Abstract
Background
Various predictors of the difficulty of total mesorectal excision for rectal cancer have been described. Although a bulky mesorectum was considered to pose technical difficulties in total mesorectal excision, no studies have evaluated the influence of mesorectum morphology on the difficulty of total mesorectal excision. Mesorectal fat area at the level of the tip of the ischial spines on magnetic resonance imaging was described as a parameter characterizing mesorectum morphology. This study aimed to evaluate the influence of clinical and anatomical factors, including mesorectal fat area, on the difficulty of total mesorectal excision for rectal cancer.
Methods
This study enrolled 98 patients who underwent robotic-assisted laparoscopic low anterior resection with total mesorectal excision for primary rectal cancer, performed by a single expert surgeon, between 2010 and 2015. Magnetic resonance imaging-based pelvimetry data were collected. Linear regression was performed to determine clinical and anatomical factors significantly associated with operative time of the pelvic phase, which was defined as the time interval from the start of rectal mobilization to the division of the rectum.
Results
The median operative time of the pelvic phase was 68 min (range 33–178 min). On univariate analysis, the following variables were significantly associated with longer operative time of the pelvic phase: male sex, larger tumor size, larger visceral fat area, larger mesorectal fat area, shorter pelvic outlet length, longer sacral length, shorter interspinous distance, larger pelvic inlet angle, and smaller angle between the lines connecting the coccyx to S3 and to the inferior middle aspect of the pubic symphysis. On multiple linear regression analysis, only larger mesorectal fat area remained significantly associated with longer operative time of the pelvic phase (p = 0.009).
Conclusions
Mesorectal fat area may serve as a useful predictor of the difficulty of total mesorectal excision for rectal cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Total mesorectal excision (TME), which represents a key technique in radical surgery for rectal cancer, requires complete dissection of the mesorectum between the visceral fascia and the pelvic fascia [1, 2]. It is difficult to perform accurate dissection in the deep and narrow space of the pelvic cavity, while ensuring both oncological safety and preservation of urogenital or anorectal function by protecting other anatomical features including the autonomic nerves [3,4,5]. Several studies have evaluated the predictors of the difficulty of TME for rectal cancer [6,7,8,9,10,11,12,13,14,15], reporting sex, body mass index, tumor size, tumor distance from the anal verge, preoperative chemoradiotherapy (CRT), visceral fat area (VFA), and pelvic dimensions among the factors associated with the difficulty of TME [6,7,8,9,10,11,12,13,14]. Although a bulky mesorectum was also considered to be associated with difficulty of TME [16], few studies have evaluated the influence of mesorectum morphology on the difficulty of TME. This scarcity of data might be related to the fact that few studies have offered a useful and simple indicator of the mesorectum morphology. Boyle et al. measured the mesorectal fat area (MFA) at the level of the tip of the ischial spines on high-resolution magnetic resonance imaging (MRI), which was considered to be an accurate representation of total mesorectal fat volume [17]. Therefore, in the present study, we aimed to evaluate the influence of clinical and anatomical factors, including MFA, on the difficulty of robotic-assisted laparoscopic low anterior resection with TME (RATME) for rectal cancer.
Materials and methods
Patient selection
Between January 2010 and December 2016, 349 patients underwent robotic-assisted laparoscopic low anterior resection for primary rectal adenocarcinoma at Shizuoka Cancer Center Hospital. Of these, 180 patients were operated on by the same expert surgeon (Y.K.). Patients who underwent tumor-specific mesorectal excision (n = 71) or multivisceral resection (n = 3), as well as those who did not undergo preoperative MRI or computed tomography (CT) at our institution (n = 8), were excluded from this analysis. The remaining 98 patients who underwent RATME by a single expert surgeon and underwent preoperative MRI or CT at our institution served as our study cohort (Fig. 1). TME was defined as complete excision of the visceral mesorectal tissue to the level of the levators, as previously described [18].
The indication for robotic surgery was rectal adenocarcinoma of clinical stage 0–IV. Robotic surgery for rectal cancer is not covered by medical insurance in Japan, and therefore it is a more costly treatment option than laparoscopic or open surgery. After providing informed consent, we performed robotic surgery in all patients who desired it. Preoperative CRT was performed only in patients for whom it was expected that it would be difficult to obtain a clear resected margin (R0) without CRT, or that shrinkage of the tumor by CRT would make anal preservation possible or would allow to avoid urinary diversion [19]. The external radiotherapy dose was 45 Gy, administered in 25 fractions to a large pelvic field over the course of 5 weeks, plus a boost of 5.4 Gy in three daily fractions, using a 4-field approach. Concomitant chemotherapy with the 5-fluorouracil derivate capecitabine (825 mg/m2) administered orally twice per day, 5 days per week. Operation was performed at 6–8 weeks after CRT. Preoperative tumor staging was performed by digital examination, colonoscopy, CT, MRI, and barium enema. Patients were staged using the tumor node metastasis (TNM) classification [20]. The patient characteristics as well as the surgical and pathological findings were recorded in a prospective database. Data collection and analysis were approved by the institutional review board of Shizuoka Cancer Center Hospital (Institutional Code: 28-J-130-28-1-3).
VFA measurement
VFA was measured using on cross-sectional CT scans obtained at the level of the umbilicus and analyzed using dedicated software (SYNAPSE VINCENT, version 4.6; Fujifilm Medical Systems., Inc. and FUJIFILM Corporation, Tokyo, Japan), according to a previously described protocol [21]. Visceral adipose tissue was determined by setting the attenuation level within the range of − 50 to − 200 Hounsfield units. The region of visceral fat was defined by automatic counter tracing, and the VFA was calculated automatically by the software.
Pelvic measurements on MRI
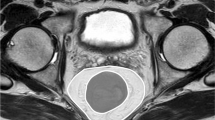
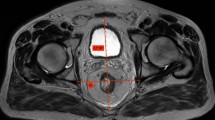
High-resolution MRI was performed using a 3.0-T system (Achieva 3.0T dStream; Royal Philips Healthcare, Amsterdam, The Netherlands). T2-weighted axial, sagittal, and coronal images with a slice thickness of 5 mm were analyzed using dedicated software (SYNAPSE version 4.1; Fujifilm Medical Systems., Inc. and FUJIFILM Corporation). We chose 11 pelvic parameters (Table 1; Figs. 2, 3, and 4) expected to potentially influence the difficulty of TME, as suggested by previous studies [6,7,8,9,10,11,12,13,14,15]. MFA was defined as the area of mesorectal fat at the level of the tip of the ischial spines, measured according to the protocol proposed by Boyle et al. [17]. Within the SYNAPSE software, the circumferences of the mesorectum and rectum were manually traced with the calibrated cursor of the “freehand region of interest tool,” and then the surface areas of the delimited mesorectum and rectum regions were obtained. MFA was calculated by subtracting the rectum area from the mesorectum area (Fig. 5). When a T3 tumor located at the level of the tip of the ischial spine transgressed the rectal wall and the outer edge of the muscularis propria could not be readily visualized, the trace was continued along a line following the contour of the outer muscularis propria [17]. A single observer (Yamaoka) made all measurements and was blinded to the surgical outcomes at the time of measurement. To assess interobserver variation, a second observer (K.T.) performed the same measurements in all patients. Not only the areas of the mesorectum and rectum were measured, but also the time taken to obtain each MFA value, and these data were compared between observers.
Pelvic measurements illustrated on a sagittal magnetic resonance image. A Pelvic inlet length: distance from the superior middle aspect of the pubic symphysis to the sacral promontory. B Pubic tubercle height: distance from the superior to the inferior middle aspect of the pubic symphysis. C Pelvic outlet length: distance from the inferior middle aspect of the pubic symphysis to the coccyx. D Sacral length: distance from the sacral promontory to the coccyx. E Sacral depth: distance from the sacral length to the deepest point of the sacral hollow
Pelvic angles illustrated on a sagittal magnetic resonance image. Angle α, the angle between the line connecting the sacral promontory to S3 and to the superior middle aspect of the pubic symphysis. Angle β, pelvic inlet angle: the angle between a line connecting the superior middle aspect of the pubic symphysis to the sacral promontory, and a line connecting the inferior middle aspect of the pubic symphysis to the coccyx. Angle γ, the angle between the lines connecting the coccyx to S3 and to the inferior middle aspect of the pubic symphysis. Angle δ, the angle between the lines connecting S3 to the sacral promontory and to the coccyx
Surgical technique
All procedures were performed using a systematic approach that included a colonic and pelvic phase, using a robotic approach described in detail elsewhere [22]. During the colonic phase, high ligation of the inferior mesenteric artery was performed via the medial-to-lateral approach. When required, splenic flexure takedown was also performed. The pelvic phase involved sharp dissection in front of the prehypogastric nerve fascia and behind Denonvilliers’ fascia to avoid autonomic nerve injury while mobilizing the rectum up to the vicinity of the tumor [23, 24]. Using linear staplers, the rectum was divided at more than 2 cm below the lower border of the tumor. After TME was completed, lateral lymph node dissection was performed in patients with clinical T3–4 lower rectal cancer on preoperative images, in accordance with the Japanese Society for Cancer of the Colon and Rectum guidelines for the treatment of colorectal cancer [2]. Anastomosis was created laparoscopically using the double-stapling technique. The main outcome of interest in this study was operative time of the pelvic phase, which was defined as the time interval from the start of rectal mobilization to the division of the rectum, and that was recorded intraoperatively.
Statistical analysis
Categorical variables are described as numbers and percentages, while continuous variables are presented as medians (range). Linear regression was performed to determine variables associated with operative time of the pelvic phase. Variables with a p value below 0.05 in the univariate analysis were entered into multivariate analysis performed using a multiple linear regression model. Variables showing significant association with the main outcome on multivariate analysis were considered risk factors, with risk thresholds set at the lower or upper quantile of the distribution, depending on whether the variable correlated negatively or positively with operative time, as described by Kim et al. [11]. Patients were categorized into two groups as follows: Easy group (no risk factors) and Difficult group (at least one risk factor). Operative outcomes were compared between the two groups. Categorical variables were compared using the Chi-square test, while continuous variables were compared using Student’s t test or the Mann–Whitney U test, as appropriate. To assess interobserver variation, the mesorectum and rectum areas for all patients were measured by two colorectal surgeons (Y. Yamaoka and K.T.), supervised by an expert colorectal surgeon (T.Y.). Interobserver agreement was evaluated using Spearman’s correlation test. All statistical analyses were performed using JMP version 13.0 (SAS Institute, Cary, NC, USA). A p value below 0.05 was considered to indicate statistical significance.
Results
Patient characteristics
Table 2 provides an overview of the clinic-pathological characteristics of the study patients. The median age was 62 years (range 29–80 years), and 71 patients (73%) were male. The median distance between the lower edge of the tumor and the anal verge was 5.0 cm (range 3.0–9.0 cm). Forty-five patients (45.9%) had tumors with clinical stage ≥ T3. An overview of 11 pelvic dimensions, VFA, and MFA is provided in Table 3. The median VFA was 126.7 cm2 (range 13.1–310.6 cm2) and the median MFA was 21.8 cm2 (range 5.9–34.4 cm2). The operative outcomes are summarized in Table 4. Fifty patients (51.0%) underwent lateral lymph node dissection and 23 patients (24%) received a diverting stoma. No patients were converted to open surgery. The median total operative time was 294 min (range 123–600 min), while the median operative time of the pelvic phase was 68 min (range 33–178 min). The median distal margin was 1.8 cm (range 0.4–6.0 cm). Two patients (2.0%) experienced local recurrence (median follow-up time, 36.0 months).
Factors Associated With the Operative Time of the Pelvic Phase
The relationship between clinico-anatomical factors and the operative time of the pelvic phase is summarized in Table 5. Univariate analysis showed that male sex, larger tumor size, larger VFA, shorter pelvic outlet length, longer sacral length, shorter interspinous distance, larger angle β, smaller angle γ, and larger MFA were significantly associated with longer operative time of the pelvic phase. Multiple linear regression analysis revealed that only larger MFA remained significantly associated with longer operative time of the pelvic phase (p = 0.009).
Operative outcomes according to procedural difficulty
As MFA was positively associated with the operative time of the pelvic phase, the threshold of the upper quartile was considered for patient stratification 26.0 cm2. Specifically, patients with MFA ≥ 26.0 cm2 were categorized into the Difficult group (n = 24) and those with MFA < 26.0 cm2 were categorized into the Easy group (n = 74). Both total operative time and the operative time of the pelvic phase were significantly longer in the Difficult group than in the Easy group. The proportion of patients who received a diverting stoma was also higher in the Difficult group (37.5 vs. 18.9%), but the difference was not significant. Other outcomes were similar between the two groups (Table 6).
Interobserver variation
Interobserver variations in the measurements of the mesorectum and rectum areas and MFA are summarized in Table 7. The Spearman’s correlation coefficient for interobserver variation in each area was more than 0.95, indicating that the measurements of the mesorectum and rectum areas and MFA were all reproducible, and the sets of measurements reported by the two observers were highly correlated (p < 0.001). The median time taken to obtain each MFA value was 51 s (range 43–72 s) for Yamaoka and 52 s (range 43–80 s) for K.T., with no significant difference between the time taken by the two observers.
Discussion
Previous studies reported that various factors were associated with the difficulty of rectal cancer surgery, including sex, tumor size, VFA, pelvic outlet dimensions, sacral length, interspinous distance, angle β, or angle γ [7,8,9,10,11,12,13,14]. Indeed, our univariate analysis also suggested that these parameters are associated with the operative time of the pelvic phase. However, to our knowledge, only one study evaluated the influence of MFA on the difficulty of TME [25]. A larger mesorectal fat area was identified as an anatomical factor significantly associated with an increase in the surgical difficulty grading for TME, which is indicated by total operative time, conversion to open surgery, use of transanal dissection, postoperative hospital stay, blood loss, and postoperative complications. In our study, we found that, on multivariate analysis, only MFA was significantly associated with the operative time of the pelvic phase. MFA was defined as the area of mesorectal fat at the level of the tip of the ischial spines, which corresponds to the level of the upper mid rectum, at 8–10 cm from the anal verge. This definition of MFA is considered to provide an accurate representation of total mesorectal fat volume [17], whereas VFA reflects the area of the greater omentum, retroperitoneal fat, mesentery, and mesocolon. Our results demonstrated that the presence of a bulky mesorectum did increase the technical difficulty of TME for rectal cancer. We speculate that the reason for the prolonged operative time is related to the fact that a large MFA causes the space between the pelvic fascia and the mesorectum to become very narrow, in which case establishing an appropriate surgical filed is more time demanding. Indeed, the median operative time of the pelvic phase was significantly longer in the Difficult group (MFA ≥ 26.0 cm2) than in the Easy group (MFA < 26.0 cm2). The proportion of patients who received a diverting stoma also tended to be higher in the Difficult group than in the Easy group. The MFA threshold of 26.0 cm2 is thus considered to be important for predicting the difficulty of TME. Moreover, the interobserver agreement was excellent for the measurements of the mesorectum and rectum areas and MFA, and it took less than one minute to obtain each MFA value. Therefore, the use of MFA in clinical practice is highly feasible.
Escal, et al. reported that the median MFA among the French was 20.7 cm2, which was similar to our result for the median MFA of 21.8 cm2 [25]. Boyle et al. reported that the mean MFA among English men and women with primary rectal cancer was 25.6 ± 7.7 and 18.4 ± 8.3 cm2, respectively [17]. In our study, the mean MFA in Japanese men and women with primary rectal cancer was 22.4 ± 5.2 and 19.8 ± 7.1 cm2, respectively. Therefore, MFA likely differs with ethnicity, as MFA was larger in English men than in Japanese men.
We evaluated the difficulty of TME performed by robotic surgery. Robotic surgery is a promising advanced technology that can overcome the inherent limitations of conventional laparoscopic surgery, including the use of straight, rigid instruments, limited degrees of freedom, an unstable camera platform with two-dimensional imaging, and poor ergonomics of the instruments in the narrow pelvic cavity with high anatomical complexity. The advantages of robotic surgery include the use of a free-moving multi-joint forceps, a motion scaling function, high-quality 3-dimensional imaging, stable camera operation, and greatly improved ergonomics [22, 26]. Several studies demonstrated, compared with conventional laparoscopic surgery or open surgery, that robotic surgery for rectal cancer provides superior short-term and long-term outcomes [21, 22, 26,27,28,29]. Moreover, patients in whom robotic surgery was considered difficult would likely not qualify as candidates for conventional laparoscopic or open surgery, or such surgeries would be even more technically challenging. Baek et al. reported that robotic surgery for rectal cancer was not influenced by pelvimetric factors known to be significantly associated with the difficulty of conventional laparoscopic surgery for rectal cancer, but these authors did not include the morphology of the mesorectum in their analysis [30]. It is expected that larger MFA is associated with higher technical difficulty of TME for rectal cancer not only in robotic surgery but also in conventional laparoscopic surgery or open surgery. Trasanal TME (TaTME) is the latest advanced surgical access technique for pelvic dissection that has attracted attention because it is expected to improve clinical, oncological, and functional outcomes through better visualization and more accurate distal TME dissection. It also has the potential to overcome patient characteristics such as obesity, male sex, or narrow pelvis, which traditionally make pelvic dissection difficult when using the abdominal approach [31, 32]. A randomized clinical trial (COLOR III) comparing TaTME and laparoscopic TME for rectal cancer is ongoing [33]. Additionally, it is necessary to examine the usefulness of TaTME in patients with a bulky mesorectum.
In our study, the operative time of the pelvic phase was employed as an indicator of difficulty of TME, which corresponds to the approach followed in previous studies [9, 11]. Other studies employed total operative time to assess the difficulty of TME [8, 12, 14]. However, total operative time is influenced by the extent of proximal lymph node dissection, mobilization of the splenic flexure, and lateral lymph node dissection. Other factors such as blood loss, rate of conversion to open surgery, and rate of anastomotic leakage were also evaluated [8, 12]. While we did record such parameters, our study sample was too small to allow for meaningful subgroup analyses focused on these parameters.
There are several limitations to this study. First, as oncological factors, pathologically evaluated TME quality, rates of positive circumferential resection margin (CRM), and local recurrence were important indicators of the difficulty of TME [6, 7, 10, 13]. However, we did not evaluate TME quality in terms of the pathology of the resected specimens because TME quality is not usually evaluated in Japan. In our study, a positive resection margin was defined as a pathologically positive surgical dissection plane or a positive proximal or distal margin of the resected specimen, which was different from the positive CRM defined as a margin at a distance of 1 mm or less from a tumor. The rates of positive resection margin and local recurrence were both too low to conduct statistical analyses. Therefore, further studies enrolling larger population samples are warranted to evaluate the influence of MFA on the oncological outcomes. Second, this was a retrospective study conducted in a single institution. A prospective study is necessary to verify the utility of MFA and validity of the threshold. Third, the learning curve in robotic surgery for rectal cancer was not taken into account. In our study sample, all patients were operated on by the same expert surgeon (Y.K.), who had performed over 500 laparoscopic colorectal resections, including more than 200 for rectal cancer, before adopting robotic-assisted surgery in clinical practice. Thus, it can be considered that the surgeon was well-informed with respect to pelvic anatomy and laparoscopic techniques involved in pelvic surgery [34].
In conclusion, our findings indicate that larger MFA was significantly associated with longer pelvic operative time in RATME for rectal cancer. MFA was useful for predicting the difficulty of TME for rectal cancer, and it should be prospectively recorded as a common clinical characteristic. Further larger studies may identify other utilities of MFA in rectal cancer surgery.
References
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery-the clue to pelvic recurrence? Br J Surg 69:613–616
Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kawano H, Kinugasa Y, Kokudo N, Murofushi K, Nakajima T, Oka S, Sakai Y, Tsuji A, Uehara K, Ueno H, Yamazaki K, Yoshida M, Yoshino T, Boku N, Fujimori T, Itabashi M, Koinuma N, Morita T, Nishimura G, Sakata Y, Shimada Y, Takahashi K, Tanaka S, Tsuruta O, Yamaguchi T, Yamaguchi N, Tanaka T, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum (2017) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. https://doi.org/10.1007/s10147-017-1101-6
Sun WM, Read NW, Katsinelos P, Donnelly TC, Shorthouse AJ (1994) Anorectal function after restorative proctocolectomy and low anterior resection with coloanal anastomosis. Br J Surg 81(2):280–284
Havenga K, Enker WE, McDermott K, Cohen AM, Minsky BD, Guillem J (1996) Male and female sexual and urinary function after total mesorectal excision with autonomic nerve preservation for carcinoma of the rectum. J Am Coll Surg 182(6):495–502
Maslekar S, Sharma A, Macdonald A, Gunn J, Monson JR, Hartley JE (2007) Mesorectal grades predict recurrences after curative resection for rectal cancer. Dis Colon Rectum 50(2):168–175
Salerno G, Daniels IR, Brown G, Norman AR, Moran BJ, Heald RJ (2007) Variations in pelvic dimensions do not predict the risk of circumferential resection margin (CRM) involvement in rectal cancer. World J Surg 31(6):1313–1320
Baik SH, Kim NK, Lee KY, Sohn SK, Cho CH, Kim MJ, Kim H, Shinn RK (2008) Factors influencing pathologic results after total mesorectal excision for rectal cancer: analysis of consecutive 100 cases. Ann Surg Oncol 15(3):721–728
Targarona EM, Balague C, Pernas JC, Martinez C, Berindoague R, Gich I, Trias M (2008) Can we predict immediate outcome after laparoscopic rectal surgery? Multivariate analysis of clinical, anatomic, and pathologic features after 3-dimensional reconstruction of the pelvic anatomy. Ann Surg 247(4):642–649
Akiyoshi T, Kuroyanagi H, Oya M, Konishi T, Fukuda M, Fujimoto Y, Ueno M, Miyata S, Yamaguchi T (2009) Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery 146(3):483–489
Killeen T, Banerjee S, Vijay V, Al-Dabbagh Z, Francis D, Warren S (2010) Magnetic resonance (MR) pelvimetry as a predictor of difficulty in laparoscopic operations for rectal cancer. Surg Endosc 24(12):2974–2979
Kim JY, Kim YW, Kim NK, Hur H, Lee K, Min BS, Cho HJ (2011) Pelvic anatomy as a factor in laparoscopic rectal surgery: a prospective study. Surg Laparosc Endosc Percutan Tech 21(5):334–339
Chen W, Li Q, Fan Y, Li D, Jiang L, Qiu P, Tang L (2016) Factors predicting difficulty of laparoscopic low anterior resection for rectal cancer with total mesorectal excision and double stapling technique. PLoS ONE 11(3):e0151773
Chen B, Zhang Y, Zhao S, Yang T, Wu Q, Jin C, He Y, Wang Z (2016) The impact of general/visceral obesity on completion of mesorectum and perioperative outcomes of laparoscopic TME for rectal cancer: A STARD-compliant article. Medicine (Baltimore) 95(36):e4462
Zhou XC, Su M, Hu KQ, Su YF, Ye YH, Huang CQ, Yu ZL, Li XY, Zhou H, Ni YZ, Jiang YI, Lou Z (2016) CT pelvimetry and clinicopathological parameters in evaluation of the technical difficulties in performing open rectal surgery for mid-low rectal cancer. Oncol Lett 11(1):31–38
Park IJ, Yu CS, Lim SB, Lee JL, Kim CW, Yoon YS, Park SH, Kim JC (2016) Is preoperative chemoradiotherapy beneficial for sphincter preservation in low-lying rectal cancer patients? Medicine (Baltimore) 95(18):e3463
Dayal S, Battersby N, Cecil T (2017) Evolution of surgical treatment for rectal cancer: a review. J Gastrointest Surg 21(7):1166–1173
Boyle KM, Chalmers AG, Finan PJ, Sagar PM, Burke D (2009) Morphology of the mesorectum in patients with primary rectal cancer. Dis Colon Rectum 52(6):1122–1129
Lowry AC, Simmang CL, Boulos P, Farmer KC, Finan PJ, Hyman N, Killingback M, Lubowski DZ, Moore R, Penfold C, Savoca P, Stitz R, Tjandra JJ (2001) Consensus statement of definitions for anorectal physiology and rectal cancer: report of the Tripartite Consensus Conference on Definitions for Anorectal Physiology and Cancer R, Washington, D.C., May 1, 1999. Dis Colon Rectum 44(7):915–919
Yamaoka Y, Kinugasa Y, Shiomi A, Yamaguchi T, Kagawa H, Yamakawa Y, Numata M, Furutani A (2017) Preoperative chemoradiotherapy changes the size criterion for predicting lateral lymph node metastasis in lower rectal cancer. Int J Colorectal Dis 32(11):1631–1637
Brierley JD, Gospodarowicz. MK, Wittekind C (2017) TNM classification of malignant tumours, 8th Edn. Wiley-Blackwell, Oxford
Shiomi A, Kinugasa Y, Yamaguchi T, Kagawa H, Yamakawa Y (2016) Robot-assisted versus laparoscopic surgery for lower rectal cancer: the impact of visceral obesity on surgical outcomes. Int J Colorectal Dis 31(10):1701–1710
Yamaguchi T, Kinugasa Y, Shiomi A, Tomioka H, Kagawa H, Yamakawa Y (2016) Robotic-assisted vs. conventional laparoscopic surgery for rectal cancer: short-term outcomes at a single center. Surg Today 46(8):957–962
Kinugasa Y, Murakami G, Suzuki D, Sugihara K (2007) Histological identification of fascial structures posterolateral to the rectum. Br J Surg 94(5):620–626
Kinugasa Y, Murakami G, Uchimoto K, Takenaka A, Yajima T, Sugihara K (2006) Operating behind Denonvilliers’ fascia for reliable preservation of urogenital autonomic nerves in total mesorectal excision: a histologic study using cadaveric specimens, including a surgical experiment using fresh cadaveric models. Dis. Colon Rectum 49(7):1024–1032
Escal L, Nougaret S, Guiu B, Bertrand MM, de Forges H, Tetreau R, Thézenas S, Rouanet P (2018) MRI-based score to predict surgical difficulty in patients with rectal cancer. Br J Surg 105(1):140–146
Yamaguchi T, Kinugasa Y, Shiomi A, Tomioka H, Kagawa H (2016) Robotic-assisted laparoscopic versus open lateral lymph node dissection for advanced lower rectal cancer. Surg Endosc 230(2):721–728
Zhang X, Wei Z, Bie M, Peng X, Chen C (2016) Robot-assisted versus laparoscopic-assisted surgery for colorectal cancer: a meta-analysis. Surg Endosc 30(12):5601–5614
Kim JY, Kim NK, Lee KY, Hur H, Min BS, Kim JH (2012) A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol 19(8):2485–2493
Kim J, Baek SJ, Kang DW, Roh YE, Lee JW, Kwak HD, Kwak JM, Kim SH (2017) Robotic resection is a good prognostic factor in rectal cancer compared with laparoscopic resection: long-term survival analysis using propensity score matching. Dis Colon Rectum 60(3):266–273
Baek SJ, Kim CH, Cho MS, Bae SU, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2015) Robotic surgery for rectal cancer can overcome difficulties associated with pelvic anatomy. Surg Endosc 29(6):1419–1424
Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, Moran B, Hanna GB, Mortensen NJ, Tekkis PP, TaTME Registry Collaborative (2017) Transanal total mesorectal excision: international registry results of the first 720 cases. Ann Surg 266(1):111–117
Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, Moran B, Hanna GB, Mortensen NJ, Tekkis PP, International TaTME Registry Collaborative (2018) Incidence and risk factors for anastomotic failure in 1594 patients treated by transanal total mesorectal excision: results from the International TaTME Registry. Ann Surg. https://doi.org/10.1097/SLA.0000000000002653
Deijen CL, Velthuis S, Tsai A, Mavroveli S, de Lange-de Klerk ES, Sietses C, Tuynman JB, Lacy AM, Hanna GB, Bonjer HJ (2016) COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc 30(8):3210–3215
Yamaguchi T, Kinugasa Y, Shiomi A, Sato S, Yamakawa Y, Kagawa H, Tomioka H, Mori K (2015) Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc 29(7):1679–1685
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Yusuke Yamaoka, Tomohiro Yamaguchi, Yusuke Kinugasa, Akio Shiomi, Hiroyasu Kagawa, Yushi Yamakawa, Akinobu Furutani, Shoichi Manabe, Kakeru Torii, Kohei Koido, and Keita Mori have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Yamaoka, Y., Yamaguchi, T., Kinugasa, Y. et al. Mesorectal fat area as a useful predictor of the difficulty of robotic-assisted laparoscopic total mesorectal excision for rectal cancer. Surg Endosc 33, 557–566 (2019). https://doi.org/10.1007/s00464-018-6331-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6331-9