Abstract
Duodenal adenocarcinomas are the most common duodenal tumors, and represent 15–25% of small bowel carcinomas. Their management differs from other small bowel tumors, with imaging playing a very important role. In this article, we provide a comprehensive review of the diagnosis and management of duodenal adenocarcinomas, emphasizing the role of the radiologist in the same.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Duodenal adenocarcinomas are the most common type of cancer affecting the duodenum and pose a challenge to the radiologists, gastroenterologists, surgeons, and oncologists [1, 2]. In the United States, it is estimated that 9160 new cases of small bowel cancers were diagnosed in 2014, with a mortality rate of 1210 persons/year, with duodenal adenocarcinomas representing 15% to 25% of the small bowel cancers [1, 3–6]. They usually present between the ages of 50–70 with a slight male predominance.
The AJCC/ TNM classification for duodenal adenocarcinomas was updated in 2010, with several changes made to the previous classification. Considering the different anatomy, presentation, and management of duodenal adenocarcinomas, we comprehensively review duodenal adenocarcinomas with respect to their latest staging, diagnostic workup, and management with an emphasis on the role of the radiologist in the multidisciplinary team approach to patient care.
Pathophysiology
Most duodenal adenocarcinomas arise from duodenal adenomas [7, 8]. Data suggest that an adenoma–carcinoma sequence similar to that described for colorectal cancers is responsible for carcinogenesis, driven by a multistep process of specific genetic changes [7, 8]. Sporadic cancers result from the stepwise accumulation of multiple somatic mutations that are believed to be acquired as a result of exposure to carcinogens within the bowel lumen. In a study evaluating 213 cases of duodenal adenocarcinoma identified from the Los Angeles County Tumor Registry, Ross et al. found that 75% of cases occurred in the second part of the duodenum, most commonly the periampullary region [9]. In addition, an increased risk of adenocarcinoma is seen in a number of familial cancer syndromes associated with adenocarcinoma of both large bowel and small bowel including hereditary nonpolyposis colorectal cancer (HNPCC), Peutz–Jeghers syndrome, and familial adenomatous polyposis (FAP). FAP patients are at increased risk for both adenomas and adenocarcinomas of the duodenum [10, 11].
Surgical anatomy
The duodenum extends from the pyloric sphincter of the stomach to the ligament of Treitz, about 25 cm in length. The duodenum is usually divided anatomically into four parts. The first segment, also known as the duodenal bulb, is located immediately distal to the pylorus. The second segment courses inferiorly from the duodenal bulb, posterior to the common bile duct, and transitions to the third segment at the inferolateral margin of the pancreatic head. The common bile duct and pancreatic duct open into the second part at the ampulla of Vater. The third segment travels horizontally across the midline, traveling between the superior mesenteric artery and the aorta. The fourth part of the duodenum extends superiorly to the ligament of Treitz, which fixes posteriorly the junction between the duodenum and the jejunum. The duodenum has no free mesentery and is covered by peritoneum only anteriorly.
The blood supply of the duodenum and pancreas is variable, but the most common anatomic configuration is described hereafter. The gastroduodenal artery (GDA) typically arises from the common hepatic artery and supplies blood directly to the first part of the duodenum. The superior pancreaticoduodenal artery typically arises from the GDA, runs along the pancreatoduodenal groove, and is an anatomic landmark between second part of the duodenum and the pancreatic head. A not infrequent variant is the presence of an accessory/ replaced right hepatic artery arising from the superior mesenteric artery, which may be seen in upto 16% cases and should be carefully noted [12]. The hepatic artery is the predominant supply of the bile ducts. The presence of aberrant hepatic arterial anatomy may be associated with a higher risk of complications such as intra-operative hemorrhage and post-operative biliary leaks. Advance information about the aberrant anatomy can help the surgeons plan appropriately and preserve the aberrant artery in most cases [12–15].
The inferior pancreaticoduodenal artery typically arises from the superior mesenteric artery and is an anatomical landmark between third part of the duodenum and uncinate process of the pancreas [16]. Small anterior lymphatic channels drain into the pancreatoduodenal lymph nodes located along the superior/inferior pancreatoduodenal arteries. These nodes drain into the pyloric lymph nodes along the gastroduodenal artery. Small posterior lymphatic channels drain into the superior mesenteric lymph nodes. Efferent lymphatic channels from these duodenal lymph nodes ultimately drain into the celiac lymph nodes.
Revised 2010 AJCC TNM staging
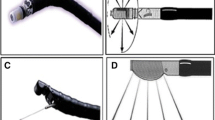
Duodenal adenocarcinomas are staged according to the American Joint Committee on Cancer Tumor, Nodes, and Metastasis (TNM) system, the most recent update of which was published in 2010, with several changes from the prior edition (Table 1) [17]. T1 lesions have been divided into T1a (invasion of lamina propria) and T1b (invasion of submucosa) to improve the value of prognostication and facilitate comparison with carcinomas involving the rest of the small bowel, which were also similarly classified . T2 lesions are defined as invasion of muscularis propria (Fig. 1), while T3 lesions are defined as invasion beyond the muscularis propria into the subserosa or into the nonperitonealized perimuscular tissue (mesentery or retroperitoneum) with extension 2 cm or less (Fig. 2). T4 lesions are defined as perforation of the visceral peritoneum or direct invasion of other organs or structures including other loops of small intestine, mesentery, more than 2 cm of retroperitoneum, or invasion of pancreas or bile duct (Figs. 3, 4). Stage I disease includes T1 and T2 lesions without nodal involvement or metastases. Stages II and III have been subdivided into stages IIA and IIB, and IIIA and stage IIIB respectively, based on prognosis. Stage IIA includes T3 lesions and stage IIB includes T4 lesions, both without nodal involvement or metastases. The N category has been changed to N1 (one to three positive lymph nodes) and N2 (four or more positive lymph nodes), leading to the division of stage III into stage IIIA and stage IIIB. Stage IV disease is defined by the presence of distant metastasis.
57-year-old male with nausea, vomiting, and abdominal pain. Axial and coronal contrast-enhanced CT (CECT) images (A, B) show an endoluminal D4 lesion (arrow) without evidence of extramural extension. There is mild dilatation of the proximal duodenum. Coronal PET image (C) demonstrates the mass to have moderate FDG uptake (arrow). Surgery confirmed T2 tumor.
56-year-old female with mild upper abdominal pain and weight loss. Axial and coronal CECT images (A, B) show thickening of the duodenal wall with possible subtle extension beyond the serosa and mild fat stranding (arrow). Locoregional adenopathy is also noted (dotted arrow in A). Surgery confirmed T3N1 disease.
42-year-old male with epigastric pain and vomiting. Axial and coronal CECT images (A, B) reveal a circumferential periampullary mass causing narrowing of the duodenal lumen (arrows), with associated adenopathy. There is loss of fat plane with the pancreatic head and CBD, with mild proximal dilatation of the CBD. The pancreatic duct was not dilated. The patient underwent Whipple’s surgery, and had a complicated post-surgical course with the development of an abscess (arrowhead in C), which was successfully drained percutaneously (image not shown). Surgery revealed T4N2 disease with positive resection margins. The patient was given adjuvant chemotherapy, but unfortunately developed recurrence after 6 months, with soft tissue developing at the surgical bed encasing the superior mesenteric vein and narrowing its lumen (D). Note the development of fatty attenuation of the liver secondary to chemotherapy. Fused axial PET/CT image confirms the presence of FDG-avid recurrent disease (E). The patient was started on second line chemotherapy, with restaging CT showing response in the form of decreased size of the soft tissue and SMV encasement (F).
49-year-old man with epigastric pain and jaundice, with a CBD stent placed at an outside institution. Axial and coronal CECT show a large periampullary mass (white arrow) involving D2 and D3 segments with invasion into the pancreas. Tumor thrombus can be seen invading the SMV and extending upto the portal vein (arrowhead). Note the CBD stent. The patient also had hepatic metastases at presentation (black arrow in B).
For duodenal adenocarcinomas, involvement of locoregional lymph nodes and invasion of adjacent structures at presentation is common. Duodenal, gastroduodenal, pancreatoduodenal, subpyloric, pyloric, hepatic, pericholedochal, and superior mesenteric nodes are considered locoregional nodes. Involvement of the celiac lymph nodes is however considered M1 disease for duodenal adenocarcinomas [17].
Clinical presentation
The diagnosis of duodenal adenocarcinoma is often made late in the course of the disease because these patients present with nonspecific symptoms such as abdominal pain (65%), weight loss, nausea, vomiting, and occult gastrointestinal tract bleeding (22%). Obstructing cancers (34%) may present with vomiting due to a gastric outlet obstruction [3]. Periampullary duodenal adenocarcinomas can present with alternate episodes of recurrent jaundice and melena due to tumor obstructing the biliary tree and then sloughing off into the bowel, causing melena [4].
Diagnostic workup and CT technique
Radiology and upper GI endoscopy play important roles for the accurate diagnosis and staging of patients with a duodenal mass, with CT being the most widely utilized imaging modality. The initial staging of such tumors is typically performed with a CT scan of the chest, abdomen, and pelvis with oral and intravenous contrast.
Since the duodenum and proximal jejunum distend within 15–20 mins of ingestion of oral contrast, positive oral contrast should be given in a split bolus at 2 h and 15 mins before the scan to well visualized the extent of soft tissue involvement [18]. Negative oral contrast is more sensitive for evaluating mucosal pathologies. However positive oral contrast is preferable as the role of CT is to demonstrate the extent of the tumor, with upper GI scopy acting as a complement and evaluating the mucosal involvement more accurately. Double-contrast studies utilizing bicarbonate granules to release carbon dioxide have also been described, although not commonly used [19, 20]. Scanning with the patient in right posterior oblique or right lateral (right side down) positions has been described to facilitate distention of the first and second parts of the duodenum if the tumor involves these locations [20]. Additional arterial phase images of the upper abdomen should be obtained on the initial scan for surgical planning, to identify vascular involvement as also variant hepatic arterial anatomy. On restaging/surveillance scans, a single phase CT of the chest, abdomen, and pelvis or only the abdomen and pelvis is usually performed. An example of a dedicated CT protocol as per current literature is given in Table 2 [14, 19–24].
CT features of the primary
The primary tumor may be seen on CT as a concentric or asymmetric irregular short-segment thickening of the bowel wall or a polypoidal or fungating mass with or without luminal narrowing [25]. Associated tumor necrosis or ulceration may also be visualized [21]. CT findings of T3 stage include transmural tumor with irregular or nodular outer border and suspicious serosal invasion and/or periduodenal fat infiltration. CT findings of T4 stage include direct invasion of adjacent organ or obliteration of the fat plane between the duodenal tumor and adjacent organs. Note must be made of vascular encasement and involvement of locoregional lymph nodes. Involvement of the superior mesenteric artery is a contraindication to surgery and the patient is given neoadjuvant treatment (Fig. 3) [21, 23]. MRI is an increasingly used useful adjunct and helps better demonstrate the relationship of the tumor to adjacent structures (Fig. 5) [24]. Although there is lack of published literature in this regard, in our experience, CT is helpful for duodenal tumors that extend laterally or inferiorly but is very difficult to interpret if the tumor invades the pancreas, particularly when secondary pancreatitis develops. MRI has a promising role for being a problem-solving tool in these situations.
58-year-old man with upper abdominal pain. Axial HASTE image (A) and post-contrast fat-suppressed T1W images (B, C) reveal a T2 hyperintense endoluminal and exophytic heterogeneously enhancing mass arising from the third part of duodenum (arrows in A and B). Note the SMV invasion and para-aortic adenopathy (arrowhead and arrow in C respectively), consistent with unresectable disease.
Periampullary tumors are defined as tumors that occur within 2 cm of the major duodenal papilla [26]. Periampullary duodenal adenocarcinomas usually show a hypoenhancing mass involving the periampullary segment of the duodenum and may extend to involve the ampulla [22, 27]. Bile duct and/ or pancreatic duct dilatation caused by obstruction of the ampulla may be seen in up to half the patients (Fig. 3) [22, 24]. A recent study of 202 patients of periampullary cancers, including 11 patients with duodenal adenocarcinoma, demonstrated an overall detection rate of 90% using a dedicated pancreatic protocol, with 10/11 duodenal adenocarcinomas detected. CT was found to be inaccurate for correctly identifying nodal involvement; 50% of patients of non-pancreatic periampullary carcinomas with enlarged nodes on CT had no nodal involvement at surgery, while 61% patients with no adenopathy on CT had metastatic involvement at surgery [22].
Role of other imaging modalities
The role of FDG PET/CT for initial diagnosis and staging of duodenal adenocarcinomas is evolving. Currently, the ability of PET/CT to detect local and distant metastatic disease has not been formally evaluated for duodenal adenocarcinomas in a large prospective study. However, several small studies reported that PET/CT allows primary cancer detection as well as local and distant lymph node involvement in duodenal tumors, making this a potentially useful tool for detecting nodal involvement in view of the limited accuracy of CT for the same [22, 28–30]. In addition, PET/CT is used for evaluating post-treatment (chemotherapy or surgery) response and restaging at the time of suspected disease recurrence [28].
Angiography plays a limited but specific role in the management of duodenal cancers. Embolization of the gastroduodenal artery is an important tool used in patients presenting with hematemesis, with a recent article suggesting utility of empiric embolization in all patients presenting with tumor-related gastrointestinal bleeding [31].
Double-contrast upper gastrointestinal barium studies were previously a commonly used modality to detect duodenal lesions and assess mucosal involvement by tumors [32]. This has however been superseded by upper GI endoscopy and CT. Further evaluation is usually with a standard upper GI endoscopy allowing direct visualization of the mucosal surface of the proximal duodenum. This is routinely performed to assess the mucosal extent of disease and obtain tissue for histopathological diagnosis. The role of tumor markers, namely carcinoembryonic antigen, is limited, as it is not sufficiently sensitive or specific for diagnostic purposes [33].
Imaging of metastatic disease
The presence of distant metastases and the development of recurrent disease have the maximum influence on survival. Patients with duodenal adenocarcinomas usually present at an advanced stage, with metastases present in more than 50% at the time of initial diagnosis [34]. The most common sites of recurrent/ metastatic disease include the liver, lung, peritoneum, and abdominal wall [3].
With regard to post-treatment surveillance of successfully treated patients, our institutional protocol at Dana Farber Cancer Institute is 6-monthly single phase CT scans for 2 years initially, followed by annual scans for 3 years.
Differential diagnosis
The most important differential diagnosis of duodenal adenocarcinoma arising from second part of the duodenum is other periampullary tumors, namely pancreatic cancer, ampullary carcinoma, and distal common bile duct cholangiocarcinoma [25]. While differentiating between them may often be possible when the site of origin is obvious on imaging, the tumors may infiltrate and involve multiple organs making definitive diagnosis difficult. Other differentials for duodenal tumors include carcinoid tumor, lymphoma, gastrointestinal stromal tumor (GIST), and metastasis. Duodenal carcinoids present as heterogeneously enhancing hypervascular focal intramural or intraluminal masses, most commonly affecting the proximal duodenum (Fig. 6A) [35, 36]. Lymphomas may present as duodenal wall thickening, intraluminal polypoidal lesions, or less commonly as an exophytic mass [37]. GISTs appear as well-circumscribed tumors with an exophytic component without adenopathy (Fig. 6B) [38]. Metastases (most commonly from melanoma) present as polypoidal lesions or as focal wall thickening, and may be diagnosed based on the history of a primary cancer and presence of other lesions [38, 39]. However, a specific diagnosis may not always be possible based on imaging, and endoscopic biopsy is necessary in most cases (Table 3).
A 62-year-old man with vague upper abdominal pain and nausea. Axial CECT shows an enhancing endoluminal soft tissue mass involving D3 (arrows), proven to be a carcinoid tumor on biopsy. B 55-year-old man undergoing CT for evaluation of left lower quadrant pain. An incidentally detected partially exophytic D2/3 junction soft tissue lesion is visualized (arrow), proven to be a GIST on biopsy.
Management of duodenal adenocarcinomas and the role of the radiologist
A multidisciplinary team approach with surgeons, oncologists, and radiologists is essential for management of duodenal adenocarcinomas. The overall management of duodenal adenocarcinomas is summarized in Fig. 7 [40–58]. Radiologists play an important role especially in preoperative assessment of tumor resectability, detection of post-operative complications, surveillance, restaging, and monitoring for drug toxicities. In the initial evaluation of duodenal adenocarcinomas, it is crucial to divide resectable locoregional diseases from unresectable and metastatic diseases because the management and prognosis of the two categories differ markedly. Imaging plays a critical role in this determination.
Locoregional duodenal adenocarcinoma
Locoregional duodenal adenocarcinomas are best managed with surgical resection. Surgical resection of the primary tumor is the treatment of choice and provides the only hope for cure for duodenal adenocarcinomas. Surgical resection includes primary tumor, investing mesentery, and regional lymph nodes at risk of metastases. Surgical resection of adequate mesentery could be limited by the proximity of the lymph nodes or cancer to the superior mesenteric artery. Pancreaticoduodenectomy (Whipple procedure) is required for duodenal adenocarcinomas involving the first and second portions of the duodenum. However, the surgical strategy for duodenal adenocarcinoma involving the third and fourth portions of the duodenum is different. In these cases, extension of duodenal adenocarcinomas into adjacent tissues is usually a more localized process compared to pancreatic cancer, and tumor-free resection margins may be obtained without resection of adjacent organs and soft tissues [43, 57, 59]. Several studies have shown no survival benefit for pancreaticoduodenectomy compared to segmental resection [42, 49, 50, 53, 60]. In addition, segmental resection can satisfy the principle of en bloc resection and reduce the morbidity of a pancreaticoduodenectomy, and is hence preferred. Hence, as long as a margin-negative resection can be obtained, segmental resection is suggested over pancreaticoduodenectomy for duodenal adenocarcinomas arising in the third and fourth portions of the duodenum to the left of the superior mesenteric artery.
Complication rate after pancreaticoduodenectomy is high, ranging from 36–41%, and the mortality rate ranges from 1–5% [61–64]. Major perioperative complications include pancreatic fistula formation (6–14%), anastomotic leakage (4%), post-operative collections, abscesses (6%), infection, hemorrhage (4%), pancreatitis (as high as 27%), and vascular thrombosis, and should be carefully looked for on the post-surgical scans (Fig. 3). Interventional radiologists play an important role by performing image-guided drainage of post-surgical collections and abscesses. Long-term complications of Whipple procedure include delayed gastric emptying (as high as 49%) and anastomotic strictures (8%) [62–64].
Adjuvant chemotherapy rather than surgery alone is recommended for patients with lymph node-positive resected duodenal adenocarcinoma. Nodal involvement and overall pathologic-stage are strong predictors of long-term survival [58]. An overall survival rate for patients with node-positive disease at 3 years is 12.5%, and an overall survival rate for patients with node-negative disease for node is 60%. In addition, positive resection margins increase the rate of local failure, while nodal involvement and transmural invasion of the bowel wall increase the rate of distant failure [41, 42]. Duodenal adenocarcinomas showed local failure rates as high as 41% to 50% after surgical resection alone [41, 42, 51, 56, 58]. Several reports have focused on the role of adjuvant therapy after curative resection of duodenal adenocarcinomas. Adjuvant chemoradiation did not decrease local recurrence or prolong overall survival [41, 56]. However, several large-scale studies reveal an increase in the use of adjuvant chemotherapy for regionally advanced duodenal adenocarcinomas [1, 48, 52]. Fluoropyrimidine-based chemoradiation, in addition to systemic chemotherapy, is recommended for patients with positive resection margins [41, 59]. Neoadjuvant therapy could be considered on a case-by case-basis in patients with bulky or locally advanced disease [51].
Unresectable and metastatic duodenal adenocarcinomas
The management of unresectable and metastatic duodenal adenocarcinomas is complex and continues to evolve. Systemic chemotherapy is recommended for patients with unresectable or metastatic duodenal adenocarcinomas who are able to tolerate it [45]. There is no standard first-line chemotherapy because of a lack of randomized trials comparing different chemotherapy regimens. A regimen combining a fluoropyrimidine with a platinum-type drug is considered a reasonable first choice [54]. The role of molecular targeted therapies including bevacizumab and cetuximab is under investigation [65–67]. It is important to recognize the not uncommon drug-associated toxicities such as development of fatty infiltration of liver, pneumonitis, and colitis on restaging scans for patients on chemotherapy (Fig. 3) [51, 57, 68]. Fatty infiltration of the liver has been reported to develop in 35% patients who are on 5-fluorouracil, a commonly used first-line fluoropyrimidine, with CT detecting the abnormality before clinical or laboratory manifestations. Other toxicities are less common. Pneumonitis usually presents as interstitial thickening or as patchy ground glass or consolidative opacities, while colitis can present as bowel wall thickening, increased enhancement, fluid-filled loops, and adjacent fat stranding [69].
For palliative therapy, palliative surgical resection of the primary tumor may be needed in patients with locally advanced unresectable or metastatic duodenal adenocarcinomas to prevent bowel obstruction or bleeding. In addition, radiation therapy may help to achieve local control, and a duodenal stent can be placed for nonsurgical palliation of duodenal obstruction [55].
Limited information is available regarding the role of metastatectomy in oligometastatic duodenal adenocarcinomas, [40, 44, 46, 47]. In a study of 1452 patients with liver metastases from a non-colorectal cancer primary that included 12 duodenal adenocarcinoma patients, Adam et al. [40] found that 5-year survival after hepatic resection was 21%. For selected patients with resectable liver metastases, a controlled primary site, and no extrahepatic metastases, hepatic resection is a reasonable option.
Conclusion
Duodenal adenocarcinomas are rare but aggressive tumors, with the radiologist playing a crucial role in its management. Imaging plays an important role in the initial diagnosis and accurate staging of duodenal adenocarcinomas, and helps differentiate resectable locoregional disease from unresectable and metastatic disease at baseline. Restaging scans of patients on treatment must be assessed for response and also to identify various toxicities secondary to chemotherapy, while post-surgical surveillance scans must be carefully evaluated for detecting local and distant sites of recurrent disease.
References
Bilimoria KY, Bentrem DJ, Wayne JD, et al. (2009) Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 249:63–71
Gabos S, Berkel J, Band P, et al. (1993) Small bowel cancer in western Canada. Int J Epidemiol 22:198–206
Dabaja BS, Suki D, Pro B, et al. (2004) Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 101:518–526
Halfdanarson TR, McWilliams RR, Donohue JH, Quevedo JF (2010) A single-institution experience with 491 cases of small bowel adenocarcinoma. Am J Surg 199:797–803
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64:9–29
Weiss NS, Yang CP (1987) Incidence of histologic types of cancer of the small intestine. J Natl Cancer Inst 78:653–656
Raghav K, Overman MJ (2013) Small bowel adenocarcinomas–existing evidence and evolving paradigms. Nat Rev Clin Oncol 10:534–544
Wheeler JM, Warren BF, Mortensen NJ, et al. (2002) An insight into the genetic pathway of adenocarcinoma of the small intestine. Gut 50:218–223
Ross RK, Hartnett NM, Bernstein L, Henderson BE (1991) Epidemiology of adenocarcinomas of the small intestine: is bile a small bowel carcinogen? Br J Cancer 63:143–145
Moertel CG, Sauer WG, Dockerty MB, Baggenstoss AH (1961) Life history of the carcinoid tumor of the small intestine. Cancer 14:901–912
Saha S, Hoda S, Godfrey R, et al. (1989) Carcinoid tumors of the gastrointestinal tract: a 44-year experience. South Med J 82:1501–1505
Stauffer JA, Bridges MD, Turan N, et al. (2009) Aberrant right hepatic arterial anatomy and pancreaticoduodenectomy: recognition, prevalence and management. HPB (Oxford) 11:161–165
Volpe CM, Peterson S, Hoover EL, Doerr RJ (1998) Justification for visceral angiography prior to pancreaticoduodenectomy. Am Surg 64:758–761
Shukla PJ, Barreto SG, Kulkarni A, et al. (2010) Vascular anomalies encountered during pancreatoduodenectomy: do they influence outcomes? Ann Surg Oncol 17:186–193
Eshuis WJ, Olde Loohuis KM, Busch OR, et al. (2011) Influence of aberrant right hepatic artery on perioperative course and longterm survival after pancreatoduodenectomy. HPB (Oxford) 13:161–167
Hernandez-Jover D, Pernas JC, Gonzalez-Ceballos S, et al. (2011) Pancreatoduodenal junction: review of anatomy and pathologic conditions. J Gastrointest Surg 15:1269–1281
Edge SBBDCC, et al. (2010) AJCC cancer staging manual, 7th edn. New York: Springer, pp 129–134
Tochetto S, Yaghmai V (2009) CT enterography: concept, technique, and interpretation. Radiol Clin N Am 47:117–132
Raptopoulos V (1989) Technical principles in CT evaluation of the gut. Radiol Clin N Am 27:631–651
Jayaraman MV, Mayo-Smith WW, Movson JS, et al. (2001) CT of the duodenum: an overlooked segment gets its due. Radiographics 21:S147–160
Farah MC, Jafri SZ, Schwab RE, et al. (1987) Duodenal neoplasms: role of CT. Radiology 162:839–843
Fong ZV, Tan WP, Lavu H, et al. (2013) Preoperative imaging for resectable periampullary cancer: clinicopathologic implications of reported radiographic findings. J Gastrointest Surg 17:1098–1106
Kazerooni EA, Quint LE, Francis IR (1992) Duodenal neoplasms: predictive value of CT for determining malignancy and tumor resectability. AJR Am J Roentgenol 159:303–309
Nikolaidis P, Hammond NA, Day K, et al. (2014) Imaging features of benign and malignant ampullary and periampullary lesions. Radiographics 34:624–641
Kim JH, Kim MJ, Chung JJ, et al. (2002) Differential diagnosis of periampullary carcinomas at MR imaging. Radiographics 22:1335–1352
Sugita R, Furuta A, Ito K, et al. (2004) Periampullary tumors: high-spatial-resolution MR imaging and histopathologic findings in ampullary region specimens. Radiology 231:767–774
Pham DT, Hura SA, Willmann JK, et al. (2009) Evaluation of periampullary pathology with CT volumetric oblique coronal reformations. AJR Am J Roentgenol 193:W202–W208
Cronin CG, Scott J, Kambadakone A, et al. (2012) Utility of positron emission tomography/CT in the evaluation of small bowel pathology. Br J Radiol 85:1211–1221
Sperti C, Pasquali C, Fiore V, et al. (2006) Clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the management of patients with nonpancreatic periampullary neoplasms. Am J Surg 191:743–748
Watanabe N, Hayashi S, Kato H, et al. (2004) FDG-PET imaging in duodenal cancer. Ann Nucl Med 18:351–353
Tandberg DJ, Smith TP, Suhocki PV, et al. (2012) Early outcomes of empiric embolization of tumor-related gastrointestinal hemorrhage in patients with advanced malignancy. J Vasc Interv Radiol 23:1445–1452
Op den Orth JO (1989) Use of barium in evaluation of disorders of the upper gastrointestinal tract: current status. Radiology 173:601–608
Zhu L, Kim K, Domenico DR, et al. (1996) Adenocarcinoma of duodenum and ampulla of Vater: clinicopathology study and expression of p53, c-neu, TGF-alpha, CEA, and EMA. J Surg Oncol 61:100–105
Ashley SW, Wells SA Jr (1988) Tumors of the small intestine. Semin Oncol 15:116–128
Seymour EQ, Griffin CN Jr, Kurtz SM (1982) Carcinoid tumors of the duodenal cap presenting as multiple polypoid defects. Gastrointest Radiol 7:19–21
Levy AD, Taylor LD, Abbott RM, Sobin LH (2005) Duodenal carcinoids: imaging features with clinical-pathologic comparison. Radiology 237:967–972
Horton KM, Fishman EK (2004) Multidetector-row computed tomography and 3-dimensional computed tomography imaging of small bowel neoplasms: current concept in diagnosis. J Comput Assist Tomogr 28:106–116
Cheng JM, Tirumani SH, Shinagare AB, et al. (2014) MDCT of primary, locally recurrent, and metastatic duodenal gastrointestinal stromal tumours (GISTs): a single institution study of 25 patients with review of literature. Clin Radiol 69:137–144
Kim SY, Ha HK, Park SW, et al. (2009) Gastrointestinal metastasis from primary lung cancer: CT findings and clinicopathologic features. AJR Am J Roentgenol 193:W197–W201
Adam R, Chiche L, Aloia T, et al. (2006) Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1452 patients and development of a prognostic model. Ann Surg 244:524–535
Bakaeen FG, Murr MM, Sarr MG, et al. (2000) What prognostic factors are important in duodenal adenocarcinoma? Arch Surg 135:635–641 (; discussion 641–632)
Barnes G Jr, Romero L, Hess KR, Curley SA (1994) Primary adenocarcinoma of the duodenum: management and survival in 67 patients. Ann Surg Oncol 1:73–78
Brucher BL, Stein HJ, Roder JD, et al. (2001) New aspects of prognostic factors in adenocarcinomas of the small bowel. Hepatogastroenterology 48:727–732
Ercolani G, Grazi GL, Ravaioli M, et al. (2005) The role of liver resections for noncolorectal, nonneuroendocrine metastases: experience with 142 observed cases. Ann Surg Oncol 12:459–466
Gibson MK, Holcroft CA, Kvols LK, Haller D (2005) Phase II study of 5-fluorouracil, doxorubicin, and mitomycin C for metastatic small bowel adenocarcinoma. Oncologist 10:132–137
Gleisner AL, Assumpcao L, Cameron JL, et al. (2007) Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer 110:2484–2492
Hemming AW, Sielaff TD, Gallinger S, et al. (2000) Hepatic resection of noncolorectal nonneuroendocrine metastases. Liver Transpl 6:97–101
Howe JR, Karnell LH, Menck HR, Scott-Conner C (1999) The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985–1995. Cancer 86:2693–2706
Joesting DR, Beart RW Jr, van Heerden JA, Weiland LH (1981) Improving survival in adenocarcinoma of the duodenum. Am J Surg 141:228–231
Kaklamanos IG, Bathe OF, Franceschi D, et al. (2000) Extent of resection in the management of duodenal adenocarcinoma. Am J Surg 179:37–41
Kelsey CR, Nelson JW, Willett CG, et al. (2007) Duodenal adenocarcinoma: patterns of failure after resection and the role of chemoradiotherapy. Int J Radiat Oncol Biol Phys 69:1436–1441
Lepage C, Bouvier AM, Manfredi S, et al. (2006) Incidence and management of primary malignant small bowel cancers: a well-defined French population study. Am J Gastroenterol 101:2826–2832
Lowell JA, Rossi RL, Munson JL, Braasch JW (1992) Primary adenocarcinoma of third and fourth portions of duodenum. Favorable prognosis after resection. Arch Surg 127:557–560
Overman MJ, Varadhachary GR, Kopetz S, et al. (2009) Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol 27:2598–2603
Piesman M, Kozarek RA, Brandabur JJ, et al. (2009) Improved oral intake after palliative duodenal stenting for malignant obstruction: a prospective multicenter clinical trial. Am J Gastroenterol 104:2404–2411
Poultsides GA, Huang LC, Cameron JL, et al. (2012) Duodenal adenocarcinoma: clinicopathologic analysis and implications for treatment. Ann Surg Oncol 19:1928–1935
Sohn TA, Lillemoe KD, Cameron JL, et al. (1998) Adenocarcinoma of the duodenum: factors influencing long-term survival. J Gastrointest Surg 2:79–87
Struck A, Howard T, Chiorean EG, et al. (2009) Non-ampullary duodenal adenocarcinoma: factors important for relapse and survival. J Surg Oncol 100:144–148
Abrahams NA, Halverson A, Fazio VW, et al. (2002) Adenocarcinoma of the small bowel: a study of 37 cases with emphasis on histologic prognostic factors. Dis Colon Rectum 45:1496–1502
van Ooijen B, Kalsbeek HL (1988) Carcinoma of the duodenum. Surg Gynecol Obstet 166:343–347
Gouma DJ, van Geenen RC, van Gulik TM, et al. (2000) Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg 232:786–795
Gervais DA, Fernandez-del Castillo C, O’Neill MJ, et al. (2001) Complications after pancreatoduodenectomy: imaging and imaging-guided interventional procedures. Radiographics 21:673–690
Raman SP, Horton KM, Cameron JL, Fishman EK (2013) CT after pancreaticoduodenectomy: spectrum of normal findings and complications. AJR Am J Roentgenol 201:2–13
Ho CK, Kleeff J, Friess H, Buchler MW (2005) Complications of pancreatic surgery. HPB (Oxford) 7:99–108
Tsang H, Yau T, Khong PL, Epstein RJ (2008) Bevacizumab-based therapy for advanced small bowel adenocarcinoma. Gut 57:1631–1632
De Dosso S, Molinari F, Martin V, et al. (2010) Molecular characterisation and cetuximab-based treatment in a patient with refractory small bowel adenocarcinoma. Gut 59:1587–1588
Santini D, Fratto ME, Spoto C, et al. (2010) Cetuximab in small bowel adenocarcinoma: a new friend? Br J Cancer 103:1305 (; author reply 1306)
Miyake K, Hayakawa K, Nishino M, et al. (2005) Effects of oral 5-fluorouracil drugs on hepatic fat content in patients with colon cancer. Acad Radiol 12:722–727
Torrisi JM, Schwartz LH, Gollub MJ, et al. (2011) CT findings of chemotherapy-induced toxicity: what radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology 258:41–56
Disclosure
Nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suh, C.H., Tirumani, S.H., Shinagare, A.B. et al. Diagnosis and management of duodenal adenocarcinomas: a comprehensive review for the radiologist. Abdom Imaging 40, 1110–1120 (2015). https://doi.org/10.1007/s00261-014-0309-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-014-0309-4