Abstract
Pancreas transplantation is considered the curative treatment for severe type 1 diabetes mellitus in selected cases. Since the first procedure in 1966, surgical techniques have been improved. The current trend among most medical centers, as well as at our Institution, is enteric drainage and systemic venous or portal anastomosis. The aim of this pictorial essay is to describe the main imaging features of pancreatic transplantation with duodenoduodenostomy drainage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreas transplantation (PT) is an established therapy for severe type 1 diabetes mellitus characterized by end-stage renal failure complications or, less often, for poorly controlled blood glucose. Ever since the first procedure in 1966, surgical techniques, as well as immunosuppression and antimicrobial prophylaxis, have been improved [1].
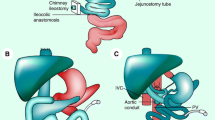
Bladder drainage of exocrine secretions (duodenocystostomy) was replaced by enteric drainage which is currently considered the standard technique [2]. Most centers employ enteric drainage with jejunum. At our Institution, between 2010 and 2018, the enteric anastomosis was duodenoduodenostomy (Fig. 1). The aim of this essay is to describe the normal pancreas graft imaging features with enteric drainage by duodenoduodenostomy as well as the main surgical complications.
Technical description of pancreatic transplantation—retroperitoneal position with duodenoduodenostomy drainage
The pancreas allograft is harvested with the donor duodenum and vascular support. The organ is placed into a fully retroperitoneal position, in which the pancreas is upright with the head in a cephalic position, and employs the duodenoduodenostomy bypass, at the level of second/third portion of the native duodenum (latero-lateral duodenoduodenostomy), which is associated with a lower rate of intestinal obstruction and provides an additional pathway for biopsy (endoscopic) [1, 3].
In PT with exocrine duodenoduodenostomy drainage, there are two types of venous anastomosis that can be performed through systemic vein (SV) or portal vein (PV). In the SV technique, the graft portal vein, normally with a very small neck, is anastomosed to the recipient inferior vena cava. In the PV technique, the graft portal vein is connected to the recipient superior mesenteric vein (Fig. 2) [4]. Anastomosis with recipient inferior vena cava allows a greater proximity between the duodenum of the recipient and donor, which reduces the incidence of vascular thrombosis [1].
Normal appearance of the pancreatic graft (*) with portal and systemic venous anastomosis on CT scan. a, b Coronal and axial reformatted CT with maximum intensity projection show the normal portal anastomosis (arrowhead). Axial and sagittal CT c, d show the normal appearance of systemic vein anastomosis on CT scan with inferior vena cava (arrow)
The arterial blood supply of the pancreas graft is usually performed after Y grafting between the superior mesenteric and splenic arteries with a segment of the bifurcation of the donor’s iliac artery, and thereafter a termino-lateral vascular anastomosis of the Y is performed with the common or external recipient iliac artery [5, 6].
Normal imaging appearance of pancreas transplants
Different imaging modalities are used to evaluate the vascular and enteric anastomosis and the parenchyma of the pancreas graft. Doppler ultrasound (US) and computed tomography (CT) are the most commonly used methods. Magnetic resonance imaging (MRI) and computed tomography angiography (CTA) are reserved for selected cases.
Normal appearance of a pancreas transplant on US is a homogeneous soft-tissue structure that is hypoechoic relative to adjacent mesenteric fat. Color and power Doppler US can demonstrate pancreas transplant perfusion and vascular anatomy, with good accuracy in the detection of venous and arterial stenosis or occlusions (Fig. 3). Arterial waveforms normally show a rapid systolic upstroke and continuous diastolic blood flow. Venous structures demonstrate a monophasic waveform within an anechoic lumen in the PV technique. However, it is not uncommon to observe a diffuse narrowing of the donor PV to the anastomosis site and this may result in relative flattening of the venous flow waveform. In pancreatic graft with SV drainage, a slight degree of cardiac phasicity may occur in the venous flow waveform [6, 7].
At unenhanced CT, the allograft appears as a homogeneous soft-tissue structure that may be isoattenuating and difficult to distinguish from hypoattenuating and nondistended bowel. The duodenum stump is often collapsed with a thick wall (Fig. 4). CTA is acquired in a multiphasic mode: angiographic and parenchymal arterial acquisitions, portal, and late phases. Contrast-enhanced CT demonstrates uniform enhancement of the normal pancreatic parenchyma and it is similar to native pancreas in all dynamic phases. The evaluation of the arterial anastomosis usually presents no obstacles once these vessels tend to be large and long. However, veins may be difficult to assess, especially when the pedicle is short (SV) or thin (PV), which is a more frequent aspect in the anastomosis with the inferior vena cava. Controversially, in these situations, the CT arterial phase or Doppler US may be a better tool than CT venous phase for the characterization of this vein. (Fig. 5).
Regarding MRI, graft appearance is the same as the native pancreas in all sequences (Fig. 6) [8].
Normal appearance of the pancreatic graft on MRI. a Axial T1-weighted fat suppressed MRI image shows the usual hyperintense of the pancreatic parenchyma (white arrows). b, c Axial T2 and fat suppressed T2-weighted MRI show hypointense signal (arrow). d Coronal contrast shows homogeneous enhancement after intravenous administration of the contrast medium (arrow). The appearance is the same as the native pancreas in all sequences
Complications
In patients with troubleshooting complications, imaging is indispensable and usually more than one method is used. The combination of Doppler US and Angio-CT is successful in most cases. Post-operative complications shall be classified according to their anatomical origin, between vascular and non-vascular [9], or in either early or late surgical time relationship. Generally, in the early post-operative period, the main objective is to rule out technical complications such as either arterial or venous thrombosis (estimated at 9%) or acute rejection (estimated at 22%). In the late post-operative period, pancreatitis or immunological complications related to chronic rejection (estimated at 28.8%) are the most feared issues [2].
Intra-abdominal fluid collection
Intra-abdominal fluid collections (abscesses, seroma, hematoma, lymphocele, pseudocyst or ascites) are common complications after PT and may occur early or at a later post-operative period (Fig. 7). In the presence of fluid in the cavity, attention shall be given to the possibility of an enteric anastomotic dehiscence or a pancreatic duct fistula secondary to focal pancreatitis, or ischemia that results in ductal rupture which may result in the development of a pseudocyst or arterial pseudoaneurysm. The use of positive oral contrast may be helpful (Fig. 8) [10].
Peripancreatic pseudocyst. Case 1: a B-mode US image shows a cyst with heterogeneous contents (arrow). b Axial unenhanced CT scan demonstrate the pancreatic pseudocyst (arrow) adjacent to pancreatic graft (dotted line). Case 2: B-mode US (c) shows anechoic fluid collections (*) perigraft. Axial reformatted d in the portal venous phase shows fluid collection (white arrows)
Bowel complication
The rate of intestinal complications following PT, which usually includes bowel obstruction, enteric leakage, and enteric anastomotic bleeding, is roughly 7%. Dehiscence in the stump duodenal anastomosis may result in fistula, with extravasation of pancreatic juice, focal chemical peritonitis, and fluid collection. Intestinal obstructions may occur by adhesions, volvulus or internal hernia (Fig. 9) [11].
Rejection and pancreatitis
Rejection is the most common cause of overall graft loss [5]. Serum markers are non-specific. Rise of serum pancreatic enzyme is the most common presentation in rejection and patients are usually asymptomatic [12, 13]. Rejection is classified as hyperacute (up to 24 h), acute (from first 24 h to 3 months), subacute (from 3 to 6 months) or chronic (> 6 months). Imaging findings in hyperacute/acute/subacute rejection are non-specific and may reveal gland enlargement/swelling, peripancreatic transplant inflammation, and heterogeneous enhancement on CT or MRI, and sometimes observe peripancreatic fluid and duodenal edema (Fig. 10) [5, 14].
Acute rejection of the pancreatic graft. Ultrasound image (a) shows an increase of the size and echogenicity of the pancreas graft (*) on B-mode with preserved arterial flow on spectral Doppler evaluation (b). Axial (c) and coronal reformatted (d) angiography CT scans reveal patent arterial (black arrow) and venous anastomosis (arrowheads), adjacent perigraft fluid collections, and fat stranding (white arrows)
In chronic rejection, a thickening of vessel wall and occlusion of the circulation and fibrosis is observed, which results in pancreatic atrophy, sometimes with areas of infarction and necrosis in the parenchyma or collections in the surgical bed of the parenchymal graft (Figs. 11, 12) [5, 14, 15]. The diagnosis may be challenging because the clinical and laboratorial are non-specific. Currently, the gold standard criteria used for the diagnosis of rejection is graft biopsy.
Chronic rejection of the pancreatic graft. Ultrasound (a) shows an increase of the echogenicity and atrophy of the gland (*) with thickening of vessel wall (white arrowhead). Coronal reformatted angiography CT with maximum intensity projection (b) shows normal appearance of the arterial (curved arrow) and venous anastomosis (white arrow) of the pancreas graft, with reduced size of the pancreatic parenchyma (arrowheads)
Chronic rejection of the pancreatic graft. Case 1: Ultrasound (a) depicts parenchymal heterogeneity with diffuse enlargement of the pancreatic graft (dotted line). Coronal CT (b) shows extensive areas of pancreatic parenchyma necrosis and irregularities in the artery (arrows). Case 2: Coronal reformatted (c) and axial CT (d) in the arterial phase show fluid collection/necrosis (white arrows) replacing the pancreas graft parenchyma, with peripheral contrast enhancement and fat tissue stranding (arrowhead)
Graft pancreatitis without association with an anatomical, immunological or infectious cause is uncommon and estimated to be around 4%. Elevation of serum pancreatic enzyme associated with inflammation of pancreas graft and perigraft tissue, without evidence of another anatomical abnormality on CT, is the classical described scenario for this pathology. However, this wide variation lies in the overlapping of imaging findings with other complications such as rejection. For that reason, some authors argue that graft pancreatitis is a diagnosis of exclusion, defined only after performing biopsies that exclude rejection (Fig. 13) [13].
Vascular complications
Vascular complications are the most common cause of early graft failure. Venous and arterial thrombosis are the most frequent complications (incidence up to 40%). Other complications are arteriovenous fistulas, stenosis, and kinking of the Y-graft [16].
In both arterial and vein acute thrombosis, there is an increase in the vessel lumen diameter, luminal thrombus, and vessel’s wall enhancement, occasionally with absence or reduction of parenchymal perfusion, which it may occur areas of necrosis and perigraft collections (Fig. 14) [9]. On the Doppler US, the parenchyma tends to become hypoechogenic and heterogeneous. In pancreas venous graft acute thrombosis there is an increased diameter of the vessel lumen and thrombus may be anechogenic (recent/acute). In chronic venous thrombosis, reduced diameter of the vessel lumen and thrombus echogenic (old/chronic) is observed. In venous thrombosis, there is no venous flow and it may lead to reverse diastolic arterial flow in Doppler [17]. On CT without contrast, in some cases, a hyperattenuation and increased vessel caliber may be seen, a finding that may represent acute thrombosis (Fig. 15) [9]. In the radiological report, it is important to mention whether thrombosis is complete or partial; whether there is or not an alteration in the perfusion of the parenchyma, and in which vessel the thrombus is located: peripheral (very distal vessel), intermediate (either parenchymal vessels or trunk of the splenic or mesenteric vein or trunk of the superior mesenteric artery or splenic artery) or central (portal vein or arterial graft) [16].
Venous anastomosis thrombosis. a, b Coronal and axial CT demonstrate thrombosis of the graft vein with superior mesenteric vein anastomosis (arrows), with non-enhancement of the parenchymal graft and area of necrosis (dotted line). Sagittal (c) and Axial (d) CT on venous phase show systemic venous anastomosis with thrombosis of the graft vein extending into the inferior vena cava (white arrow and arrowhead) with graft edema and areas of necrosis (*)
Different cases of arterial acute thrombosis with pancreatic graft necrosis. Color Doppler US (a) demonstrates thrombus and undetectable flow in the artery branches of the graft. Sagittal and axial CT (b) without contrast show a mild hyperattenuation and increased vessel caliber, a finding that may represent an acute thrombosis (arrow). Coronal CT (c) shows thrombus of the distal stump of the superior mesenteric artery of the donor (arrow). Coronal CT MIP on the late arterial phase (d) shows thrombus in the graft artery near the anastomotic site with the right common iliac artery (white arrow)
Arterial pseudoaneurysms are usually secondary to infections or procedures. Early diagnosis is important due to the risk of rupture and bleeding. On Doppler US, there is an oval image adjacent to the pancreas graft or vessels involved in anastomotic, anechoic or heterogeneous, with biphasic pattern flow (to-and-fro), demonstrating a swirling pulsatile color Doppler pattern that has been linked to the classic idea of “yin and yang”. In CT and MRI, there is an intense arterial enhancement and it is occasionally possible to characterize communication with the vessels of the pancreas graft (Fig. 16) [17, 18].
Conclusion
Prior knowledge of normal imaging features of the pancreas graft as well as the complications related specifically to a PT with enteric drainage are fundamental prerequisites for an accurate contribution to the diagnostic investigation and to support medical decision-making.
References
Ferrer J, Molina V, Rull R, et al. Pancreas Transplantation: Advantages of a Retroperitoneal Graft Position. Cirugía Española (English Edition). 2017;95(9):513-520.
Walter M, Jazra M, Kykalos S, et al. 125 Cases of duodenoduodenostomy in pancreas transplantation: a single-centre experience of an alternative enteric drainage. Transplant International. 2014;27(8):805-815.
Perosa M, Noujaim H, Ianhez LE, et al. Experience with 53 portal-duodenal drained solitary pancreas transplants. Clinical Transplantation. 2014;28(2):198-204.
Bazerbachi F, Selzner M, Marquez MA, et al. Portal Venous Versus Systemic Venous Drainage of Pancreas Grafts: Impact on Long-Term Results. American Journal of Transplantation. 2011;12(1):226-232.
Vandermeer FQ, Manning MA, Frazier AA, Wong-You-Cheong JJ. Imaging of Whole-Organ Pancreas Transplants. RadioGraphics. 2012;32(2):411-435.
Dillman JR, Elsayes KM, Bude RO, Platt JF, Francis IR. Imaging of Pancreas Transplants: Postoperative Findings With Clinical Correlation. Journal of Computer Assisted Tomography. 2009;33(4):609-617.
Heyneman LE, Keogan MT, Tuttle-Newhall JE, Porte RJ, Leder RA, Nelson RC. Pancreatic Transplantation Using Portal Venous and Enteric Drainage: The Postoperative Appearance of a New Surgical Procedure. Journal of Computer Assisted Tomography. 1999;23(2):283-290.
Tolat PP, Foley WD, Johnson C, Hohenwalter MD, Quiroz FA. Pancreas Transplant Imaging: How I Do It. Radiology. 2015;275(1):14-27.
Low G, Crockett AM, Leung K, et al. Imaging of Vascular Complications and Their Consequences Following Transplantation in the Abdomen. RadioGraphics. 2013;33(3):633-652.
França M, Certo M, Martins L, et al. Imaging of pancreas transplantation and its complications. Insights into Imaging. 2010;1(5-6):329-338.
Lall CG, Sandrasegaran K, Maglinte DT, Fridell JA. Bowel complications seen on CT after pancreas transplantation with enteric drainage. AJR Am J Roentgenol 2006;187(5):1288–1295.
Ferrero PG, Pozo JCD. Imaging in pancreas transplantation complications: Temporal classification. Journal of Medical Imaging and Radiation Oncology. 2018;62(4):504-511.
Redfield RR, Kaufman DB, Odorico JS. Diagnosis and Treatment of Pancreas Rejection. Current Transplantation Reports. 2015;2(2):169-175.
Heller M, Bhargava P. Imaging in pancreatic transplants. Indian Journal of Radiology and Imaging. 2014;24(4):339.
Humar A, Khwaja K, Ramcharan T, et al. Chronic rejection: the next major challenge for pancreas transplant recipients. Transplantation. 2003;76(6):918-923.
Hakeem A, Chen J, Iype S, et al. Pancreatic allograft thrombosis: Suggestion for a CT grading system and management algorithm. American Journal of Transplantation. 2017;18(1):163-179.
Liu Y, Akisik F, Tirkes T, et al. Value of magnetic resonance imaging in evaluating the pancreatic allograft transplant complications. Abdominal Imaging. 2015;40(7):2384-2390.
Tan M, Carlo AD, Stein LA, Cantarovich M, Tchervenkov JI, Metrakos P. Pseudoaneurysm Of The Superior Mesenteric Artery After Pancreas Transplantation Treated By Endovascular Stenting. Transplantation. 2001;72(2):336-338.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ribeiro, B.J., Yoshimi, N.T., Fortes, C.D.F.M. et al. Pancreatic transplantation with duodenoduodenostomy drainage: technique, normal radiological appearance and complications. Abdom Radiol 45, 479–490 (2020). https://doi.org/10.1007/s00261-019-02267-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02267-x