Abstract
Small bowel transplantation is a surgical technique reserved for patients with end-stage intestinal failure. Despite its inherent technical difficulties, it has emerged as the standard of care for these patients. This article reviews the background and different surgical techniques for this procedure and then fully describes the spectrum of imaging findings of pancreatic and biliary complications, which have a prevalence of up to 17%, after this procedure based on 23-year single-center experience. The pancreaticobiliary complications encountered in our experience and discussed in this article include: ampullary stenosis, biliary cast, choledocholithiasis, bile leak, recurrent cholangitis, acute pancreatitis, chronic pancreatitis, and pancreatic duct fistula. Familiarity with the broad spectrum of PB complications and their variable manifestations will help radiologists to accurately diagnose these complications which have relatively high morbidity and mortality in these immune-compromised patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Small bowel transplantation (SBTX) is a technically challenging procedure reserved for patients with irreversible small bowel failure who can no longer be maintained on total parenteral nutrition (TPN) [1–3]. Intestinal failure is commonly caused by an abnormally short length of small bowel (short gut syndrome). Other less common causes include dysmotility syndromes, extensive mesenteric desmoid tumors, enterocyte dysfunction, and extensive portomesenteric thrombosis. To date, less than 2500 SBTX have been performed in the United States [4], with over 600 cases performed in our institution [3]. Since the first successful intestinal transplantation in 1987 [5], there has been marked improvement in patient and graft survival rate due to advances in surgical techniques, improved postoperative care, and introduction of novel immunosuppression regimens [1, 3, 6]. Tacrolimus-based regimens, introduced in early 1989, played a significant role with marked improvement in prevention and control of acute cellular rejection [7]. Published reports from experienced centers show 1- and 5-year survivals of 90% and 60%, respectively [1]. Survival rates, however, remain below those for solid organ transplantations.
Pancreaticobiliary (PB) complications of SBTX are not uncommon [8]. In a recent study, 17% of patients who received either combined liver and small bowel transplantation (L/SB) or multivisceral transplantation (MVT) grafts developed PB complications [8]. To our knowledge, detailed description of these complications has not been reported in the radiology literature. The purpose of this pictorial essay is to review the nomenclature and different surgical techniques (with emphasis on their effect on PB anatomy) and to describe the spectrum of imaging findings of PB complications after SBTX based on 23-year single-center experience.
Nomenclature and types of small bowel-containing allografts
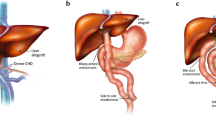
Depending on patient’s underlying disease, comorbidities (especially status of liver), age, anatomy, and other surgical considerations, different surgical approaches of SBTX are utilized. The three main prototypes are isolated SBTX, L/SB, and MVT (Fig. 1). The L/SB and MVT grafts are also referred to as composite intestinal allografts. The term MVT is used whenever the allograft includes the stomach. MVT graft may or may not include the liver, referred to as “full” or “modified” MVT, respectively [9].
Common types of small bowel transplantation. Schematic drawing shows the anatomy of grafts in isolated small bowel (A), combined liver and small bowel (B), and full multivisceral (C) transplantations. Native organs are colored in red and transplanted organs are in turquoise (adapted from Ref. [17]; with permission of Future Medicine Ltd.).
The isolated SBTX, containing the jejunum and ileum only, is performed in patients with preserved liver function; it has been the most widely used technique accounting for 44% of all SBTX in the United States [4]. Since the PB tree and native papilla remain intact in this technique, the risk of PB complications is relatively low.
L/SB is indicated in patients with intestinal failure and end-stage liver disease (due to long-term TPN) or in patients with extensive portomesenteric thrombosis [10]. Different techniques of biliary anastomosis have been used for L/SB. These techniques evolved from Roux-en-Y choledochojejunostomy (the original technique in 1990s) to newer techniques with en-bloc retrieval of liver, duodenum, jejunum, and ileum preserving the graft papilla and eliminating the need for biliary reconstruction [10]. In the latter cases, the entire or part of the transplanted pancreas is contained in the allograft such that patients will have two duodena and two pancreata (native and transplanted for each) (Fig. 2). Transection of the graft pancreas introduces a risk of graft pancreatic duct fistula or leak. Hence, new techniques use the entire pancreas for the allograft rather than just the head of pancreas [8]. The native common bile duct is transected and oversewn in this technique.
A 33-year-old male with history of modified multivisceral transplantation for pseudo-obstruction syndrome. The native pancreaticoduodenal complex was preserved in this patient to avoid biliary reconstruction. The patient has two duodena and two pancreata. A Axial contrast-enhanced CT through the upper abdomen shows the transplanted pancreas (encircled). B Axial contrast-enhanced CT through the mid abdomen, in a lower section than (A), shows the native pancreas (encircled) which is placed inferior and anterior to the transplanted one. The vascular conduit made by donor aorta (carrel’s patch) is annotated by an arrow. C MIP coronal MRCP image better shows relationship between native pancreatic duct (outlined by arrows) and transplanted pancreatic duct (annotated by dotted arrows). The native pancreatic duct joins the biliary tree.
MVT is indicated in patients with complex abdominal pathologies including massive polyposis, extensive desmoid tumors, locally aggressive neoplasms, generalized intestinal myopathy/neuropathy, traumatic loss of viscera, and complete thrombosis of splanchnic vessels [10]. In full MVT, the native organs are explanted and the donor stomach, duodenum, jejunum, ileum, pancreas, and liver are transplanted en bloc; hence, the graft PB tree and papilla remain intact. The native liver remains in place in modified MVT. In the original technique, the native biliary tree is reconstructed via choledocho-choledochal anastomosis. The native pancreaticoduodenal complex is excised and the transplanted pancreas is in continuity with the graft duodenum through an intact papilla. In a variation of this technique (introduced in 2000), the native pancreaticoduodenal complex is preserved and the native duodenum is anastomosed to the graft duodenum in side-to-side fashion (piggyback). Patient will have two duodena and two pancreata with the native pancreas usually positioned superior and posterior to the transplanted one. The biliary tree remains in continuity with the native duodenum through intact papilla.
Spectrum of pancreaticobiliary (PB) complications
Rejection and infection are by far the most common complications after SBTX [3, 4]. Other complications include those related to surgery (such as abscess, anastomotic leak, adhesion, etc.), vascular complications, post-transplantation lymphoproliferative disorder (PTLD), and graft-vs.-host-disease. Once thought to be less common, PB complications of SBTX are recently shown to be relatively common [8]. In that study, 44 out of 271 (17%) patients who received either L/SB or MVT grafts developed PB complications: 20 had biliary complications, 19 had pancreatic complications, and 5 had combined PB complications [8]. The same study showed that the risk of PB complications was significantly higher in MVT than in L/SB (25% vs. 9%). No PB complications were found in recipients of isolated SBTX in this cohort.
The spectrum of PB complications with the incidence and their imaging findings are detailed below.
Ampullary stenosis
Ampullary stenosis, which is a well-known complication post-liver transplantation, is thought to be caused by denervation of the common bile duct and sphincter of Oddi during transplantation surgery [11, 12]. It was the most common biliary complication in our cohort, seen in 3% of patients. All cases occurred in the graft ampulla [8]. These patients present with abnormal (obstructive pattern) liver function tests and are usually asymptomatic due to denervation, in contradistinction to this entity in non-transplant patients. Imaging studies show marked biliary ductal dilatation (Fig. 3). Ultrasound is the initial test to look for biliary ductal dilatation in patients with pain or abnormal liver function test. MR cholangiopancreatography (MRCP) is the preferred non-invasive method to delineate the entire biliary tree. Endoscopic retrograde cholangiopancreatography (ERCP) is similar to MRCP in its diagnostic capability (to exclude obstructive causes such as stone or biliary cast) but has therapeutic advantages as well (such as sphincterotomy and removal of biliary stones and casts) [11]. It should be noted that isolated biliary ductal dilatation, in the absence of abnormal liver function tests, is nonspecific and of unclear clinical significance (since all these patients had cholecystectomy).
Ampullary stenosis. A 45-year-old female with history of full multivisceral transplantation for short gut syndrome caused by thrombosis of superior mesenteric artery. The patient was found to have elevated serum bilirubin level. US was performed to evaluate the biliary tree. A Transverse Duplex US image through the porta hepatis shows moderate dilatation of common duct (annotated by asterisk) and intrahepatic ducts. B Coronal projection from ERCP also demonstrates moderate dilatation of intra- and extrahepatic bile ducts to the level of ampulla. No choledocholithiasis was seen. Sphincterotomy was performed and subsequent blood works showed normalization of serum bilirubin level.
Bile duct casts and stones
Bile casts and stones are seen in patients following biliary reconstruction, such as modified MVT with choledocho-choledochal anastomosis or L/SB with choledochojejunostomy (Figs. 4, 5). Bile casts were seen in 2% of patients in our cohort [8]. While the pathogenesis is not fully understood, the proposed etiologies include sloughing of biliary epithelium (due to prolonged cold ischemic time or acute cellular rejection), biliary infection, biliary stasis, alteration of the bile milieu, and use of postoperative biliary drainage tubes [13, 14]. These patients present with jaundice, abnormal (obstructive pattern) liver function tests, or signs of cholangitis. Ultrasound is the first line modality to detect biliary ductal dilatation but may not be able to show intraductal filling defects. MRCP offers excellent resolution for the evaluation of biliary tree and can depict intraductal filling defects with high sensitivity (Fig. 4). Addition of T1-weighted MRI may further enhance the sensitivity to detect biliary casts which tend to show high T1 signal intensity (Fig. 4) [15]. ERCP is the preferred intervention to remove biliary casts or stones. A biliary sphincterotomy is followed by removal of casts with an extraction balloon or basket.
Biliary cast. A 22-year-old female with history of combined liver and small bowel transplantation for cryptogenic enteritis presented with elevated serum bilirubin and alkaline phosphatase. A Coronal image from respiratory-triggered 3-D MRCP shows irregularity of the extrahepatic duct and filling defects in the duct. B Axial T1W MR image through the porta hepatis demonstrates T1 hyperintensity within the duct (arrow in B) compatible with biliary cast/stone. The patient underwent ERCP for treatment. C Coronal projection from ERCP again shows irregularity of the extrahepatic duct and intraductal filling defects (arrow in C) compatible with biliary cast syndrome. The biliary casts were removed by balloon sweep method.
Choledocholithiasis. A 64-year-old female with history of full multivisceral transplantation for short gut syndrome from a complication of gastric bypass surgery. Axial image from unenhanced CT through the porta hepatis shows several peripherally calcified stones (arrow) within the common bile duct.
Bile leak
Bile leak may develop at different sites including the duct-to-duct anastomosis, the T-tube insertion site, or the cut surface of liver. Bile leak is seen in patients with biliary reconstruction (i.e., L/SB with Roux-en-Y choledochojejunostomy or modified MVT with choledocho-choledochal anastomosis). In our cohort, bile leaks were present in 2% of patients [8]. ERCP is appropriate to delineate the anatomy and to document the diagnosis (Fig. 6). Hepatobiliary scintigraphy and MRI with use of hepatobiliary agent (such as gadoxetate disodium) are additional imaging modality options to confirm the diagnosis in selected cases [16]. While mild forms of leak can be managed by biliary stenting during the ERCP and possibly image-guided drainage of biloma, more complex cases may require open surgery and reconstruction.
Bile leak. A 30-year-old male with history of Gardner’s syndrome status post modified multivisceral transplantation. CT (not shown) demonstrated an intra-abdominal collection. The patient underwent ERCP (28 days after the surgery) because of clinical concern for bile leak. A Frontal projection from ERCP shows extraluminal contrast (arrows) compatible with leak at the biliary anastomosis. A biliary stent was placed. B Follow-up ERCP after removal of stent showed normal-caliber extrahepatic duct with resolution of leak.
Recurrent cholangitis
One patient in our cohort presented with recurrent cholangitis likely related to enterobiliary reflux [8]. The patient had L/SB with Roux-en-Y choledochojejunostomy. Enteroclysis in this patient showed dilatation of native duodenum and allograft jejunum with marked reflux of contrast into the pancreaticobiliary limb as well as the biliary tree (Fig. 7).
Recurrent ascending cholangitis due to enterobiliary reflux. A 29-year-old male with history of bowel ischemia (due to trauma) status post combined small bowel and liver transplantation. Frontal projection from enteroclysis shows marked reflux of enteric contrast into the pancreatobiliary limb and biliary tree (arrow).
Acute pancreatitis
Acute pancreatitis represents the most common PB complication in patients after an L/SB or MVT transplant (5.5% in our cohort [8]). In the majority of cases, the pancreatic allograft was involved. Use of histidine–tryptophan–ketoglutarate (HTK) solution for preservation of graft pancreas has been associated with higher incidence of acute pancreatitis after reperfusion than the use of University of Wisconsin solution [3]. Several cases of acute pancreatitis of native pancreas were also noted in our cohort [8]. The exact causes for pancreatitis in the native pancreas are uncertain, with possible mechanisms including altered perfusion (due to post-surgical changes in vascular anatomy), ischemia, and other causative agents.
Necrotizing and interstitial pancreatitis were seen with equal incidence. Since the majority of imaging was done without intravenous contrast (a cautionary measure to protect renal function), the assessment for the presence of pancreatic necrosis was often inadequate. The diagnosis of necrotizing pancreatitis was evidenced by the findings on re-exploration. All of these patients underwent debridement. The common findings of acute pancreatitis on CT include enlargement of the pancreas, peripancreatic fluid, and disproportionate peripancreatic stranding (Fig. 8). On MRI, the common findings are signal alteration of the pancreatic parenchyma (especially decreased signal on T1-weighted sequences) and peripancreatic fluid (better seen on fat-suppressed T2-weighted sequence) (Fig. 9).
Acute interstitial pancreatitis of allograft. A 44-year-old female with history of full multivisceral transplantation for short gut syndrome caused by thrombosis of celiac and superior mesenteric arteries. Axial image from contrast-enhanced CT through the pancreas shows enlargement of the pancreatic allograft (asterisk) and peripancreatic fluid compatible with acute pancreatitis. No areas of necrosis are seen on CT. The patient had elevated serum amylase and lipase levels.
Acute on chronic pancreatitis of allograft. A 44-year-old male with history of combined small bowel and pancreas transplantation for short gut syndrome caused by volvulus. The patient presented with pain and had elevated serum amylase and lipase levels. A Axial T2W MR image through the pancreas allograft shows peripancreatic fluid, mild pancreatic atrophy, and dilatation of pancreatic duct (arrow in A); changes compatible with acute on chronic pancreatitis. B Thick-slab 2D MRCP image in coronal oblique plane demonstrates marked dilatation of the entire main pancreatic duct and the side branches. C Frontal projection from ERCP again depicts dilatation of main (white arrow) and side-branch (black arrow) pancreatic ducts.
Since many patients with composite intestinal transplant might have both the native and transplanted pancreas glands, it is important for the radiologist to distinguish between the two in the report and convey the findings accordingly.
Chronic pancreatitis
Chronic pancreatitis was less common than acute pancreatitis, seen only in two patients (<1%) in our cohort [8]: one involved the native pancreas and the other the transplanted pancreas. The patient with chronic pancreatitis of the native pancreas developed worsening pancreatic parenchymal calcifications 11 months after isolated SBTX along with progressive parenchymal atrophy (Fig. 10). CT, MRI, and ERCP showed marked dilatation of the main pancreatic duct. One patient had a stone in the pancreatic duct. Both patients underwent ERCP and stenting of the pancreatic duct.
Chronic pancreatitis of native pancreas. A 36-year-old male with history of combined small bowel and liver transplantation for short gut syndrome caused by Crohn’s disease. A Axial image from unenhanced CT through the native pancreas shows several intraductal and parenchymal pancreatic calcifications (arrows in A). B Thick-slab 2D MRCP image in coronal oblique plane better delineates the large intraductal stone (solid white arrow) with irregularity and dilatation of the upstream duct. Also note the additional intraductal filling defect (dashed arrow). The patient underwent ERCP for removal of stone. C Frontal projection image from ERCP again shows the large intraductal stone (black arrow in C) and dilatation of upstream pancreatic duct (white arrow in C).
Pancreatic duct fistula
Pancreatic fistula was seen in 2% of patients in our cohort [8]. The underlying causes include allograft splenectomy, necrotizing pancreatitis, and allograft pancreatic transection. The diagnosis is confirmed by ERCP or exploratory laparotomy (Fig. 11). The diagnosis may be suspected in CT or MRI where intra-abdominal fluid collections are in continuity with the pancreas.
Pancreatic duct fistula. A 52-year-old male with history of full multivisceral transplantation for short gut syndrome caused by portomesenteric venous thrombosis. A left upper quadrant drain catheter was placed for 3 months after the surgery because of persistent output. The drain output was positive for amylase indicating a leak from pancreatic duct. Frontal projection image from ERCP delineates contrast leaking from the pancreatic tail (black arrow). The pancreatic duct is annotated by the white arrows. The patient was taken back to the operating room for surgical repair of pancreatic duct fistula.
Such patients can be managed by stenting of the allograft pancreatic duct or by surgical debridement and distal pancreatectomy. These patients frequently require percutaneous catheter drainage of the intra-abdominal fluid collection.
Conclusion
Despite its inherent surgical complexities and technical difficulties, SBTX has emerged as the standard of care for patients with intestinal failure with improved outcomes. PB complications are not uncommon after small bowel transplant with composite visceral grafts. Familiarity with the broad spectrum of PB complications and their variable manifestations will help radiologists to accurately diagnose these complications which have relatively high morbidity and mortality. Early diagnosis of PB complications is crucial for prompt management of these immune-compromised recipients which will lead to better therapeutic outcome. Understanding surgical technique and operative anatomy is also essential not only for the accurate diagnosis of complications but also for effective multidisciplinary team approach including radiologic and/or surgical intervention.
References
Abu-Elmagd KM, Kosmach-Park B, Costa G, et al. (2012) Long-term survival, nutritional autonomy, and quality of life after intestinal and multivisceral transplantation. Ann Surg 256:494–508
Abu-Elmagd K, Bond G (2002) The current status and future outlook of intestinal transplantation. Minerva Chir 57:543–560
Abu-Elmagd KM, Costa G, Bond GJ, et al. (2009) Five hundred intestinal and multivisceral transplantations at a single center: major advances with new challenges. Ann Surg 250:567–581
Organ precurement and transplantation netword (OPTN). US Department of Health and Human Services. http://www.optn.transplant.hrsa.gov/. Accessed 25 Nov 2014.
Starzl TE, Rowe MI, Todo S, et al. (1989) Transplantation of multiple abdominal viscera. J Am Med Assoc 261:1449–1457
Fishbein TM (2009) Intestinal transplantation. N Engl J Med 361:998–1008
Abu-Elmagd K (2003) The history of intestinal transplantation. In: Nakim NSPV (ed) The history of organ and cell transplantation. London: Imperial College Press, p 171
Papachristou GI, Abu-Elmagd KM, Bond G, et al. (2011) Pancreaticobiliary complications after composite visceral transplantation: incidence, risk, and management strategies. Gastrointest Endosc 73:1165–1173
Abu-Elmagd KM (2011) The small bowel contained allografts: existing and proposed nomenclature. Am J Transpl 11:184–185
Nickkholgh A, Contin P, Abu-Elmagd K, et al. (2013) Intestinal transplantation: review of operative techniques. Clin Transpl 27(Suppl 25):56–65
Safdar K, Atiq M, Stewart C, Freeman ML (2009) Biliary tract complications after liver transplantation. Expert Rev Gastroenterol Hepatol 3:183–195
Pascher A, Neuhaus P (2006) Biliary complications after deceased-donor orthotopic liver transplantation. J Hepatobiliary Pancreat Surg 13:487–496
Ayoub WS, Esquivel CO, Martin P (2010) Biliary complications following liver transplantation. Dig Dis Sci 55:1540–1546
Pfau PR, Kochman ML, Lewis JD, et al. (2000) Endoscopic management of postoperative biliary complications in orthotopic liver transplantation. Gastrointest Endosc 52:55–63
Kinner S, Umutlu L, Dechene A, et al. (2012) Biliary complications after liver transplantation: addition of T1-weighted images to MR cholangiopancreatography facilitates detection of cast in biliary cast syndrome. Radiology 263:429–436
Banzo I, Blanco I, Gutierrez-Mendiguchia C, et al. (1998) Hepatobiliary scintigraphy for the diagnosis of bile leaks produced after T-tube removal in orthotopic liver transplantation. Nucl Med Commun 19:229–236
Abu-Elmagd K, Bond G (2003) Gut failure and abdominal visceral transplantation. Proc Nutr Soc 62:727–737
Conflict of interest
The authors have no relevant financial interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borhani, A.A., Dasyam, A.K., Papachristou, G. et al. Radiologic features of pancreatic and biliary complications following composite visceral transplantation. Abdom Imaging 40, 1961–1970 (2015). https://doi.org/10.1007/s00261-014-0338-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-014-0338-z