage T4 disease. 42 patients were defined as N0, while 34 were defined as N1, patients. 12 patients were categorized as N2. The mean total fat volume (TFV) was 11,074 ± 4208 ml (min–max 2973–21,988 ml), while the mean visceral fat volume (VFV) was 4213 ± 1777 ml (min–max 1130–9297 ml), and the mean subcutenous far volume (SFV) was 6885 ± 2780 ml (min–max 1843–14,397 ml). The mean MRV was found to be 88.9 ± 44.3 ml (min–max 21.2 ± 269.0 ml). Detailed data are summarized in Table 1.
No statistically significant relationship was found between the mesorectal fat tissue volume (MRV) and patient age. But there was a positive correlation between the MRV and TFV, VFV, body mass index (BMI), weight, and height. The highest correlation was with the VFV, and the r coefficient was calculated as 0.666. Detailed findings are demonstrated at Table 2.
The patients were categorized into certain groups according to their responses to nCRT. These groups were as follows: no response, minimal response, partial or near-complete response, and complete response. As the response rate to nCRT increased, so did the MRV, TFV, VFV, and SFV values (Table 3).
Patients with no or minimal pathological response were evaluated as response-negative cases; while patients with partial or complete response were evaluated as response-positive cases. The mean MRV values were found to be 69.6 ± 31.0 ml for the response-negative groups and 105.8 ± 47.5 ml for the response-positive groups (p < 0.005). No statistically significant association was found between the pathological response rates and the BMI, TFV, VFV, and SFV values. The pathological response to nCRT has been found to decrease as the N stage increases (p < 0.05). The region of rectal involvement, T stage, age, and gender have been shown to be factors that influence the pathological response rate to nCRT (p < 0.05) (Table 4).
A cut-off value of ≥ 69.4 for MRV predicted the response to nCRT, with 82.9% sensitivity and 58.5% specificity [AUC: 0.757 (0.653–0.842), p < 0.001] in receiver operating characteristic (ROC) curve analysis (Graphic 1).
No statistically significant association was found between the MRV values and the T stages of the disease, whereas it was shown a statistically significant negative relationship exists between MRV and tumor N stage (p < 0.05) (Table 5).
Discussion
Certain parameters are used for predicting the pathological response to neoadjuvant chemoradiotherapy. Studies indicate that early N stage and small tumor size, together with low CEA values, can be used as a parameter in the prediction of response to nCRT [18,19,20]. These parameters may occasionally lead to varying results; nevertheless, we have also other options in hand, for this prediction task including MRI sequences such as diffusion-weighted imaging (DWI), T2-weighted imaging (T2WI), and dynamic contrast MR imaging [21,22,23]. To the best of our knowledge, this is the first study to investigate the role of the mesorectal adipose tissue in the prediction of the pathological response to nCRT for the treatment of rectal cancer. A previous study has focused on the effects of TFV, VFV, and SFV on the survival rate in rectal cancer patients [24]. Another study has revealed that the survival rates increase as the MRV increases [25]. In our study, however, we have investigated the effects of MRV on the response to nCRT in patients with rectal cancer.
The amount of mesorectal adipose tissue is more affected by foreign fat accumulation than the natural bone structure around the pelvis [25]. Therefore, as the total body fat increases, the mesorectal adipose tissue volume also increases. There is also a positive correlation between MRV and VFV, TFV, and SFV (p < 0.05). The correlation coefficient between MRV and VFV is rather high. This may well be attributed to the fact that MRV is a component of VFV. This positive correlation between the VFV and MRV has also been demonstrated in various previous studies [26]. A valuable result of our study was the increased response to nCRT at higher MRV values. Although our study has not aimed to investigate the possible effects of TFV, VFV, and SFV on the pathological response to nCRT, the study has shown that these factors do not affect the pathological response. However, Lee et al. [27] demonstrated that the subcutaneous and visceral adipose tissue volumes, MRV is a component of VFV, have an effect on the survival of rectum cancer patients. We think that further studies are needed to investigate the effects of the visceral and subcutaneous fat volumes on the pathological response to nCRT.
No statistically significant association was found between the MRV and the T stages of the tumor. On the other hand, a negative correlation was found between the MRV and the N stage of the tumor: the MRV was found to decrease as the N stage increased (p < 0.005). Torkzad et al. [28] have shown that nodal involvement was less in patients with high mesorectal volumes, in a multinational study they performed on 267 rectal cancer patients. The fact that a high MRV is associated with a higher response rate to nCRT may be explained by the buffer effect of the fat compartment against the local invasion and intrafascial micro lymph-node-metastasis of the tumor [29, 30]. In other words, it may be said that as the N stage increases, the MRV decreases; and this gives way to an increase in tumor burden and decrease in response. On the other hand, it is possible that the N stage may have increased due to the initially low MRV present at the beginning of the disease. Both of these circumstances may cause this result. Another hypothesis we suggest to explain this situation is that a local excretion of some forms of immune cytokines from the mesorectum due to the stimulation created by nCRT may be contributing to the effect of the therapy. Histopathological studies are needed to prove this hypothesis.
Our study has some limitations: (1) tumor sizes have not been taken into consideration. We consider that the tumor size may not have a large effect on the radiation dose. The presence of lymph nodes increases the radiation dose applied. Besides, in the presence of lymph nodes, radiation application is not focused on the tumoral area. All these factors led us to think that the tumor size may be neglected. Thus, a study has reported that tumor size is not a predictor of pathological response to nCRT [31]. (2) The single-center structure of our study is a limitation of its own. The number of our complete pathological-response patients is quite close to the numbers in the literature; but still, the rather low total number of our patients is a limiting factor in dividing them into two distinct groups, one with a full, and the other one with no, pathological response. This limitation is crucial in terms of statistical sample grouping. (3) There were also certain limitations on assessment and evaluation. The images we evaluated were static, frozen images. This is rather intriguing, because it may lead to a false suggestion that the mesorectum is an anatomically stable space and not influenced by the bodily habits of the individual [26]. Since there is no established reference point designated for the measurement of MRV, both the individual characteristics and the differences in experience of the evaluators may lead to false results. Thus, there is a certain need for standardization. In our study, evaluations were performed by two radiologists, utilizing the same reference points. We assume this has reduced the possibility of erring in measurements, to the minimums. These validations, however, need to be conducted within an interobserver structure.
Conclusions
Our study has demonstrated that MRV may be used as a novel parameter in predicting the pathological response to nCRT in patients with locally advanced rectum cancer. Routine MRV measurements may be performed easily on CT images of rectal cancer patients. It has been noted that the pathological response rate increases as the MRV exceeds 69.4 ml. The AUC value for MRV has been assessed as 0.757 (95% CI 0.653–0.842). We suggest that the fixation of the nutritional status of the patients could be of benefit for the final outcome of the therapy.
References
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683-691. doi: https://doi.org/10.1136/gutjnl-2015-310912.
Siegel RL, Kimberly D Miller KD, Jemal A. Cancer statistics, CA Cancer J Clin. 2020 Jan;70(1):7-30. doi: https://doi.org/10.3322/caac.21590
van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM-K, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled. Randomized Controlled Trial TME trial. 2011;12(6):575–582. doi: https://doi.org/10.1016/S1470-2045(11)70097-3
van de Velde JH Cornelis, Boelens PG, Josep MB, Jan C-W, Andres C, Lennart B, et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum, Eur J Cancer. 2014; 50(1):1-34. https://doi.org/10.1016/j.ejca.2013.06.048
Monique Maas, Patty J Nelemans, Vincenzo Valentini, Prajnan Das, Claus Rödel, Li-Jen Kuo, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010; 11(9):835–844. doi: https://doi.org/10.1016/S1470-2045(10)70172-8
Påhlman L, Bohe M, Cedermark B, Dahlberg M, Lindmark G, Sjödahl R, et al. The Swedish rectal cancer registry. Br J Surg. 2007; 94(10):1285-1292. doi: https://doi.org/10.1002/bjs.5679.
Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, et al. Magnetic resonance imaging–detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011; 29(28):3753-3760. doi: https://doi.org/10.1200/JCO.2011.34.9068
Patel UD, Brown G, Rutten H, West N, Sebag-Montefiore D, Glynne-Jones R,et al. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2012; 19(9):2842-2852. doi: https://doi.org/10.1200/JCO.2011.34.9068
Hötker AM, Tarlinton L, Mazaheri Y, Woo KM, Gönen M, Saltz LB, et al. Multiparametric MRI in the assessment of response of rectal cancer to neoadjuvant chemoradiotherapy: A comparison of morphological, volumetric and functional, MRI parameters. Eur Radiol. 2016; 26(12):4303-4312. doi: https://doi.org/10.1007/s00330-016-4283-9
Kim SH, Chang HJ, Kim DY, Park JW, Baek JY, Kim SY, et al., What Is the Ideal Tumor Regression Grading System in Rectal Cancer Patients after Preoperative Chemoradiotherapy?, Cancer Res Treat. 2016 Jul; 48(3): 998–1009.
Santos MD, Silva C, Rocha A, Matos E, Nogueira C, Lopes C. Prognostic Value of Mandard and Dworak Tumor Regression Grading in Rectal Cancer: Study of a Single Tertiary Center, ISRN Surg. 2014 Mar 4;2014:310542. doi: https://doi.org/10.1155/2014/310542
Cui Y, Yang X, Shi Z, Yang Z, Du X, Zhao Z, et al. Radiomics analysis of multiparametric MRI for prediction of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol. 2019; 29(3):1211-1220. doi: https://doi.org/10.1007/s00330-018-5683-9
Yi X, Pei Q, Zhang Y, Zhu H, Wang Z, Chen C, et al. MRI-based radiomics predicts tumor response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Front Oncol. 2019;26;9:552-562 https://doi.org/10.3389/fonc.2019.00552
Shu Z, Fang S, Ye Q, Mao D, Cao H, Pang P, et al. Prediction of efficacy of neoadjuvant chemoradiotherapy for rectal cancer: the value of texture analysis of magnetic resonance images, Abdom Radiol. 2019; 44(11):3775-3784. doi: https://doi.org/10.1007/s00261-019-01971-y
Ryan JE, Warrier SK, Lynch AC, Herio AG. Assessing pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a systematic review. Colorectal Dis. 2015; 17(10):849-861. https://doi.org/10.1111/codi.13081
Kidron D, Lahav L, Berkovich L, Mishaeli M, Haj N, Shmuel Y. Lack of Pathological Response of Rectal Cancer to Neoadjuvant Chemoradiotherapy is Associated with Poorer Long-Term Oncological Outcomes. Clinical Oncology and Research 2019; 2(5):1-6. doi: https://doi.org/10.31487/j.COR.2019.5.18
Akay S, Urkan M, Balyemez U, Erşen M, Taşar MJD. Is visceral obesity associated with colorectal cancer? The first volumetric study using all CT slices. Diagn Interv Radiol. 2019; 25(5):338-345. doi: https://doi.org/10.5152/dir.2019.18350.
Garland ML, Vather R, Bunkley N, Pearse M, Bissett IP. Clinical tumour size and nodal status predict pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Int J Colorectal Dis 2014; 29: 301–7.
Qiu HZ, Wu B, Xiao Y, Lin GL. Combination of differentiation and T stage can predict unresponsiveness to neoadjuvant therapy for rectal cancer. Colorectal Dis 2011; 13: 1353–60.
Huh JW, Kim HR, Kim YJ. Clinical prediction of pathological complete response after preoperative chemoradiotherapy for rectal cancer. Dis Colon Rectum 2013; 56: 698–703.
Gollub MJ, Tong T, Weiser M, Zheng J, Gonen M, Zakian KL. Limited accuracy of DCE-MRI in identification of pathological complete responders after chemoradiotherapy treatment for rectal cancer. Eur Radiol. 2017;27(4):1605-1612. doi: https://doi.org/10.1007/s00330-016-4493-1
Sassen NS, M de Booij, Sosef M, Berendsen R, Lammering G, Clarijs R, et al. Locally advanced rectal cancer: is diffusion weighted MRI helpful for the identification of complete responders (ypT0N0) after neoadjuvant chemoradiation therapy? Eur Radiol . 2013 Dec;23(12):3440–9. doi: https://doi.org/10.1007/s00330-013-2956-1
Jung SH, Heo SH, Kim JW, Jeong YY, Shin SS, Soung MG, et al. Predicting response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer: Diffusion‐weighted 3 tesla MR imaging J Magn Reson Imaging. 2012 Jan;35(1):110–6. doi: https://doi.org/10.1002/jmri.22749.
Clark W, Siegel EM, Chen YA, Zhao X, Parsons CM, Jonathan M, et al. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg. 2013; 216(6):1070-1081 https://doi.org/10.1016/j.jamcollsurg.2013.01.007
Yoon J, Chung YE, Lim JS, Kim M-J. Quantitative assessment of mesorectal fat: New prognostic biomarker in patients with mid-to-lower rectal cancer. Eur Radiol. 2019; 29(3):1240-1247. doi: https://doi.org/10.1007/s00330-018-5723-5
Allen S, Gada V, Blunt DM. Variation of mesorectal volume with abdominal fat volume in patients with rectal carcinoma: assessment with MRI. BrJ Radiol 2007; 80(952):242-247. doi: https://doi.org/10.1259/bjr/66311683
Lee KH, Kang B-K, Ahn BKJ. Higher visceral fat area/subcutaneous fat area ratio measured by computed tomography is associated with recurrence and poor survival in patients with mid and low rectal cancers. Int J Colorectal Dis. 2018; 33(9):1303-1307. doi: https://doi.org/10.1007/s00384-018-3065-z
Torkzad MR, Hansson KA, Lindholm J, Martling A, Blomqvist L. Significance of mesorectal volume in staging of rectal cancer with magnetic resonance imaging and the assessment of involvement of the mesorectal fascia. Eur Radiol . 2007; 17(7):1694-1699 doi: https://doi.org/10.1007/s00330-006-0521-x
Sprenger T, Rothe H, Becker H, Beissbarth T, Homayounfar K, Gauss K, et al. Lymph node metastases in rectal cancer after preoperative radiochemotherapy: impact of intramesorectal distribution and residual micrometastatic involvement. Am J Surg Pathol. 2013; 37(8):1283-1299doi: 0.1097/PAS.0b013b3182886ced
Boyle KM, Chalmers AG, Finan PJ, Sagar PM, Burke D. Morphology of the mesorectum in patients with primary rectal cancer. Dis Colon Rectum. 2009; 52(6):1122-1129. doi:https://doi.org/10.1007/DCR.0b013e31819ef62f.
García-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003; 46(3):298–304. doi: https://doi.org/10.1007/s10350-004-6545-x
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dilek, O., Akkaya, H., Parlatan, C. et al. Can the mesorectal fat tissue volume be used as a predictive factor in foreseeing the response to neoadjuvant chemoradiotherapy in rectum cancer? A CT-based preliminary study. Abdom Radiol 46, 2415–2422 (2021). https://doi.org/10.1007/s00261-021-02951-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-02951-x