Abstract
Purpose
Microvascular invasion (MVI), which is difficult to diagnose before surgery, is a major factor affecting postoperative recurrence in patients with hepatocellular carcinoma (HCC). The relationship between the radiological tumor capsule and MVI is controversial. This study aimed to evaluate the association between the tumor capsule and MVI.
Methods
We searched Medline (by PubMed) and Embase (by OvidSP). Two review authors independently screened titles and abstracts, selected studies about MVI prediction with radiologic tumor capsule and studies with enough data for extracted, assessed the methodological quality and collected data. Summary results were presented as the diagnostic odds ratio (DOR), sensitivity, specificity, and 95% confidence interval.
Results
Fifteen studies with 2038 patients were included; fourteen studies, including 1331 patients, with no significant heterogeneity indicated no relationship between absent tumor capsule and MVI [DOR = 0.90 (0.64, 1.26)]. Six studies, including 541 patients, with no significant heterogeneity showed incomplete capsule could be used to predict MVI of HCC preoperatively [DOR = 1.85 (1.13, 3.04)]. The overall sensitivity and specificity estimate were 0.50 (0.37, 0.64) and 0.64 (0.53, 0.74), respectively. Eight studies, including 1349 patients, with highly significant heterogeneity revealed that complete capsule could be a protective factor for MVI [DOR = 1.97 (1.01, 3.86)].
Conclusions
For MVI of HCC, incomplete tumor capsule is a risk factor, while a complete tumor capsule might be a protective factor. However, absent capsule doesn’t show significant relationship with MVI. This might be due to combination of the risk and protective effects of present capsule in MVI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer death worldwide, especially in developing countries. It is the sixth leading cause of cancer death among men in developed countries [1]. Radical resection and liver transplantation are currently recognized as the most effective HCC treatments. Microvascular invasion (MVI), also called microvascular cancer embolism, refers to the microscopic observation of cancer cell nest in the vessels covered by endothelial cells [2]. MVI’s presence shortened disease-free survival and overall survival at 3 years after liver transplantation. It also effects disease-free survival at 3 years after liver resection [3]. When MVI exists, the margin of surgery needs to be expanded, and combined systemic treatment is recommended to improve the possibility of cure [4]. However, MVI is rarely diagnosed preoperatively, and histopathological examination has a certain degree of sampling error and false negative results. Many promising radiologic markers are used to predict MVI, such as tumor capsule, a non-smooth tumor margin, peritumoral enhancement, radiogenomic biomarkers, and texture analysis. The relationship between radiologic tumor capsule and MVI is highly controversial. Lim et al. [5] suggested that the presence and structural integrity of HCC capsules was closely correlated to the absence of MVI. Other authors reported that the presence of a fibrous capsule was a strong predictor of portal venous invasion by HCC [6]. Meanwhile, others stated the capsule did not show significant correlation with MVI [7]. The tumor capsule on preoperative imaging can be either complete, incomplete, or absent. This systematic review aimed to identify relationships between these three categories of tumor capsules and their roles as predictive MVI factors. The tumor capsule was divided into three groups to investigate the correlation between capsule and MVI. The first was that comparison between absent capsule and present (including complete and incomplete) capsule. The second was that comparison between incomplete capsule and not-incomplete (including complete and absent) capsule. The third is that comparison between complete capsule and not-complete (including incomplete and absent) capsule. Sub-analyses of each group were provided to estimate potential effects of MRI versus CT.
Methods
Literature search strategy
We searched Medline (by PubMed) and Embase (by OvidSP) up to October 12, 2018. The references included in the literature were also searched to locate additional studies. “Hepatocellular carcinoma” was used as a medical search heading (MeSH term) and free term. “Microvascular invasion” was used as a free term combined with the search terms “capsule,” “pseudocapsule,” “involucrum,” “encapsulation,” “envelope,” and “peplo.” There are no restrictions on the published language or type of research.
Inclusion and exclusion criteria
Studies about preoperative MVI prediction with a radiological tumor capsule on CT or MRI were included. Studies were required to provide enough data to obtain a diagnostic 2 × 2 table or odds ratio (OR) values. Excluded studies were duplications, non-diagnostic tests, non-human trails, case reports, reviews, conference abstracts, non-primary HCC, histopathological capsule, macrovascular invasion, non-extractable data, and anti-tumor therapy trials.
Quality assessment and data extraction
Two review authors (F.Z. with 2 years of abdominal expertise and F.Y. with 8 years of abdominal expertise) independently screened titles and abstracts, selected studies about MVI prediction with radiological tumor capsule on CT or MRI and studies with enough data for extracted, assessed the quality of included studies, and collected data. When disagreements arose, the authors discussed the issues, or a third author (W.X.C. with more than 30 years of abdominal expertise) was asked to solve the problem. We used the Quality Assessment of Diagnostic Accuracy studies (QUADAS-2) tool provided by the Cochrane Collaboration to assess methodological quality of individual studies. Each individual question was categorized as “yes,” “no,” or “unclear,” and risk of bias was comprehensively assessed for each study.
The two authors independently extracted the following data from each included study: first author’s last name, publication year, country, mean age, gender, sample size, capsule status, microvascular invasion, macrovascular invasion, preoperative anti-tumor therapy, surgery method, mass types and imaging method. Data were extracted as true positives, false positives, false negatives, and true negatives to form a diagnostic 2 × 2 table or a direct OR value.
Statistical analysis
Stata V.13 (StataCorp LP, College Station, Texas) was used for statistical analysis in addition to the threshold that was obtained from Meta-Disc 1.4. Review Manager 5.3 was used to create quality assessment chart. Summary results of the association between tumor capsule and MVI were presented as the diagnostic odds ratio (DOR), sensitivity, specificity, and 95% confidence interval. A random effects model was used for all summary analysis. p ≤ 0.05 was considered statistically significant. A heterogeneity χ2 test was used to analyze the statistical heterogeneity among the included studies, with p < 0.1 indicating heterogeneity. I2 > 50% was considered significant heterogeneity among studies. Heterogeneity due to the threshold effect was investigated using the Spearman correlation coefficient. We performed Egger’s + test to detect publication bias.
Results
Study selection
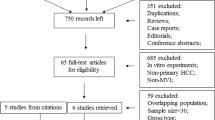
Through our search strategies, 451 articles were retrieved. Studies that were duplications, non-diagnostic tests, non-human trials, case reports, reviews, conference abstracts, and related to non-primary HCC were excluded (430 studies). We read the full texts of remaining 21 potentially eligible studies and excluded 13 studies. Eight studies only evaluated the histopathological capsule, not the radiological capsule. Three studies had data that could not be extracted [5, 8, 9], one study enrolled patients with macrovascular invasion [10]; and one study was a review [11]. We found eight studies from reference lists and excluded one because it included patients with anti-tumor therapy [12]. Finally, we included 15 articles, eight from the searched studies, and seven from the references. (Fig. 1).
Study characteristics
Of the 15 included studies, there were nine from China, three from South Korea, one from Japan, one from America and one from Italy. Six studies included only solitary tumors, and nine studies included both solitary and multiple tumors. Seven studies excluded patients with macrovascular invasion, and the remaining studies did not indicate whether patients with macrovascular invasion were included. Nine studies excluded patients who underwent anti-tumor therapy before surgery, and the remaining studies did not indicate whether patients with anti-tumor therapy were included. Eight studies included only patients with hepatectomy, two studies included only patients with liver transplantation, four studies included patients with both resection and transplantation, and one study did not provide the surgical method. Eleven articles evaluated patients by magnetic resonance imaging (MRI) only, three studies evaluated patients by computed tomography (CT) only, and one study evaluated patients by both MRI and CT.
The included studies characteristics were listed in Table 1 [7, 13,14,15,16,17,18,19,20,21,22,23,24,25,26]. For the study by Lei et al. [26], we contacted the author but could not obtain the original data; OR value was used for analysis. For the study by Ariizumi et al. [22], we extracted the data separately for CT and MRI, which were both used to assess MVI. The 15 studies included 2038 patients with HCC. At least 2100 tumors were included in this review, which 711 tumors were pathologically diagnosed as MVI-positive and 1389 tumors were diagnosed as MVI-negative. (Ariizumi et al. [22] analyzed the same patients using both CT and MRI, so the total number of patients, tumors and capsules were extracted and analyzed as two separate studies). The quality of the included studies according to QUADAS-2 guidelines is listed in Fig. 2.
Accuracy of tumor capsule for predicting MVI
The relationship between absent tumor capsule and MVI was assessed in 14 studies [7, 13,14,15,16,17,18,19,20,21,22,23,24,25], including 1331 patients with HCC and at least 1393 tumors. Of these tumors, 500 were positive for MVI (161 tumors with present capsule and 339 tumors with absent capsule) and 893 were negative for MVI (303 tumors with present capsule and 590 tumors with absent capsule). There was no significant heterogeneity among the included studies (χ2 = 19.90, p = 0.133; I2 = 29.7%). Meta-analysis with a random effects model showed no statistically significant relationship between the absence of tumor capsule and MVI [DOR = 0.90 (0.64, 1.26), p = 0.537] (True negatives referred to the tumor with absent capsule and MVI). The result of Egger’s + test showed no publication bias (p = 0.546). A subgroup analysis of MRI versus CT was performed, and the results showed no relationship between absent capsule and MVI in both groups. In MRI group, eleven studies including 965 patients showed no heterogeneity (χ2 = 8.71, p = 0.56; I2 = 0.0%). In CT group, Four studies including 366 patients showed significant heterogeneity (χ2 = 6.80, p = 0.079; I2 = 55.9%) (Fig. 3).
The relationship between incomplete tumor capsule and MVI was assessed in seven studies [13, 14, 18, 20,21,22,23], including 642 patients with HCC and at least 660 tumors. Of these tumors, 242 were positive for MVI (131 tumors with incomplete capsule and 111 tumors with not-incomplete capsule) and 418 were negative (140 tumors with incomplete capsule and 278 tumors with not-incomplete capsule). There was very high heterogeneity among the studies (χ2 = 32.60, p = 0.000; I2 = 78.5%). The Spearman correlation coefficient was − 0.096 (p = 0.820), indicating that heterogeneity was not caused by the threshold effect. Meta-analysis with a random effect model showed a statistically significant relationship between incomplete capsule and MVI [DOR = 2.74 (1.22, 6.15), p = 0.015]. The summary sensitivity and specificity estimates were 0.56 (0.42–0.70) and 0.68 (0.56–0.79). By observing the forest plot (attached in Fig. 1 of Electronic supplementary material), we found heterogeneity was caused by study of Reginelli et al. [18]. While reading the full text, we failed to determine the cause of clinical heterogeneity in this study. After excluding the study [18] with high heterogeneity, the meta-analysis was performed again. The remaining six studies [13, 14, 20,21,22,23] assessed the relationship between incomplete capsule and MVI, including 541 patients with HCC and at least 559 tumors. Of these tumors, 210 were positive for MVI (105 tumors with incomplete capsule and 105 tumors with not-incomplete capsule) and 349 were negative (133 tumors with incomplete capsule and 216 tumors with not-incomplete capsule). There was no significant heterogeneity among included studies (χ2 = 9.47, p = 0.149; I2 = 36.7%). Meta-analysis with a random effect model showed a statistically significant relationship between incomplete capsule and MVI [DOR = 1.85 (1.13, 3.04), p = 0.015]. The overall sensitivity and specificity estimates were 0.50 (0.37, 0.64) and 0.64 (0.53, 0.74), respectively. A subgroup analysis of MRI versus CT was performed, and different results were obtained in MRI and CT groups. In MRI group, there was significant heterogeneity (χ2 = 9.06, p = 0.06; I2 = 55.8%), and five studies including 378 patients with 396 HCC showed that incomplete tumor capsule was a predictor for MVI [DOR = 1.98 (1.00, 3.93), p = 0.051]. In CT group, there was no heterogeneity (χ2 = 0.37, p = 0.54; I2 = 0.0%), Two studies including 163 patients with 163 HCC showed that incomplete tumor capsule has no correlation with MVI [DOR = 1.70 (0.79, 3.68), p = 0.178]. (Fig. 4).
Eight studies [13, 14, 18, 20,21,22,23, 26] were assessed the relationship between complete tumor capsule and MVI, including 1349 patients with HCC and at least 1367 tumors. Of these tumors, 453 were positive for MVI and 914 were negative. Meanwhile, 776 HCCs with not-complete tumor capsule and 591 HCCs with complete capsule. There was significant heterogeneity among the included studies (χ2 = 34.91, p = 0.000, I2 = 77.1%). Meta-analysis with a random effect model showed a statistically significant relationship between complete tumor capsule and MVI (DOR = 1.97 (1.01, 3.86), p = 0.048) (True negatives referred to the tumor with complete capsule and MVI). Only OR was extracted without the original data in the study by Lei et al. [26], so there was no way to detect the threshold effect and the combined effect. A subgroup analysis of MRI versus CT was performed, and different results were obtained in MRI and CT groups. In MRI group, there was significant heterogeneity (χ2 = 10.61, p = 0.06; I2 = 52.9%), and six studies including 1085 patients with 1,103 HCC showed that complete tumor capsule was a protective factor for MVI [DOR = 2.05 (1.19, 3.55), p = 0.01]. In CT group, there was severe heterogeneity (χ2 = 23.84, p = 0.00; I2 = 91.6%), Three studies including 264 patients with 264 HCC showed that complete tumor capsule has no correlation with MVI [DOR = 1.86 (0.17, 20.25), p = 0.611]. (The forest plot was listed in Fig. 2 of Electronic supplementary material).
Discussion
Previous systematic reviews by Hu et al. [27, 28] assessed the relationships between tumor margin, peritumoral enhancement, peritumoral hypo-intensity on hepatobiliary phase and MVI. Our systematic review assessed the relationship between tumor capsule and MVI and found that incomplete tumor capsule on preoperative imaging revealed microvascular invasion in HCC, and complete capsule might prevented the recurrence of MVI. Meanwhile, there was no statistically significant correlation between the absent radiological tumor capsule and MVI.
Ariizumi et al. [22] included patients with both CT- and MR-enhanced scans in the study. However, the two methods showed inconsistent results at univariate analysis. MRI showed a significant correlation between incomplete tumor capsule and MVI, while CT showed no statistically significant difference of incomplete capsule between the positive-MVI and negative-MVI groups. The results were consistent with our subgroup analysis results. Several investigators [7, 13, 17] have reported that the absent of radiological capsule did not show significant correlation with MVI, which was consistent with our conclusion before and after subgroup analysis. It is likely that the capsule is both a protective factor and a risk factor. Meanwhile, the overall effects of present tumor capsules including unfavorable effects of incomplete tumor capsules and favorable effects of complete tumor capsules were cancelled out. As a result, the relationship between the absent tumor capsule and MVI was not statistically significant. The subgroup of MRI showed no heterogeneity, suggesting that the result of no relationship between absent capsule and microvascular invasion was very reliable. This can provide important reference value for the prediction of MVI in the future.
As can be seen from our subgroup analysis results, the results of the MRI group were consistent with those before subgroup analysis, while the incomplete capsule and complete capsule showed no correlation with microvascular invasion in the CT group. We consider that the possible reasons are as follows. Firstly, the image-forming principle of MRI is different from that of CT. MRI shows better contrast resolution to soft tissue, which further leads to clearly separate the capsule from surrounding tissue. Secondly, Contrast enhancement CT examination generally includes arterial phase, portal venous phase and delayed phase. While MRI examination includes early artery phase, late artery phase, early portal venous phase, late portal venous phase and delayed phase. Multiphases scanning of MRI may make the judgment of the capsule more accurate. Thirdly, when evaluating the relationship between incomplete capsule and MVI, only two studies were included in the CT group. When evaluating the relationship between complete capsule and MVI, only three studies were included in the CT group. The small sample sizes of CT group may also be the reason affecting the results. It can be seen from the results of our subgroup analysis that in the future, MRI may be better than CT in diagnosing MVI through capsule.
Our systematic review had several limitations. First, retrospective studies were included in our studies, and patient selection could introduce some bias. Second, although incomplete capsule could predict MVI in the MRI group, we need to be aware that the lower limit of the confidence interval for DOR was 1.0, which was statistically significant, but may fluctuate to the left or right due to a new research, and might further leading to the change of our results. Third,we failed to find out the cause of heterogeneity. Fourth, differences in subjective judgment of capsules could lead to differences in the results among different studies, which could further influence our meta-analysis results. Finally, pathological examination should have been considered the best practice for MVI diagnosis, but no standard and generally adopted pathological examination protocol existed, which possibly resulted in underestimating the MVI rate [29].
Lim et al. [5] suggested that there was a correlation between the size of HCC and the presence of capsule, capsules being more common when the size of HCCs was 2.0–9.9 cm in diameter. Evidence [21, 26, 30] had indicated that tumor size was a important factor for MVI prediction and tumor size can be controlled easily in the study. Therefore, we assume if future studies could further refine tumor size to improve the diagnosis accuracy of capsule for MVI. The sensitivity and specificity of incomplete capsules to predict MVI of HCC were not high. Therefore, it is hoped that the diagnostic efficiency of capsule for MVI of HCC will be improved by combing with other imaging features, especially in MR examination. This will be of great clinical significance for guiding further treatment and improving the survival rate of HCC patients. Heterogeneity reduces the reliability of our results, so prospective studies with big sample sizes are needed in the future. Further research subdividing the integrity of capsule on MRI, such as classification by the percentage of capsule in tumor, and combining imaging with pathological findings of capsule may provide great value for MVI diagnosis.
Conclusion
Incomplete tumor capsule is a risk factor, while a complete tumor capsule may be a protective factor for MVI. However, there is no correlation between absent radiological tumor capsule and MVI. This might be due to combination of the risk and protective effects of capsule in MVI. Future prospective researches with large sample sizes, limited tumor size or subdivided capsule integrity in MRI may provide more information for the diagnosis of MVI.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA: a cancer journal for clinicians 65 (2):87-108. https://doi.org/10.3322/caac.21262
CCong WM, Bu H, Chen J, Dong H, Zhu YY, Feng LH, Chen J (2016) Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol 22 (42):9279-9287. https://doi.org/10.3748/wjg.v22.i42.9279
Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK (2013) A Systematic Review of Microvascular Invasion in Hepatocellular Carcinoma: Diagnostic and Prognostic Variability. Annals of Surgical Oncology 20 (1):325-339. https://doi.org/10.1245/s10434-012-2513-1
Tsai TJ, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, Hsia CY, Wu CW (2000) Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery 127 (6):603-608. https://doi.org/10.1067/msy.2000.105498
Lim JH, Choi D, Park CK, Lee WJ, Lim HK (2006) Encapsulated hepatocellular carcinoma: CT-pathologic correlations. European Radiology 16 (10):2326-2333
Adachi E, Maeda T, Kajiyama K, Kinukawa N, Matsumata T, Sugimachi K, Tsuneyoshi M (1996) Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivariate analyses of 232 resected cases without preoperative treatments. Cancer 77 (10):2022-2031. https://doi.org/10.1002/(sici)1097-0142(19960515)77:10<2022::aid-cncr9>3.0.co;2-s
CChou CT, Chen RC, Lee CW, Ko CJ, Wu HK, Chen YL (2012) Prediction of microvascular invasion of hepatocellular carcinoma by pre-operative CT imaging. Br J Radiol 85 (1014):778-783. https://doi.org/10.1259/bjr/65897774
Zhao H, Hua Y, Dai T, He J, Tang M, Fu X, Mao L, Jin H, Qiu Y (2017) Development and validation of a novel predictive scoring model for microvascular invasion in patients with hepatocellular carcinoma. European Journal of Radiology 88:32-40
Zhang W, Wang P, Wang L, Liu L, Chen J, Su D (2018) Preoperative computed tomography and serum alpha-fetoprotein to predict microvascular invasion in hepatocellular carcinoma. Medicine (Baltimore) 97 (27):e11402. https://doi.org/10.1097/md.0000000000011402
Ahn SJ, Kim JH, Park SJ, Kim ST, Han JK (2019) Hepatocellular carcinoma: preoperative gadoxetic acid-enhanced MR imaging can predict early recurrence after curative resection using image features and texture analysis. Abdominal radiology (New York) 44 (2):539-548. https://doi.org/10.1007/s00261-018-1768-9
An C, Kim MJ (2018) Imaging features related with prognosis of hepatocellular carcinoma. Abdom Radiol 44:509–516. https://doi.org/10.1007/s00261-018-1758-y
Witjes CD, Willemssen FE, Verheij J, van der Veer SJ, Hansen BE, Verhoef C, de Man RA, Ijzermans JN (2012) Histological differentiation grade and microvascular invasion of hepatocellular carcinoma predicted by dynamic contrast-enhanced MRI. J Magn Reson Imaging 36 (3):641-647. https://doi.org/10.1002/jmri.23681
Kim H, Park MS, Choi JY, Park YN, Kim MJ, Kim KS, Choi JS, Han KH, Kim E, Kim KW (2009) Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol 19 (7):1744-1751. https://doi.org/10.1007/s00330-009-1331-8
Chou CT, Chen RC, Lin WC, Ko CJ, Chen CB, Chen YL (2014) Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. AJR American journal of roentgenology 203 (3):W253-259. https://doi.org/10.2214/ajr.13.10595
Xu P, Zeng M, Liu K, Shan Y, Xu C, Lin J (2014) Microvascular invasion in small hepatocellular carcinoma: is it predictable with preoperative diffusion-weighted imaging? J Gastroenterol Hepatol 29 (2):330-336. https://doi.org/10.1111/jgh.12358
Ahn SY, Lee JM, Joo I, Lee ES, Lee SJ, Cheon GJ, Han JK, Choi BI (2015) Prediction of microvascular invasion of hepatocellular carcinoma using gadoxetic acid-enhanced MR and 18F-FDG PET/CT. Abdominal Imaging 40 (4):843-851. https://doi.org/10.1007/s00261-014-0256-0
Lee S, Kim SH, Lee JE, Sinn DH, Park CK (2017) Preoperative gadoxetic acid–enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. Journal of Hepatology 67 (3):526-534. https://doi.org/10.1016/j.jhep.2017.04.024
Reginelli A, Vanzulli A, Sgrazzutti C, Caschera L, Serra N, Raucci A, Urraro F, Cappabianca S (2017) Vascular microinvasion from hepatocellular carcinoma: CT findings and pathologic correlation for the best therapeutic strategies. Medical oncology (Northwood, London, England) 34 (5):93. https://doi.org/10.1007/s12032-017-0949-7
Yang C, Wang H, Sheng R, Ji Y, Rao S, Zeng M (2017) Microvascular invasion in hepatocellular carcinoma: is it predictable with a new, preoperative application of diffusion-weighted imaging? Clinical Imaging 41:101-105. https://doi.org/10.1016/j.clinimag.2016.10.004
Zhou X, Wang G, Gao Y, Yu L, Li W, Jiao L, Li Z (2017) Predict microvascular invasion in hepatocellular carcinoma by dynamic contrast-enhanced magnetic resonance imaging in patients with hepatitis B virus. International Journal of Clinical and Experimental Medicine 10 (8):11728-11738
Huang M, Liao B, Xu P, Cai H, Huang K, Dong Z (2018) Prediction of Microvascular Invasion in Hepatocellular Carcinoma: Preoperative Gd-EOB-DTPA-Dynamic Enhanced MRI and Histopathological Correlation. Contrast Media & Molecular Imaging 2018:9674565. https://doi.org/10.1155/2018/9674565
Ariizumi S-i, Kitagawa K, Kotera Y, Takahashi Y, Katagiri S, Kuwatsuru R, Yamamoto M (2011) A non-smooth tumor margin in the hepatobiliary phase of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging predicts microscopic portal vein invasion, intrahepatic metastasis, and early recurrence after hepatectomy in patients with hepatocellular carcinoma. Journal of Hepato-Biliary-Pancreatic Sciences 18 (4):575-585. https://doi.org/10.1007/s00534-010-0369-y
Wang WT, Yang L, Yang ZX, Hu XX, Ding Y, Yan X, Fu CX, Grimm R, Zeng MS, Rao SX (2018) Assessment of Microvascular Invasion of Hepatocellular Carcinoma with Diffusion Kurtosis Imaging. Radiology 286(2):571-580. https://doi.org/10.1148/radiol.2017170515
Chandarana H, Robinson E, Hajdu CH, Drozhinin L, Babb JS, Taouli B (2011) Microvascular invasion in hepatocellular carcinoma: Is it predictable with pretransplant MRI? American Journal of Roentgenology 196 (5):1083-1089
Zhao W, Liu W, Liu H, Yi X, Hou J, Pei Y, Liu H, Feng D, Liu L, Li W (2018) Preoperative prediction of microvascular invasion of hepatocellular carcinoma with IVIM diffusion-weighted MR imaging and Gd-EOB-DTPA-enhanced MR imaging. PloS one 13 (5):e0197488. https://doi.org/10.1371/journal.pone.0197488
Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, Wang K, Wan X, Lau WY, Wu M, Shen F (2016) Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the milan criteria
Hu HT, Shen SL, Wang Z, Shan QY, Huang XW, Zheng Q, Xie XY, Lu MD, Wang W (2018) Peritumoral tissue on preoperative imaging reveals microvascular invasion in hepatocellular carcinoma: a systematic review and meta-analysis. Abdominal Radiology 43 (12):3324-3330. https://doi.org/10.1007/s00261-018-1646-5
Hu H, Zheng Q, Huang Y, Huang XW, Lai ZC, Liu J, Xie X, Feng ST, Wang W (2017) A non-smooth tumor margin on preoperative imaging assesses microvascular invasion of hepatocellular carcinoma: A systematic review and meta-analysis. Scientific Reports 7 (1):15375. https://doi.org/10.1038/s41598-017-15491-6
Hu HT, Wang Z, Kuang M, Wang W (2018) Need for normalization: the non-standard reference standard for microvascular invasion diagnosis in hepatocellular carcinoma. World journal of surgical oncology 16 (1):50. https://doi.org/10.1186/s12957-018-1347-0
Kaibori M, Ishizaki M, Matsui K, Kwon AH (2010) Predictors of microvascular invasion before hepatectomy for hepatocellular carcinoma. J Surg Oncol 102 (5):462-468. https://doi.org/10.1002/jso.21631
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, F., Yang, F., Li, J. et al. Incomplete tumor capsule on preoperative imaging reveals microvascular invasion in hepatocellular carcinoma: a systematic review and meta-analysis. Abdom Radiol 44, 3049–3057 (2019). https://doi.org/10.1007/s00261-019-02126-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02126-9