Abstract
Surgical emergencies associated with duodenal neoplasms pose a substantial challenge. In this chapter, we present an overview of common duodenal neoplasms, followed by a discussion of the presentation and management of three primary surgical emergencies: obstruction, perforation, and bleeding.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
6.1 Introduction
Surgical emergencies associated with duodenal neoplasms pose a substantial challenge. In this chapter, we present an overview of common duodenal neoplasms, followed by a discussion of the presentation and management of three primary surgical emergencies: obstruction, perforation, and bleeding.
6.2 Neoplasms of the Duodenum
6.2.1 Primary Duodenal Malignancies
Small bowel cancers are rare, contributing to an estimated 0.6% of all new cancer cases and 0.3% of all cancer deaths in the United States in 2021 [1]. The distribution of these lesions across the small intestine varies widely by histologic subtype; over 50% of small bowel adenocarcinomas arise in the duodenum, while neuroendocrine tumors (NETs), lymphomas, and gastrointestinal stromal tumors (GISTs) occur less frequently in this location (15–20%, respectively). In contrast, most NETs arise in the ileum [2, 3]. Incidence of these tumors has not changed significantly over time [4], with the exception of a marked increase in the diagnosis of NETs over the last 2–3 decades [3]. Overall, duodenal malignancies comprise about 25% of all small bowel cancers [5]. Duodenal neoplasms may be difficult to diagnose, as they are not screened for routinely and often only present with nonspecific symptoms such as abdominal pain, nausea, vomiting, and/or indolent weight loss [6, 7]. In the emergent setting, histologic subtypes may present in any fashion, but GISTs most often manifest with bleeding, lymphomas most commonly with perforation, and adenocarcinomas most often with obstruction [6].
6.2.1.1 Adenocarcinoma
The duodenum is the site of more than half of all intestinal adenocarcinomas accounting for nearly 60% of all duodenal malignancies [2]. A single-center series by Halfdanarson et al. suggested that duodenal tumors present at an earlier stage than jejunal or ileal tumors, likely owing to earlier onset of symptoms from higher flow obstruction [8]. Risk factors for small bowel adenocarcinoma include inflammatory bowel disease, celiac disease, and familial polyposis syndromes [9]. In the absence of powerful evidence supporting systemic or regional nonsurgical therapies, surgical resection is often a treatment priority. Notwithstanding, many patients present with locally advanced or disseminated disease precluding complete resection, and a broadening experience supports first-line systemic therapy in patients with higher risk or metastatic disease [3].
6.2.1.2 Neuroendocrine Tumors (NETs)
Neuroendocrine tumors (NETs) of the small intestine were traditionally referred to as carcinoids, though the term NET is increasingly favored and encompasses both low-grade, more indolent tumors and higher grade lesions [10, 11]. These tumors account for 15–20% of primary duodenal malignancies [2, 3]. Approximately one-third of NETs are functional, the majority of which are gastrinomas or somatostatinomas [11, 12]. Risk factors for NETs include smoking, alcohol use [13], and multiple endocrine neoplasia type 1 (MEN-1) [14]. Endoscopic resection may be adequate for small nonfunctional NETs, but larger tumors and gastrinomas often require operative management, frequently including regional lymph node removal. A more permissive approach to localized NETs of the duodenum may be appropriate in patients with MEN-1 who often have multifocal disease [12]. A landmark study on the Zollinger-Ellison syndrome demonstrated that, even among the small proportion of MEN-1 patients free of disease immediately after operation, almost all recurred at 5 years, suggesting limited impact of surgery in this population other than for palliation [15].
6.2.1.3 Lymphomas
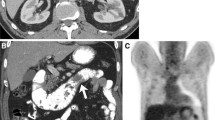
Small bowel lymphomas are rare (0.2–0.5 per 100,000 in the United States) and primarily present in the jejunum and ileum [16] (Fig. 6.1). Lymphomas comprise approximately 10% of all duodenal malignancies [2, 3]. The histologic subtypes of duodenal lymphomas vary significantly and are beyond the scope of this chapter. The mainstay of first-line treatment for all small bowel lymphomas is chemotherapy, with the notable addition of H. pylori treatment for mucosa-associated lymphoid tissue tumors (MALTs) [17, 18]. There is a role, in selected cases, for surgical palliation of symptoms or to improve candidacy for systemic therapy [6].
6.2.1.4 Gastrointestinal Stromal Tumors (GISTs)
GISTs are the most common GI sarcoma and are more commonly diagnosed through increased recognition over the past two decades [19]. Approximately 28% of all GISTs are located in the small intestine; a quarter of these arise in the duodenum. Six percent of duodenal malignancies are GISTs [2, 19]. Surgical resection is the treatment of choice for localized disease; negative margin resection is the goal. Lymphadenectomy is unnecessary as GISTs rarely metastasize to lymph nodes, and this may allow for more conservative surgical approaches. The tyrosine kinase inhibitor imatinib is active against the majority of GISTs and may be indicated in the adjuvant and/or neoadjuvant setting [20].
6.2.2 Benign Duodenal Neoplasms
Benign neoplasms including lipomas, adenomas, leiomyomas, and other entities are relatively uncommon in the duodenum. They are often incidental findings or present with nonspecific symptoms of abdominal pain, nausea, and/or vomiting. Adenomas are the most common of benign lesions. While periampullary location may complicate treatment approaches, many of these tumors can be managed with endoscopic or limited operative resection [21, 22].
6.2.3 Extension of a Pancreatic Malignancy
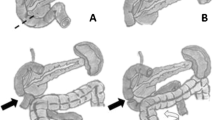
Pancreatic malignancies may infiltrate or compress the duodenum. Pancreatic cancer accounts for 3% of all new cancer cases and 8% of cancer deaths [1]. Up to 80% of patients with pancreatic cancer present with metastatic or locally advanced disease; 10–25% of patients develop symptoms of duodenal or gastric outlet obstruction at some point in their course [23]. The treatment for duodenal obstruction traditionally included operative gastrojejunostomy [24], but advances in endoscopic approaches have afforded alternatives including plastic or self-expanding metal stents [25] (Fig. 6.2). Decompressive gastrostomy tubes placed in surgery, by endoscopy, or by interventional radiology may also provide palliation in patients with particularly poor prognoses [23].
6.2.4 Metastatic Disease to the Duodenum
Metastatic disease to the small bowel is relatively rare. Melanoma is the most common malignancy to metastasize to the gastrointestinal tract; the stomach or duodenum is involved in 5–50% of these cases [26, 27]. Other potential primary cancers to metastasize to the duodenum include colon, lobular breast, pancreatic, lung, and renal cell carcinomas [7]. Similarly to primary duodenal tumors, metastases may present with obstruction or bleeding, though the latter is uncommon [28, 29].
6.3 Surgical Emergencies
6.3.1 Intestinal Obstruction
6.3.1.1 Presentation
Patients presenting with an obstructing mass in the duodenum may manifest a combination of abdominal pain, bloating, and vomiting [30]. The likelihood of an obstructive presentation is dependent on the type and location of malignancy; for example, about 25% of duodenal adenocarcinomas present with obstruction, but this is less common if the tumor is located near the ampulla [31]. Vomiting is a hallmark of obstructive presentations, occurring in up to 80% of patients [32], and may be large volume and projectile in nature [33]. A shorter duration and rapid progression of abdominal pain may indicate a benign etiology rather than malignancy [32], and pain associated with peptic stricture may be more colicky in nature [33]. Weight loss is commonly endorsed by patients with gastroduodenal malignancy that has been present long enough to cause obstruction [32].
6.3.1.2 Physical Exam and Laboratory Findings
On examination, patients with a duodenal obstruction may display vague epigastric tenderness. A “succussion splash,” or a splashing sound audible through a stethoscope when the abdomen is rocked or tapped, may be present, indicating gastroduodenal accumulation of contents. Mild diffuse abdominal distention may be present, though this is unlikely to be diffuse as the distal bowel will be decompressed. Patients may appear dehydrated or malnourished. If patients have been vomiting, laboratory examination may reflect hypokalemia and/or a hypochloremic metabolic alkalosis [34, 35].
6.3.1.3 Imaging
A variety of imaging techniques may demonstrate the gastric outlet obstruction resulting from a duodenal mass. Plain-film X-ray may reveal a “double-bubble” sign indicating a distended stomach adjacent to a distended duodenum [36]. Similarly, a fluoroscopic upper GI series may demonstrate partial or complete obstruction at some segment of the duodenum. However, most commonly, a computed tomography (CT) scan is readily available and used to make the diagnosis of an obstructive duodenal mass. Computed tomography offers several imaging characteristics that may help differentiate the various types of duodenal masses. GISTs are often relatively large, lobular, well-circumscribed, vascular masses [37], while lipomas have the appearance (density) of fat and often appear intraluminal on imaging due to their size despite their submucosal location [38]. Adenocarcinomas may have an “apple-core” appearance with associated narrowing or thickening of the duodenal wall, with or without ulceration or invasion into adjacent structures. If there is question as to the extent of local invasion of adjacent structures or encasement of vessels, or if the lesion is periampullary, an MRI can be helpful [39]. NETs tend to occur in the proximal portion of the duodenum (first or second segments) and appear as focal intraluminal masses [40]. In the setting of clinical intestinal obstruction, it may be useful to perform a CT of the abdomen with oral contrast to radiographically evaluate for complete or partial obstruction. Oral contrast should be preferentially administered via a nasogastric tube and subsequently followed with rapid evacuation to avoid high-volume emesis and aspiration.
6.3.1.4 Management
As in any case of gastrointestinal obstruction, a nasogastric tube for gastric decompression is warranted. Electrolyte abnormalities (particularly Mg2+, Ca2+, PO4−, and K+) as well as volume depletion should be aggressively corrected. Surgical management should focus on both decompression and restoration of gastrointestinal continuity, with or without resection of the primary lesion. If the patient’s condition allows for pathologic diagnosis and oncologic staging, resection may be indicated, and if the patient is safely able to tolerate a definitive operation, an oncological operation should be performed. Otherwise, palliative surgical management with gastrojejunal bypass is often the chosen approach. If the latter is performed, it is important that the patient be maintained on acid-suppressive therapy postoperatively [30]. If bypass is not feasible, gastrostomy tube placement for drainage with or without a jejunostomy tube for feeding may be helpful. Alternatively, endoscopic stenting of the duodenal obstruction can be considered [41] (Fig. 6.3). This approach is best suited for patients with extremely poor prognosis and life expectancy (<6 months), including those with widely disseminated metastatic disease upon presentation. Duodenal stenting is not without complication risk, as stents may migrate or cause perforation or bleeding, or may also obstruct [23, 25, 42]. Depending on the patient and expected survival, these risks may be mitigated through the use of diverse types of stents (i.e., covered vs uncovered) [43].
A subset of patients presenting with duodenal obstruction deserve special consideration: those with some concurrent degree of biliary obstruction. These patients may additionally and/or concurrently require a biliary bypass (thus a “double bypass”) with a Roux limb anastomosed to both the bile duct and the stomach [24]. Outcomes for gastrojejunostomy with or without biliary bypass are reasonable given the often debilitated and malnourished nature of this patient population; however, this procedure has definite inherent risks. An analysis of the American College of Surgeons National Surgical Quality Improvement Project (ACS NSQIP) data from 2005 to 2011 identified a 20% 30-day morbidity rate when this operation was undertaken for patients with unresectable pancreatic cancer. This was found to be higher than in patients who underwent laparotomy alone, though no difference in mortality was detected, reflecting the grave prognosis for most patients with unresectable periampullary cancer [44]. Unsurprisingly, emergent operation was associated with increased morbidity [45].
6.3.2 Duodenal Perforation
6.3.2.1 Presentation
Perforation of a duodenal malignancy may occur after an extended period of obstruction, from an aggressive necrotic tumor and/or in the context of neoadjuvant, adjuvant, or palliative therapy (i.e., radiation or chemotherapy). Patients that develop a perforation present with sudden onset of severe epigastric pain and/or diffusely throughout the abdomen, particularly if it involves the intraperitoneal portion of the duodenum. Conversely, a retroperitoneal or contained duodenal perforation may present with more indolent and subtle symptoms including malaise, nausea/vomiting, and fever. Patients with intraperitoneal perforations presenting soon after onset may have more localized pain; if later, pain may be more diffuse. The pain may radiate to the right shoulder secondary to irritation of the right diaphragm from accumulating of subdiaphragmatic succus or gastric contents [46]. In some cases, perforations may remain contained or “self-sealed,” in which case the pain may actually diminish with time and be nearly resolved upon presentation. Patients may also report a history of weight loss or food intolerance leading up to the acute presentation [47]. In the case of an actively treated duodenal malignancy, perforation in this setting may result from tissue necrosis occurring secondary to treatment (i.e., following chemotherapy for lymphoma) [48].
6.3.2.2 Physical Exam and Laboratory Findings
Patients can exhibit abdominal tenderness, with or without peritonitis (including guarding and rebound tenderness). Depending on the duration of symptoms, this may be accompanied with signs of sepsis and shock, including fever, tachycardia, hypotension, and hyperlactatemia [47]. It is worth noting, however, that these are the signs and symptoms of any free intraperitoneal perforation, including that of the stomach and colon. Given that the duodenum is, in part, a retroperitoneal structure, some perforations may be contained and not cause peritonitis [49].
Laboratory workup should include a complete blood count, looking in particular for a leukocytosis, and a lactic acid elevation, particularly for patients who are clinically in shock. In those with an unidentified etiology for hollow viscus perforation, studies for other potential causes (i.e., H. pylori, gastrin levels) may be helpful, though these are less useful in the setting of known malignancy [47]. If malignancy is suspected based on history or imaging at the time of presentation, tumor markers such as CEA and CA 19-9 can be obtained to guide future surveillance [31].
6.3.2.3 Imaging
Upright or lateral decubitus abdominal radiographs may demonstrate pneumoperitoneum, though the sensitivity of this finding is less than 80% [46]. While in some cases such findings in themselves may be sufficient to proceed directly to laparotomy, in the absence of extreme hemodynamic instability and when at a center with rapid access to cross-sectional imaging, it is reasonable to obtain a CT scan to help rule out other sources of hollow viscus perforation and to help plan the operative intervention [50]. In the setting of perforation, a discrete tumor may not always be identifiable on CT imaging, but if a tumor is visible, adenocarcinoma will most often appear as a focal area of wall thickening. GISTs, on the other hand, will appear as exophytic masses with heterogeneous enhancement with or without ulceration, while lymphomas will appear with homogenous enhancement and may have clear lymph node involvement [50]. Even small bubbles of gas surrounding any mass suggests perforation, as does extravasation of an oral contrast agent [47]. Other findings suspicious for perforation include mesenteric fat stranding locally, bowel wall thickening, or bowel wall discontinuity [51]. Live fluoroscopic examination may be useful, but more time consuming than CT imaging, which has a sensitivity of 96% or greater for the diagnosis of hollow viscus perforation [52]. Albeit less sensitive, abdominal sonography may be useful in detecting free fluid [53].
6.3.2.4 Management
Broad-spectrum antibiotics should be administered early as mortality in septic shock rises steadily for every hour delay in antibiotic administration [54]. The patient should be resuscitated promptly while awaiting definitive management. This should continue intraoperatively and not delay surgical intervention which, if possible, should involve resection of the tumor. However, the indications to resect in the setting of perforation may be limited, particularly with a mass of unknown pathology or in the setting of extraduodenal extension or distant metastasis. Even when technically feasible, malnutrition, hemodynamic instability, and organ dysfunction (e.g., worsening acute kidney injury) represent relative contraindications to a more extensive resection [55, 56].
Intraluminal content spillage and contamination must be controlled early, even though definitive management may be delayed for a subsequent intervention (“damage control”) [57]. Definitive management of duodenal perforations can be achieved by primary closure and/or omental flap or patch (Cellan-Jones or Graham patch) [58, 59]. This is traditionally done via laparotomy but is increasingly being done laparoscopically in those familiar with the technique and in stable patients [60]. When the tumor itself perforates, these approaches often fail as the tissue is tenuous and will not hold stitches. In this case, alternative surgical management is required, and exclusion and bypass may be necessary. Pyloric exclusion involves closing the pylorus (either internally through a gastrotomy or by stapling externally across) and restoring bowel continuity with a gastrojejunal bypass [58, 61]. There is little data supporting this technique in the setting of perforated malignancy, and the benefit of pyloric exclusion in traumatic injury has also been called into question [62]. Notwithstanding, the significant challenges associated with a perforated duodenal tumor sometimes necessitate creative solutions including closure, reinforcement with vascularized tissue, exclusion, bypass, or duodenal drainage [63]. The latter can sometimes be accomplished with placement of a distal jejunostomy tube directed retrograde accompanied by extraluminal drains around the perforated bowel segment. Additionally, in the setting of failed attempt at closure or patch of a duodenal leak, percutaneous transhepatic biliary drainage may be helpful to divert bile.
6.3.3 Duodenal Bleeding
6.3.3.1 Presentation
Patients presenting with bleeding duodenal lesions may manifest similar signs and symptoms as those with any upper gastrointestinal bleed including those of simple peptic ulcers. They may present with a primary complaint of hematemesis and/or melena or experience symptoms of hypovolemia, such as lightheadedness. Most often, bleeding is slow, and occult and microcytic anemia is the only indication [64]. Melena is a somewhat sensitive sign, as it may reflect as little as 100 mL of luminal bleeding. Hematochezia may also be present, particularly if the bleed is brisk [53]. Importantly, patients may suffer an intraperitoneal or retroperitoneal duodenal bleed and never display findings of intraluminal blood [48]. Bleeding is a more common presenting symptom in patients with GISTs, as compared with other tumors [6].
6.3.3.2 Physical Exam and Laboratory Findings
On examination, patients will often have painless bleeding with hematemesis, melena, or hematochezia per rectum as described above. If the hemorrhage is brisk, the patient will also demonstrate signs of hemorrhagic shock with signs of volume depletion, such as pallor and cool, clammy extremities [65]. Vital signs may reveal tachycardia with or without hypotension, depending on the class of shock [66, 67]. It is important to realize that hypotension may not manifest until 30% of the patient’s blood volume has been lost, otherwise termed class III or IV hemorrhagic shock [67]. Urine output may be decreased [68]. Laboratory examination is likely to show a low hemoglobin, though it may be normal initially. Other laboratory evidence of ongoing bleeding may include acute kidney injury with increased creatinine and electrolyte derangements [65]. In the setting of an acute bleed, anemia will more likely be normocytic, while in the setting of chronic low-grade bleeding, the anemia will be microcytic as with iron deficiency [68]. Additional laboratory abnormalities may include elevations in lactate, secondary to tissue hypoperfusion [67].
6.3.3.3 Imaging
As in the case of any upper gastrointestinal bleed, diagnosis and management mostly occur in parallel. Often, the preferred initial maneuver (after resuscitation) is upper endoscopy, as this can be both diagnostic and therapeutic [53]. Alternatively when endoscopy is not available or bleeding is too profuse to allow proper endoscopic visualization, CT angiography (CTA) is a rapid and often very accessible option. Though not the traditional first-line investigative option, CTA sensitivity and positive predictive value have improved, and this may be a reasonable place to start in the absence of other options [53]. In this context, oral contrast (i.e., Gastrografin) should be avoided in favor of intravenous contrast alone [69]. The sensitivity of CTA in gastrointestinal bleeds is about 50%, with a slightly greater sensitivity for acute as opposed to chronic bleeds [70]. Data on tumor hemorrhage in particular is sparse, but for all GI bleeds, a minimum hemorrhage rate of 0.3–0.5 cc/min is required for CTA detection [71]. Other modalities for detection of upper GI bleeding include visceral angiography, which also detects bleeding at the same rates [72], and nuclear scintigraphy, which is significantly more sensitive (minimum bleeding rate detection at 0.02–0.05 cc/min) but not offering much utility in the setting of a bleeding duodenal mass that is likely visible on endoscopy [73].
6.3.3.4 Management
As with any GI bleed, the first priority is prompt evaluation of hemodynamic status, remembering that the airway may be in jeopardy in the patient with active hematemesis and may need to be secured prior to further management. Particularly in the setting of acute hemorrhage and significant volume loss, ensuring adequate intravenous access is essential to allow for resuscitation [53, 64]. Importantly, there is some evidence that a restrictive transfusion strategy (transfusion trigger 7 g/dL) is associated with better outcomes than a liberal transfusion strategy (9 g/dL), even in upper GI bleeding patients [74]. Another randomized study demonstrated similar outcomes between transfusion thresholds of 8 g/dL and 10 g/dL, suggesting that at a minimum, a restrictive strategy may be safe [75]. For patients in acute hemorrhagic shock, permissive hypotension may result in less blood products transfused and may confer a survival benefit [76]. Coagulopathy should be corrected promptly. There is controversy regarding the use of tranexamic acid (TXA) in the setting of upper GI bleeding. Though there have been meta-analyses suggesting some benefit for GI bleeding in general (upper and lower, primarily upper in the included studies) [77, 78], the HALT-IT trial, an international, randomized, placebo-controlled trial in upper GI bleeds, found no benefit [79].
After stabilization, the primary goal should be nonoperative management of acute bleeding, in an attempt to temporize and ultimately plan an elective definitive operation (if indicated) [61]. As noted above, the first step in this process should be an upper endoscopy, not only to identify the site of bleeding but also to attempt to achieve hemostasis through the use of endoscopic clipping, submucosal epinephrine injection, cautery, or application of topical hemostatic agents [61, 80].
Endoscopic management of recurrent duodenal tumor hemorrhage can be entertained, but no data exists to support or recommend it. One might, however, extrapolate from bleeding ulcer data, which suggests that repeated attempts at endoscopic management may be beneficial [81]. When the bleeding surface has high-risk features (i.e., exposed vessel) or when there is a diffuse area of devitalized necrotic tissue, trans-arterial embolization may be the more ideal method for definitive bleeding control [61, 82, 83]. It is worth noting that, although rebleeding rates are high, in the short term, bleeding often either stops with endoscopic intervention or is self-limited [84]. This gives providers time to develop more appropriate long-term strategies, which may include up-front surgical resection in oncologic fashion or neoadjuvant treatment, which may in itself help ameliorate bleeding [85]. In the case of unresectable tumors, nonoperative management strategies may help with both tumor shrinkage and palliation of bleeding. These may include imatinib for GISTs [86] or radiation for other malignancies [87].
6.4 Special Considerations
6.4.1 Metastatic Disease
In certain cases, the surgical emergency may be the index presentation of the patient’s malignancy. In some, gross metastatic disease may be readily apparent, either on preoperative imaging or intraoperatively. Surgical management of the acute issue should not deviate from the approaches described above in the face of metastatic disease. Bleeding must be controlled, perforation must be managed, and obstruction must be relieved. However, the presence of metastatic disease warrants an up-front goal-of-care discussion and might favor less invasive modalities for definitive management. For example, an obstruction that might have been manageable with a distal gastrectomy might be better managed with a gastrojejunostomy or a duodenal stent [25, 42, 43]. As discussed above, bleeding may better be managed directly with angioembolization [82, 83]. Perforation, in many cases, will mandate operation regardless of cancer stage; however, every attempt should be made to limit intervention in cases when operation is not expected to prolong life [61].
6.5 Anatomic Considerations
6.5.1 Involvement of the Ampulla
Surgical emergencies of the duodenum may be complicated by involvement of the ampulla of Vater. In cases of tumor bleeding or perforation involving the ampulla or periampullary duodenum, the approach should be the same as for metastatic disease. Less invasive or complex options are preferred, as outcomes from emergent pancreaticoduodenectomy are poor, with perioperative mortality that varies by indication but may be as high as 20% and a complication rate of 90% [88, 89]. In the case of bleeding, endoscopic or interventional radiology management should be used, and in the case of perforation, exclusion and bypass should be favored over oncologic resection [61].
Decision-making may be slightly more complex in the case of an obstructing ampullary tumor, mandating some attention to the bile duct. Indeed, as described earlier, biliary obstruction is a common presentation of duodenal and pancreatic head malignancies, with 70% of pancreatic cancers presenting with jaundice [90, 91]. As noted above, both duodenal stents [92] and biliary stents [91] are well-accepted options if the tumor is unresectable (Fig. 6.4).
6.5.2 Enteric Access
If in the operating room for one of the above surgical emergencies, one should consider placing enteral access (i.e., jejunostomy tube) prior to closing the laparotomy. This is particularly true for the patient undergoing operation for duodenal obstruction as there is a significant incidence of delayed gastric emptying after palliative gastrojejunostomy [93]. This evidence has been used by some to advocate the use of stenting over gastrojejunostomy [94, 95], but in cases where the decision has already been made to perform an operation, a jejunostomy tube may make sense. On the other hand, more recent literature suggests important morbidity from prophylactic jejunostomy tube placement [96], both following pancreaticoduodenectomy [97, 98] and after resection for gastric cancer [99]. It is unclear, however, whether these data make a legitimate argument against jejunostomy tube placement once already in surgery, as there may be confounding by indication in that surgeons may opt to place a tube in sicker and more frail patients. The issue remains controversial, but there is likely a population of patients for whom a feeding jejunostomy should be considered.
6.5.3 Goals of Care
Patients presenting with duodenal surgical emergencies are at high perioperative risk and are often found to have advanced disease. In general, emergency surgery carries a significantly greater mortality (12.5% vs. 2.7%) and morbidity (32.8% vs. 12.7%) risk than elective general surgery [100]. Data on oncologic surgical emergencies is limited, but these risks are likely even higher in patients with malignancies [101]. Beyond the perioperative phase, one must also consider the patient’s long- and short-term prognosis prior to undertaking surgical intervention. Adequate communication regarding goals of care with the patient and his/her loved ones prior to major surgery has long been problematic [102], particularly in emergency surgery [103], but is of utmost importance [104]. A frank preoperative discussion should occur between the surgeon, patient, oncologist when possible, and family where the risks, prognosis, and goals of care are explicitly stated and all questions answered.
6.6 Conclusion
A variety of duodenal malignancies may present with obstruction, perforation, or bleeding, requiring prompt resuscitation and consideration of operative or nonoperative interventions. While general principles are largely similar to those applicable in non-oncologic emergency surgery, the extent of disease, prognosis, preexisting conditions and nutritional status, long-term treatment plan, and the patient’s goals of care may complicate decision-making. Careful consideration will be needed to proceed to optimal surgical care individualized to the patient, the tumor, and the complication.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Raghav K, Overman MJ. Small bowel adenocarcinomas—existing evidence and evolving paradigms. Nat Rev Clin Oncol. 2013;10(9):534–44.
Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249(1):63–71.
Kerremans RP, Lerut J, Penninckx FM. Primary malignant duodenal tumors. Ann Surg. 1979;190(2):179.
Hatzaras I, Palesty JA, Abir F, et al. Small-bowel tumors: epidemiologic and clinical characteristics of 1260 cases from the Connecticut tumor registry. Arch Surg. 2007;142(3):229–35.
Catena F, Ansaloni L, Gazzotti F, et al. Small bowel tumours in emergency surgery: specificity of clinical presentation. ANZ J Surg. 2005;75(11):997–9.
Minardi AJ Jr, Zibari GB, Aultman DF, McMillan RW, McDonald JC. Small-bowel tumors. J Am Coll Surg. 1998;186(6):664–8.
Halfdanarson TR, McWilliams RR, Donohue JH, Quevedo JF. A single-institution experience with 491 cases of small bowel adenocarcinoma. Am J Surg. 2010;199(6):797–803.
Aparicio T, Zaanan A, Svrcek M, et al. Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis. 2014;46(2):97–104.
Mullen JT, Wang H, Yao JC, et al. Carcinoid tumors of the duodenum. Surgery. 2005;138(6):971–8.
Massironi S, Campana D, Partelli S, et al. Heterogeneity of duodenal neuroendocrine tumors: an Italian multi-center experience. Ann Surg Oncol. 2018;25(11):3200–6.
Sato Y, Hashimoto S, Mizuno K-I, Takeuchi M, Terai S. Management of gastric and duodenal neuroendocrine tumors. World J Gastroenterol. 2016;22(30):6817–28.
Chen CC, Neugut AI, Rotterdam H. Risk factors for adenocarcinomas and malignant carcinoids of the small intestine: preliminary findings. Cancer Epidemiol Biomark Prevent. 1994;3(3):205–7.
Hoffmann KM, Furukawa M, Jensen RT. Duodenal neuroendocrine tumors: classification, functional syndromes, diagnosis and medical treatment. Best Pract Res Clin Gastroenterol. 2005;19(5):675–97.
Norton JA, Fraker DL, Alexander HR, et al. Surgery to cure the Zollinger–Ellison syndrome. N Engl J Med. 1999;341(9):635–44.
Pan SY, Morrison H. Epidemiology of cancer of the small intestine. World J Gastrointest Oncol. 2011;3(3):33.
Na HK, Won SH, Ahn JY, et al. Clinical course of duodenal mucosa-associated lymphoid tissue lymphoma: comparison with gastric mucosa-associated lymphoid tissue lymphoma. J Gastroenterol Hepatol. 2021;36(2):406–12.
Lu PW, Fields AC, Yoo J, et al. Surgical management of small bowel lymphoma. J Gastrointest Surg. 2021;25(3):757–65.
Lee N, Tang D, Jayaraman S. Penetrating cardiac trauma and the use of emergent extracorporeal membrane oxygenation and therapeutic hypothermia: when cooler heads prevail. Trauma Case Rep. 2015;1(9–12):95–8.
Colombo C, Ronellenfitsch U, Yuxin Z, et al. Clinical, pathological and surgical characteristics of duodenal gastrointestinal stromal tumor and their influence on survival: a multi-center study. Ann Surg Oncol. 2012;19(11):3361–7.
Perez A, Saltzman JR, Carr-Locke DL, et al. Benign nonampullary duodenal neoplasms. J Gastrointest Surg. 2003;7(4):536–41.
Kemp CD, Russell RT, Sharp KW. Resection of benign duodenal neoplasms. Am Surg. 2007;73(11):1086–91.
Perone JA, Riall TS, Olino K. Palliative care for pancreatic and periampullary cancer. Surg Clin N Am. 2016;96(6):1415.
Singh SM, Longmire WP Jr, Reber HA. Surgical palliation for pancreatic cancer. The UCLA experience. Ann Surg. 1990;212(2):132–9.
Jeurnink SM, Steyerberg EW, van Hooft JE, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71(3):490–9.
Liang KV, Sanderson SO, Nowakowski GS, Arora AS. Metastatic malignant melanoma of the gastrointestinal tract. Mayo Clin Proc. 2006;81(4):511–6.
Benedeto-Stojanov D, Nagorni A, Živković V, Milanović J, Stojanov D. Metastatic melanoma of the stomach and the duodenum. Arch Oncol. 2006;14(1–2):60–1.
Adamo R, Greaney PJ, Witkiewicz A, Kennedy EP, Yeo CJ. Renal cell carcinoma metastatic to the duodenum: treatment by classic pancreaticoduodenectomy and review of the literature. J Gastrointest Surg. 2008;12(8):1465–8.
Nazareno J, Taves D, Preiksaitis H-G. Metastatic breast cancer to the gastrointestinal tract: a case series and review of the literature. World J Gastroenterol. 2006;12(38):6219–24.
Dempsey DT. Pyloroplasty and gastrojejunostomy. In: Fischer JE, editor. Fischer’s mastery of surgery. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2012.
Grandhi MS, Schulick RD. Duodenal cancer (including intestinal type ampullary cancer). In: Morita SY, Balch CM, Klimberg VS, Pawlik TM, Posner MC, Tanabe KK, editors. Textbook of complex general surgical oncology. New York: McGraw-Hill Education; 2018.
Khullar SK, DiSario JA. Gastric outlet obstruction. Gastrointest Endosc Clin N Am. 1996;6(3):585–603.
Soybel DI, Landman WB. Ileus and bowel obstruction. In: Mulholland MW, Lillemoe KD, editors. Greenfield’s surgery: scientific principles and practice. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2011.
Gan S-I. Gastric outlet obstruction in adults. Waltham: UpToDate; 2020.
McQuaid KR. Complications of peptic ulcer disease. In: Papadakis MA, McPhee SJ, Rabow MW, editors. Current medical diagnosis treatment 2021. New York: McGraw-Hill Education; 2021.
Shen Y-C, Lin Y-S. Double-bubble sign in an adult patient. Gastroenterology. 2017;153(5):1191–2.
Cai P-Q, Lv X-F, Tian L, et al. CT characterization of duodenal gastrointestinal stromal tumors. Am J Roentgenol. 2015;204(5):988–93.
Thompson WM. Imaging and findings of lipomas of the gastrointestinal tract. Am J Roentgenol. 2005;184(4):1163–71.
Suh CH, Tirumani SH, Shinagare AB, et al. Diagnosis and management of duodenal adenocarcinomas: a comprehensive review for the radiologist. Abdom Imaging. 2015;40(5):1110–20.
Levy AD, Taylor LD, Abbott RM, Sobin LH. Duodenal carcinoids: imaging features with clinical-pathologic comparison. Radiology. 2005;237(3):967–72.
Roses RE, Folkert IW, Krouse RS. Malignant bowel obstruction: reappraising the value of surgery. Surg Oncol Clin N Am. 2018;27(4):705–15.
Dormann A, Meisner S, Verin N, Wenk LA. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004;36(06):543–50.
Woo SM, Kim DH, Lee WJ, et al. Comparison of uncovered and covered stents for the treatment of malignant duodenal obstruction caused by pancreaticobiliary cancer. Surg Endosc. 2013;27(6):2031–9.
Roses RE, Tzeng C-WD, Ross MI, Fournier KF, Abbott DE, You YN. The palliative index: predicting outcomes of emergent surgery in patients with cancer. J Palliat Med. 2014;17(1):37–42.
Bartlett EK, Wachtel H, Fraker DL, et al. Surgical palliation for pancreatic malignancy: practice patterns and predictors of morbidity and mortality. J Gastrointest Surg. 2014;18(7):1292–8.
Mulholland MW. Gastroduodenal ulceration. In: Mulholland MW, Lillemoe KD, Doherty GM, Maier RV, Simeone DM, Upchurch Jr GR, editors. Greenfield’s surgery: scientific principles and practice. 5th ed. Philadelphia: Lippincott Wlliams & Wilkins; 2011.
Chikunguwo SM, Maher JW. Perforated duodenal ulcer. In: Fischer JE, editor. Fischer’s mastery of surgery. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2012.
Bosscher MRF, van Leeuwen BL, Hoekstra HJ. Surgical emergencies in oncology. Cancer Treat Rev. 2014;40(8):1028–36.
Wong C-H, Chow PKH, Ong H-S, Chan W-H, Khin L-W, Soo K-C. Posterior perforation of peptic ulcers: presentation and outcome of an uncommon surgical emergency. Surgery. 2004;135(3):321–5.
Gosangi B, Rocha TC, Duran-Mendicuti A. Imaging spectrum of duodenal emergencies. Radiographics. 2020;40(5):1441–57.
Borofsky S, Taffel M, Khati N, Zeman R, Hill M. The emergency room diagnosis of gastrointestinal tract perforation: the role of CT. Emerg Radiol. 2015;22(3):315–27.
Reich H, Chou D, Melo N. Perforated hollow viscus. In: Butler KL, Harisinghani M, editors. Acute care surgery: imaging essentials for rapid diagnosis. New York: McGraw-Hill Education; 2015.
Britt LD, Peitzman A, Barie P, Peitzman A. Acute care surgery. Philadelphia: Wolters Kluwer Health; 2012.
Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77.
Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000;7(9):705–12.
Dorcaratto D, Heneghan H, Fiore B, et al. Segmental duodenal resection: indications, surgical techniques and postoperative outcomes. J Gastrointest Surg. 2015;19(4):736–42.
Rotondo MF, Schwab CW, McGonigal MD, et al. ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35(3):375–82.
Ansari D, Torén W, Lindberg S, Pyrhönen H-S, Andersson R. Diagnosis and management of duodenal perforations: a narrative review. Scand J Gastroenterol. 2019;54(8):939–44.
Ricci JL, Turnbull ADM. Spontaneous gastroduodenal perforation in cancer patients receiving cytotoxic therapy. J Surg Oncol. 1989;41(4):219–21.
Sanabria A, Villegas MI, Morales Uribe CH. Laparoscopic repair for perforated peptic ulcer disease. Cochrane Database Syst Rev. 2013;2:CD004778.
Folkert IW, Roses RE. Value in palliative cancer surgery: a critical assessment. J Surg Oncol. 2016;114(3):311–5.
Seamon MJ, Pieri PG, Fisher CA, et al. A 10-year retrospective review: does pyloric exclusion improve clinical outcome after penetrating duodenal and combined pancreaticoduodenal injuries? J Trauma Acute Care Surg. 2007;62(4):829–33.
Berne TV, Donovan AJ. Nonoperative treatment of perforated duodenal ulcer. Arch Surg. 1989;124(7):830–2.
Schirmer BD. Bleeding duodenal ulcer. In: Fischer JE, editor. Fischer’s mastery of surgery. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2012.
Feinman M, Haut ER. Upper gastrointestinal bleeding. Surg Clin N Am. 2014;94(1):43–53.
Gutierrez G, Reines H, Wulf-Gutierrez ME. Clinical review: hemorrhagic shock. Crit Care. 2004;8(5):373.
Cannon JW. Hemorrhagic shock. N Engl J Med. 2018;378(4):370–9.
Kamboj AK, Hoversten P, Leggett CL. Upper gastrointestinal bleeding: etiologies and management. Mayo Clin Proc. 2019;94(4):697–703.
Polotsky M, Vadvala HV, Fishman EK, Johnson PT. Duodenal emergencies: utility of multidetector CT with 2D multiplanar reconstructions for challenging but critical diagnoses. Emerg Radiol. 2020;27(2):195–203.
Amarteifio E, Sohns C, Heuser M, Püsken M, Lange B, Obenauer S. Detection of gastrointestinal bleeding by using multislice computed tomography acute and chronic hemorrhages. Clin Imaging. 2008;32(1):1–5.
Wells ML, Hansel SL, Bruining DH, et al. CT for evaluation of acute gastrointestinal bleeding. Radiographics. 2018;38(4):1089–107.
Walker TG, Salazar GM, Waltman AC. Angiographic evaluation and management of acute gastrointestinal hemorrhage. World J Gastroenterol. 2012;18(11):1191–201.
Soto JA, Park SH, Fletcher JG, Fidler JL. Gastrointestinal hemorrhage: evaluation with MDCT. Abdom Imaging. 2015;40(5):993–1009.
Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21.
Jairath V, Kahan BC, Gray A, et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic, open-label, cluster randomised feasibility trial. Lancet. 2015;386(9989):137–44.
Tran A, Yates J, Lau A, Lampron J, Matar M. Permissive hypotension versus conventional resuscitation strategies in adult trauma patients with hemorrhagic shock: a systematic review and meta-analysis of randomized controlled trials. J Trauma Acute Care Surg. 2018;84(5):802–8.
Lee P-L, Yang K-S, Tsai H-W, Hou S-K, Kang Y-N, Chang C-C. Tranexamic acid for gastrointestinal bleeding: a systematic review with meta-analysis of randomized clinical trials. Am J Emerg Med. 2021;45:269–79.
Dionne JC, Oczkowski SJW, Hunt BJ, et al. Tranexamic acid in gastrointestinal bleeding: a systematic review and meta-analysis. Crit Care Med. 2022;50(3):e313–9.
Roberts I, Shakur-Still H, Afolabi A, et al. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled trial. Lancet. 2020;395(10241):1927–36.
Hwang JH, Fisher DA, Ben-Menachem T, et al. The role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest Endosc. 2012;75(6):1132–8.
Lau JYW, Sung JJY, Lam Y-h, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;340(10):751–6.
Koo HJ, Shin JH, Shin S, Yoon H-K, Ko G-Y, Gwon DI. Efficacy and clinical outcomes of transcatheter arterial embolization for gastrointestinal bleeding from gastrointestinal stromal tumor. J Vasc Interv Radiol. 2015;26(9):1297–304.
Loffroy R, Rao P, Ota S, De Lin M, Kwak B-K, Geschwind J-F. Embolization of acute nonvariceal upper gastrointestinal hemorrhage resistant to endoscopic treatment: results and predictors of recurrent bleeding. Cardiovasc Intervent Radiol. 2010;33(6):1088–100.
Ofosu A, Ramai D, Latson W, Adler DG. Endoscopic management of bleeding gastrointestinal tumors. Ann Gastroenterol. 2019;32(4):346–51.
Krishnamurthy G, Singh H, Sharma V, Savlania A, Vasishta RK. Therapeutic challenges in the management of bleeding duodenal gastrointestinal stromal tumor: a case report and review of literature. J Gastrointest Cancer. 2019;50(1):170–4.
Liu Q, Kong F, Zhou J, Dong M, Dong Q. Management of hemorrhage in gastrointestinal stromal tumors: a review. Cancer Manag Res. 2018;10:735–43.
Kondoh C, Shitara K, Nomura M, et al. Efficacy of palliative radiotherapy for gastric bleeding in patients with unresectable advanced gastric cancer: a retrospective cohort study. BMC Palliat Care. 2015;14(1):37.
Tsai C-Y, Lai B-R, Wang S-Y, et al. The impact of preoperative etiology on emergent pancreaticoduodenectomy for non-traumatic patients. World J Emerg Surg. 2017;12(1):21.
Gulla A, Tan WP, Pucci MJ, et al. Emergent pancreaticoduodenectomy: a dual institution experience and review of the literature. J Surg Res. 2014;186(1):1–6.
Lillemoe KD, Pitt HA. Palliation: surgical and otherwise. Cancer. 1996;78(3):605–14.
Kruse EJ. Palliation in pancreatic cancer. Surg Clin N Am. 2010;90(2):355–64.
Oh SY, Edwards A, Mandelson M, et al. Survival and clinical outcome after endoscopic duodenal stent placement for malignant gastric outlet obstruction: comparison of pancreatic cancer and nonpancreatic cancer. Gastrointest Endosc. 2015;82(3):460–8.
Doberneck RC, Berndt GA. Delayed gastric emptying after palliative gastrojejunostomy for carcinoma of the pancreas. Arch Surg. 1987;122(7):827–9.
Jeurnink SM, Steyerberg EW, van’t Hof G, van Eijck CHJ, Kuipers EJ, Siersema PD. Gastrojejunostomy versus stent placement in patients with malignant gastric outlet obstruction: a comparison in 95 patients. J Surg Oncol. 2007;96(5):389–96.
Chandrasegaram MD, Eslick GD, Mansfield CO, et al. Endoscopic stenting versus operative gastrojejunostomy for malignant gastric outlet obstruction. Surg Endosc. 2012;26(2):323–9.
Zapas JL, Karakozis S, Kirkpatrick JR. Prophylactic jejunostomy: a reappraisal. Surgery. 1998;124(4):715–20.
Padussis JC, Zani S, Blazer DG, Tyler DS, Pappas TN, Scarborough JE. Feeding jejunostomy during Whipple is associated with increased morbidity. J Surg Res. 2014;187(2):361–6.
Nussbaum DP, Zani S, Penne K, et al. Feeding jejunostomy tube placement in patients undergoing pancreaticoduodenectomy: an ongoing dilemma. J Gastrointest Surg. 2014;18(10):1752–9.
Patel SH, Kooby DA, Staley CA III, Maithel SK. An assessment of feeding jejunostomy tube placement at the time of resection for gastric adenocarcinoma. J Surg Oncol. 2013;107(7):728–34.
Havens JM, Peetz AB, Do WS, et al. The excess morbidity and mortality of emergency general surgery. J Trauma Acute Care Surg. 2015;78(2):306–11.
Dumont F, Mazouni C, Bitsakou G, et al. A pre-operative nomogram for decision making in oncological surgical emergencies. J Surg Oncol. 2014;109(7):721–5.
Pecanac KE, Kehler JM, Brasel KJ, et al. “It’s big surgery”: preoperative expressions of risk, responsibility and commitment to treatment after high-risk operations. Ann Surg. 2014;259(3):458.
Cooper Z, Courtwright A, Karlage A, Gawande A, Block S. Pitfalls in communication that lead to nonbeneficial emergency surgery in elderly patients with serious illness description of the problem and elements of a solution. Ann Surg. 2014;260(6):949–57.
Hatchimonji JS, Huston-Paterson HH, Dortche K, et al. Do we know our patients’ goals? Evaluating preoperative discussions in emergency surgery. Am J Surg. 2020;220(4):861–2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hatchimonji, J.S., Roses, R.E., Pascual, J.L. (2023). Duodenum. In: Tarasconi, A., Bui, S., Chirica, M., Roth, G., Nahmias, J. (eds) Oncologic Surgical Emergencies. Hot Topics in Acute Care Surgery and Trauma. Springer, Cham. https://doi.org/10.1007/978-3-031-36860-8_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-36860-8_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-36859-2
Online ISBN: 978-3-031-36860-8
eBook Packages: MedicineMedicine (R0)