Abstract
Background
Histologic microvascular invasion (MVI) substantially worsens the prognosis of patients with hepatocellular carcinoma, and can only be diagnosed postoperatively. Preoperative assessment of MVI by imaging has been focused on tumor-related features, while peritumoral imaging features have been indicated elsewhere to be more accurate. The aim of the present study is to evaluate the association between peritumoral imaging features and MVI.
Methods
Literature search was performed using the PubMed, Embase, and Cochrane Library databases. Summary results of the association between peritumoral imaging features and MVI were presented as the odds ratio (OR) and the 95% confidence interval. Meta-regression and subgroup analyses were performed when heterogeneity was detected. Diagnostic accuracy analysis was also conducted for identified features.
Results
Ten studies were included in the analysis. Moderate and low heterogeneities were found among the seven studies on peritumoral enhancement and four studies on peritumoral hypointensity on HBP, respectively. Summary results revealed a significant association between MVI and peritumoral enhancement (OR 4.04 [2.23, 7.32], p < 0.05), and peritumoral hypointensity on HBP (OR 10.62 [5.31, 21.26], p < 0.05). Diagnostic accuracy analysis revealed high specificity (0.90-0.94) but low sensitivity (0.29–0.40) for both features to assess MVI.
Conclusion
The two peritumoral imaging features are significantly associated with MVI. The two features highly suggest MVI only when present with a high false negative rate. Promotion of their diagnostic efficiency can be a worthwhile task for future research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Hepatocellular carcinoma (HCC) is considered as a cause of increasing cancer-related death worldwide. Microvascular invasion (MVI) is defined as tumoral invasion into the portal radicle vein, the large capsule vessel, or a vascular space lined by endothelial cells, and is only microscopically detectable [1]. HCC patients with MVI have significantly poorer postoperative survival rate (overall or disease-free survival) [2, 3], even for patients with small solitary tumors [4, 5]. MVI is diagnosed postoperatively by histology and requires extensive sampling, with detectable rate varying in the range of 15–57.1% [6]. Attempts have been made to preoperatively evaluate patients for MVI, and imaging test has been a promising way. Peritumoral tissue is the first to be affected by MVI, vessels of which furthermore serves as the main blood dissemination path from which portal vein tumor thrombosis (PVTT), intrahepatic and extrahepatic metastasis originate [1, 7, 8]. Therefore, imaging features involving peritumoral tissue may reveal a direct association with MVI compared to those of the tumor itself.
Peritumoral enhancement is defined as the existence of a detectable, arterial-enhancing portion adjacent to, but separate from, the tumor border, as identified on arterial phase images, which later became isointense with the background liver parenchyma in the delayed phase [9]. Further, peritumoral hypointensity on HBP is defined as an irregular, wedge-shaped, or flame-like hypointense area of liver parenchyma located outside of the tumor margin [10]. Their association with MVI has been controversially reported [9, 11,12,13,14,15,16,17]. Hence, we conducted an analysis of the available evidence to reach a more conclusive result considering the relationship between peritumoral imaging features and MVI.
Materials and methods
Search strategy
We searched the PubMed, Embase, and Cochrane Library databases to find relevant studies. The last search update was performed on December 1, 2017. We used MeSH and free words such as “hepatocellular carcinoma,” “microvascular invasion,” and “peritumoral” and their variations. No restriction including language was applied. Potentially eligible citations from retrieved articles were reviewed to identify additional studies. A detailed search strategy is presented in the supplementary materials (Online Resource).
Inclusion and exclusion criteria
Studies providing data on the association between peritumoral imaging features and histological confirmed MVI in HCC patients were included. Data should be reported using 2 × 2 tables, relative risks (RR), odds ratios (OR), or other forms of data sufficient for the estimations. We excluded reviews, studies without valid data, and trials with overlapping populations.
Data extraction and quality assessment
Two authors (Q.Y.S. and X.W.H.) independently reviewed the included studies and extracted the relevant data. Discrepancies were resolved by consensus with a third author (M.K.), whose decision was considered final. The data that were recorded included the first author, country, year of publication, study design, sample size, and possible sources of bias discussed below. These variables included the mean tumor size, the presence of single or multiple tumors, the application of preoperative antitumor treatment, and the imaging modality applied. The level of evidence was evaluated in accordance with the Newcastle–Ottawa Scale (NOS) [18], establishing a consensus among all three of the authors (Q.Y.S., X.W.H. and M.K.).
Statistical analysis
We used STATA version 14.0 (Stata Corporation, College Station, TX, USA) for data analysis. The random-effects model was applied. Results were presented as the OR and the 95% confidence interval with a two-sided probability. A p < 0.05 was considered statistically significant. We used the Cochran Q test to assess the heterogeneity among studies. A value of I2 ≥ 50% suggested considerable heterogeneity. A meta-regression was applied to detect possible sources of bias from the extracted parameters. A subgroup analysis was conducted based on the results of meta-regression, and sensitivity analysis was conducted by removing one study each time as well as the studies with a low NOS score. Publication bias was evaluated using Egger’s test [19] and funnel plots.
Results
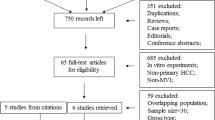
A total of 55 studies were identified using our search strategy (Fig. 1). After the title and abstract were reviewed, studies that were not related, duplicates, reviews, and editorials were excluded, leaving 16 for further screening. After a full text review, 3 studies were excluded as conference abstracts that were published later as full records [20,21,22], 4 for having no valid data after confirmation by email contact with the authors [23,24,25,26]. One study was included from the citation [17]. Finally 10 studies were included in the analysis, and their characteristics are listed in Table 1.
All of the 10 included studies were retrospective studies, with an NOS score that ranged from 7 to 10. Of these studies, five were from Korea, four from China, and one from Japan. Seven studies were on peritumoral enhancement, and four on peritumoral hypointensity on HBP. In total, 441 pathologically diagnosed MVI-positive patients and 722 MVI-negative patients were included in the analysis. The mean tumoral diameter of the patients who were included in each study ranged from 1.4 cm to 4.4 cm. Six studies included patients with a solitary HCC, while four had both solitary and multiple tumors. The two studies by Chou et al. [11, 15] used CT scans to assess peritumoral enhancement, while others applied MRI. Patients who received preoperative antitumor treatment were excluded in six studies, while not reported in four studies.
The summary result revealed a significant association between peritumoral enhancement and MVI, with an OR 4.04 [2.23, 7.32], and p < 0.05 (Fig. 2). The inconsistency index I2 was 45.4%, indicating moderate heterogeneity. Meta-regression indicated a possible source of bias in the origin of study (p < 0.05), the imaging test applied (p < 0.05), and the tumor number included (p = 0.02). Subgroup analysis revealed statistically significant results in the group from Korea (OR 7.60 [4.39, 13.17]) and China (OR 2.09 [1.12, 3.92]), and in the solitary (OR 4.32 [1.93, 9.66]) and multiple (OR 3.45 [1.21, 9.78]) tumor groups. When stratified by imaging test, the result of the studies using MRI was statistically significant (OR 4.98 [2.76, 8.99]), but was not significant for those using CT (OR 2.01 [0.62, 6.52]) (Table 2). Sensitivity analysis and Egger’s test showed no small-study effects (p = 0.58). We attempted to apply peritumoral enhancement as the only diagnostic criteria for MVI, which yielded a sensitivity of 0.29 and specificity of 0.90 (Fig. 3). The peritumoral hypointensity on HBP was also significantly associated with MVI, with an OR = 10.62 [5.31, 21.26], and p < 0.05 (Fig. 4). The heterogeneity was low (I2 = 0). A diagnostic analysis revealed similar results with low sensitivity (0.40) and high specificity (0.94) (Fig. 5).
The summary result considering association between peritumoral enhancement and MVI. The point estimates of sensitivity and specificity for each study are shown as solid boxes, and the size of each solid box indicates its weight in the analysis. Error bars are 95% confidence intervals. The estimated OR is 4.04 [2.23, 7.32], with p < 0.05. Moderate heterogeneity is indicated with I2 being 45.4%
The summary result considering association between peritumoral hypointensity on HBP and MVI. The point estimates of sensitivity and specificity for each study are shown as solid boxes, and the size of each solid box indicates its weight in the analysis. The estimated OR is 10.62 [5.31, 21.26], with p < 0.05. Low heterogeneity is indicated with I2 being 0%
Discussion
Our meta-analysis indicated significant association between peritumoral imaging features and MVI, but poor diagnostic accuracy. In clinical practice, when MVI is indicated preoperatively, the diagnostic accuracy can be improved with intentional expanded sampling. Adjuvant treatments such as sorafenib [27, 28] or trans-catheter arterial chemoembolization (TACE) [29] can be applied, as these approaches have been reported to improve the survival of HCC patients with MVI. Imaging method has been promising to assess MVI preoperatively, given the discovery of associations between imaging features and proteomics characteristics and gene signatures, and further association with a pathologically diagnosed MVI [30,31,32,33]. Kusano et al. [34] discovered a high level of expression of placental growth factor (PlGF) and vascular endothelial growth factor receptor (VEGFR-1) in adjacent noncancerous tissue associated with MVI. This elevated expression may induce peritumoral pathologic angiogenesis and potentiate vascular invasion [35]. Peritumoral enhancement may represent compensatory arterial hyperperfusion, after changes in hemodynamic perfusion with obstruction of the peritumoral microvessels by tumor thrombi [36]. Peritumoral hypointensity on HBP reflects decreased peritumoral uptake of the contrast agent on MRI. Nishie [37] suggested that this finding may be caused by Kupffer cells and hepatocyte injury in tumorous AP shunts in which the associated portal branches are obstructed.
We noticed that in the subgroup analysis of studies on peritumoral enhancement by country, studies from China revealed a significant association (I2 = 0, p = 0.02), while each single study reported an opposite result. A possible explanation is the increased statistical power with a bigger sample size. Only 2 studies used CT as the imageological method to assess peritumoral enhancement and reported nonsignificant results. Is this a result of small-study effects, or of essentially different meaning of peritumoral enhancement between CT and MRI? We think more studies are needed for further analysis. The diagnostic analysis revealed a high specificity but low sensitivity for both features, indicating them to be not very clinically useful. They highly suggest MVI when present, while a high false negative rate is problematic. Important specified information may be missed by the naked eye. At the same time, heterogeneity certainly exists among reviewers and studies, as image interpretation is operator dependent.
The radiomics method is a new research technique that is objective and informative with the assistance of artificial intelligence software in extracting high-throughput advanced quantitative features. Imaging signatures involving shape/size-based, histogram-based, filtere-based, and textural features are uncovering deep disease characteristics that cannot be appreciated visually [38]. Braman et al. [39] found peritumoral radiomic features on pretreatment breast cancer dynamic contrast-enhanced MRI to be robust predictors for a complete pathological response to neoadjuvant chemotherapy, both with and without a priori knowledge of the receptor status. In addition, Prasanna et al. [40] reported several peritumoral radiomic features on preoperative MRI to be more predictive of survival in glioblastoma multiforme than features from enhancing tumors, necrotic regions, or known clinical factors. By means of radiomics method on peritumoral tissue, preoperative MVI assessment in HCC can hopefully become more accurate and reliable in future studies.
There are limitations in our analysis. First, positive result publication bias is a major concern, especially for peritumoral hypointensity on HBP which is almost the only feature studied and reported in all of the included studies. Nonsignificant or unfavorable study results may be missed, especially when the study number is limited and Egger’s test is not applicable to assess publication bias. Second, as image interpretation is reviewer dependent, biases from interpreting imaging features between studies may result in heterogeneity, although this finding is partially explained by meta-regression results. Furthermore, the low sensitivities of both peritumoral features to diagnosis MVI limit their clinical applicability. Future studies are needed to work on this issue.
Conclusions
In summary, peritumoral imaging features are significantly associated with MVI. The features highly suggest MVI only when present with a high false negative rate. Promotion of their diagnostic efficiency can be a worthwhile task for future research.
References
Roayaie S, Blume IN, Thung SN, et al. (2009) A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 137(3):850–855. https://doi.org/10.1053/j.gastro.2009.06.003
Fan ST, Poon RT, Yeung C, et al. (2011) Outcome after partial hepatectomy for hepatocellular cancer within the Milan criteria. Br J Surg 98(9):1292–1300. https://doi.org/10.1002/bjs.7583
Sumie S, Nakashima O, Okuda K, et al. (2014) The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol 21(3):1002–1009. https://doi.org/10.1245/s10434-013-3376-9
Du M, Chen L, Zhao J, et al. (2014) Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer 14:38. https://doi.org/10.1186/1471-2407-14-38
Yamashita Y, Tsuijita E, Takeishi K, et al. (2012) Predictors for microinvasion of small hepatocellular carcinoma <= 2 cm. Ann Surg Oncol 19(6):2027–2034. https://doi.org/10.1245/s10434-011-2195-0
Rodríguez-Perálvarez M, Luong TV, Andreana L, et al. (2013) A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol 20(1):325–339. https://doi.org/10.1245/s10434-012-2513-1
Llovet JM, Schwartz M, Mazzaferro V (2005) Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis 25(2):181–200. https://doi.org/10.1055/s-2005-871198
Jun L, Zhenlin Y, Renyan G, et al. (2012) Independent factors and predictive score for extrahepatic metastasis of hepatocellular carcinoma following curative hepatectomy. Oncologist 17(7):963–969. https://doi.org/10.1634/theoncologist.2011-0447
Kim H, Park MS, Choi JY, et al. (2009) Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol 19(7):1744–1751. https://doi.org/10.1007/s00330-009-1331-8
Kim KA, Kim MJ, Jeon HM, et al. (2012) Prediction of microvascular invasion of hepatocellular carcinoma: usefulness of peritumoral hypointensity seen on gadoxetate disodium-enhanced hepatobiliary phase images. JMRI. 35(3):629–634. https://doi.org/10.1002/jmri.22876
Chou CT, Chen RC, Lee CW, et al. (1014) Prediction of microvascular invasion of hepatocellular carcinoma by pre-operative CT imaging. Br J Radiol 2012(85):778–783. https://doi.org/10.1259/bjr/65897774
Xu P, Zeng M, Liu K, et al. (2014) Microvascular invasion in small hepatocellular carcinoma: is it predictable with preoperative diffusion-weighted imaging? J Gastroenterol Hepatol (Australia) 29(2):330–336. https://doi.org/10.1111/jgh.12358
Shin SK, Kim YS, Shim YS, et al. (2017) Peritumoral decreased uptake area of gadoxetic acid enhanced magnetic resonance imaging and tumor recurrence after surgical resection in hepatocellular carcinoma: a STROBE-compliant article. Medicine 96(33):e7761. https://doi.org/10.1097/md.0000000000007761
Ahn SY, Lee JM, Joo I, et al. (2015) Prediction of microvascular invasion of hepatocellular carcinoma using gadoxetic acid-enhanced MR and (18)F-FDG PET/CT. Abdom Imaging 40(4):843–851. https://doi.org/10.1007/s00261-014-0256-0
Chou CT, Chen RC, Lin WC, et al. (2014) Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. Am J Roentgenol 203(3):W253–W259. https://doi.org/10.2214/ajr.13.10595
Lee S, Kim SH, Lee JE, Sinn DH, Park CK (2017) Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol 67(3):526–534. https://doi.org/10.1016/j.jhep.2017.04.024
Yang C, Wang H, Sheng R, et al. (2016) Microvascular invasion in hepatocellular carcinoma: is it predictable with a new, preoperative application of diffusion-weighted imaging? Clin Imaging 41:101–105. https://doi.org/10.1016/j.clinimag.2016.10.004
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Tsujita E, Yamashita Y, Takeishi K, et al. (2013) The clinicopathological impact of gross classification on solitary small hepatocellular carcinoma. Hepatogastroenterology 60(127):1726–1730
Lee S, Kim SH, Lee JE (2017) Single hepatocellular carcinoma: preoperative imaging biomarker for predicting microvascular invasion on gadoxetic acid-enhanced MR imaging. Hepat Int 11(1):S370–S371. https://doi.org/10.1007/s12072-016-9783-9
Renzulli M, Buonfiglioli F, Brocchi S, et al. (2016) Imaging features of microvascular invasion in hepatocellular carcinoma developed in HCV-related cirrhosis after direct-acting antiviral therapy. Hepatology 64(1):223A–224A
Shin SK, Kim YS, Shim YS, et al. (2017) Clinical significance of the peri-tumoral decreased uptake area on hepatobiliary phase of gadoxetic acid-enhanced MRI in hepatocellular carcinoma. Hepat Int 11(1):S375. https://doi.org/10.1007/s12072-016-9783-9
Kobayashi T, Aikata H, Daijo K, et al. (2016) Comparison of 18F-FDG PET-CT and Gd-EOB-DTPA-enhanced MRI for prediction of microvascular invasion and outcome after curative resection for early-stage hepatocellular carcinoma. Hepatology 64(1):218A
Robinson E, Chandarana H, Droxhinin L, et al. (2009) MRI features of HCC do not predict microvascular invasion in liver transplant for hepatocellular carcinoma. Liver Transpl 15:S111
Zhang W, Lai SL, Chen J, et al. (2017) Validated preoperative computed tomography risk estimation for postoperative hepatocellular carcinoma recurrence. World J Gastroenterol 23(35):6467–6473. https://doi.org/10.3748/wjg.v23.i35.6467
Nishie A, Yoshimitsu K, Irie H, et al. (2009) Radiological detectability of minute hepatic venous invasion in hepatocellular carcinoma. Eur J Radiol 70(3):517–524. https://doi.org/10.1016/j.ejrad.2008.02.021
Mendez-Sanchez N, Vasquez-Fernandez F, Zamora-Valdes D, Uribe M (2008) Sorafenib, a systemic therapy for hepatocellular carcinoma. Ann Hepatol 7(1):46–51
Wang SN, Chuang SC, Lee KT (2014) Efficacy of sorafenib as adjuvant therapy to prevent early recurrence of hepatocellular carcinoma after curative surgery: a pilot study. Hepatol Res 44(5):523–531. https://doi.org/10.1111/hepr.12159
Sun JJ, Wang K, Zhang CZ, et al. (2016) Postoperative adjuvant transcatheter arterial chemoembolization after R0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann Surg Oncol 23(4):1344–1351. https://doi.org/10.1245/s10434-015-5008-z
Banerjee S, Wang DS, Kim HJ, et al. (2015) A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology 62(3):792–800. https://doi.org/10.1002/hep.27877
Allen JC, Nault JC, Zhu G, et al. (2016) The transcriptomic G1-G6 signature of hepatocellular carcinoma in an Asian population Association of G3 with microvascular invasion. Medicine (United States). https://doi.org/10.1097/md.0000000000005263
Pote N, Alexandrov T, Le Faouder J, et al. (2013) Imaging mass spectrometry reveals modified forms of histone H4 as new biomarkers of microvascular invasion in hepatocellular carcinomas. Hepatology 58(3):983–994. https://doi.org/10.1002/hep.26433
Minguez B, Hoshida Y, Villanueva A, et al. (2011) Gene-expression signature of vascular invasion in hepatocellular carcinoma. J Hepatol 55(6):1325–1331. https://doi.org/10.1016/j.jhep.2011.02.034
Kusano H, Han J, Bulthuis M, et al. (2013) Microvascular invasion in hepatocellular carcinoma is defined by tumor characteristics and aberrant angiogenesis in peritumoral tissue. Hepatology 58(4):1214A. https://doi.org/10.1002/hep.26883
Semelka RC, Hussain SM, Marcos HB, Woosley JT (2000) Perilesional enhancement of hepatic metastases: correlation between MR imaging and histopathologic findings-initial observations. Radiology 215(1):89–94. https://doi.org/10.1148/radiology.215.1.r00mr2989
Matsui O, Kobayashi S, Sanada J, et al. (2011) Hepatocellular nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging 36(3):264–272. https://doi.org/10.1007/s00261-011-9685-1
Nishie A, Asayama Y, Ishigami K, et al. (2014) Clinicopathological significance of the peritumoral decreased uptake area of gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid in hepatocellular carcinoma. J Gastroenterol Hepatol 29(3):561–567. https://doi.org/10.1111/jgh.12423
Yip SS, Aerts HJ (2016) Applications and limitations of radiomics. Phys Med Biol 61(13):R150–R166. https://doi.org/10.1088/0031-9155/61/13/r150
Braman NM, Etesami M, Prasanna P, et al. (2017) Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res 19(1):57. https://doi.org/10.1186/s13058-017-0846-1
Prasanna P, Patel J, Partovi S, Madabhushi A, Tiwari P (2016) Radiomic features from the peritumoral brain parenchyma on treatment-naive multi-parametric MR imaging predict long versus short-term survival in glioblastoma multiforme: preliminary findings. Eur Radiol. https://doi.org/10.1007/s00330-016-4637-3
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclosure
The authors declare that they have no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, HT., Shen, SL., Wang, Z. et al. Peritumoral tissue on preoperative imaging reveals microvascular invasion in hepatocellular carcinoma: a systematic review and meta-analysis. Abdom Radiol 43, 3324–3330 (2018). https://doi.org/10.1007/s00261-018-1646-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1646-5