Abstract
Purpose
A four-parameter risk model that included cardiac iodine-123 metaiodobenzylguanidine (MIBG) imaging and readily available clinical parameters was recently developed for prediction of 2-year cardiac mortality risk in patients with chronic heart failure. We sought to validate the ability of this risk model to predict post-discharge clinical outcomes in patients with acute decompensated heart failure (ADHF) and to compare its prognostic value with that of the Acute Decompensated Heart Failure National Registry (ADHERE) and Get With The Guidelines-Heart Failure (GWTG-HF) risk scores.
Methods
We studied 407 consecutive patients who were admitted for ADHF and survived to discharge, with definitive 2-year outcomes (death or survival). Cardiac MIBG imaging was performed just before discharge. The 2-year cardiac mortality risk was calculated using four parameters, namely age, left ventricular ejection fraction, New York Heart Association functional class, and cardiac MIBG heart-to-mediastinum ratio on delayed images. Patients were stratified into three groups based on the 2-year cardiac mortality risk: low- (< 4%), intermediate- (4–12%), and high-risk (> 12%) groups. The ADHERE and GWTG-HF risk scores were also calculated.
Results
There was a significant difference in the incidence of cardiac death among the three groups stratified using the 2-year cardiac mortality risk model (p < 0.0001). The 2-year cardiac mortality risk model had a higher C-statistic (0.732) for the prediction of cardiac mortality than the ADHERE and GWTG-HF risk scores.
Conclusion
The 2-year MIBG-based cardiac mortality risk model is useful for predicting post-discharge clinical outcomes in patients with ADHF.
Trial registration number
UMIN000015246, 25 September 2014.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute decompensated heart failure (ADHF) is a leading cause of hospitalisation worldwide [1]. Despite advances in diagnosis and management, the clinical outcomes in patients admitted for ADHF remain poor [2]. The risk stratification of patients with heart failure (HF) is of paramount importance for proper management [3]. The use of risk models to estimate the likelihood of adverse events can help clinicians plan disease management and select patients suitable for advanced therapies, leading to improvements in patient outcomes and cost-effectiveness of care [4, 5]. Although numerous risk models exist for patients with HF, there is currently no established risk model that can be applied to patients with ADHF [6]. The Acute Decompensated Heart Failure National Registry (ADHERE) and Get With The Guidelines-Heart Failure (GWTG-HF) risk scores have been validated as ADHF in-hospital mortality risk scores [7, 8], and they have also recently been shown to predict post-discharge outcomes [9]. However, most of the risk scores for patients with ADHF only predict short-term prognosis; there is no validated risk score to predict long-term prognosis in patients with ADHF [6].
Increased cardiac sympathetic nerve activity (CSNA) is associated with poor prognosis in patients with HF [10,11,12,13,14]. Cardiac iodine-123 (123I) metaiodobenzylguanidine (MIBG) imaging is the most widely used method for assessing CSNA. Recently, a four-parameter risk model, including cardiac MIBG imaging and readily available clinical parameters, was developed and validated for the prediction of 2-year cardiac mortality risk in patients with chronic HF (CHF) using a Japanese CHF database that consisted of 1322 patients [15, 16]. However, there is no information available on the usefulness of the 2-year MIBG-based cardiac mortality risk model for the prediction of post-discharge prognosis in patients with ADHF. Therefore, we sought to validate the ability of this model to predict post-discharge clinical outcomes in patients with ADHF, and to compare its prognostic value with that of the ADHERE and GWTG-HF risk scores.
Material and methods
Participants
We analysed patients who were enrolled in our ongoing single-centre, prospective cohort registry, “Osaka Prefectural trial: Acute heart failure syndrome Registry (OPAR)” (clinical registration with the University hospital Medical Information Network: UMIN000015246). The registry included consecutive patients who were admitted for ADHF, which was diagnosed according to the Framingham criteria [17], and who survived to discharge [18]. Only the first admission of each patient during the study period was registered. We excluded patients with acute coronary syndrome, malignancy with a predicted life expectancy < 6 months, severe valvular or coronary artery disease that required surgical treatment during hospitalisation or immediately after discharge, and those who underwent chronic haemodialysis. In addition, we excluded patients who did not undergo cardiac MIBG imaging or echocardiography at discharge, those who had been taking drugs known to interfere with cardiac MIBG uptake, those with missing data for the calculation of the ADHERE or GWTG-HF risk scores, those without definitive 2-year outcomes (death or survival), those who were considered inappropriate for this study by their primary physicians due to difficulties during follow-up and predicted poor adherence, and those who withdrew informed consent. Patients with Parkinson’s disease were also excluded because the disease is known to interfere with cardiac MIBG uptake. Enrolment occurred between October 2011 and January 2017. The study was carried out in accordance with the principles outlined in the Declaration of Helsinki, and the Institutional Ethics Committee approved the study protocol. Written informed consent was obtained from all participants prior to enrolment.
Data collection
We collected data on clinical variables, including age, sex, aetiology of HF, New York Heart Association (NYHA) functional class, body mass index, comorbidities, prior HF admissions, category of HF, systolic and diastolic blood pressures, heart rates, oral medications, and the use of device therapy. All patients underwent cardiac 123I-MIBG imaging, echocardiography, and venous blood sampling. Cardiac MIBG imaging and echocardiography were performed after stabilisation of the HF symptoms (just before discharge), and venous blood was drawn at admission. Echocardiography was performed according to standard techniques using a commercially available machine, as previously reported [19]. The estimated glomerular filtration rate (eGFR) was calculated using the modified isotope dilution mass spectrometry traceable Modification of Diet in Renal Disease Study equation with a Japanese coefficient [20].
Cardiac 123I-MIBG imaging
Myocardial imaging with 123I-MIBG (MyoMIBG-I 123 Injection; FUJIFILM Toyama Chemical, Tokyo, Japan) was performed using a conventional rotating gamma camera (BrightView; Philips, Amsterdam, The Netherlands) equipped with a low-energy type cardiac high-resolution collimator. Patients were placed in the supine position. A 111-MBq dose of 123I-MIBG was injected intravenously at rest following an overnight fast. Initial and delayed image acquisition was performed in the anterior chest view 20 min (early) and 200 min (late) after isotope injection. All images were reviewed by two independent observers who were blinded to the clinical data. As previously reported [21], the heart-to-mediastinum ratio (HMR) on initial (early HMR) and delayed images (late HMR) was determined from the pixel count (counts/pixel) in a visually drawn region of interest over the entire left ventricular (LV) myocardium divided by the pixel count in a 7 × 7 pixel region of interest in the upper mediastinum. The cardiac washout rate of MIBG was calculated from the initial and delayed images with correction for radioactive decay of 123I and background substraction.
Two-year mortality risk model
Similar to previous reports, a multivariate logistic model, including age, LV ejection fraction (LVEF), NYHA functional class (I–II vs. III–IV) at discharge, and MIBG late HMR, was used to calculate the 2-year probability of cardiac death (unit %/2 years) due to pump failure death (PFD), sudden cardiac death (SCD), and/or acute myocardial infarction [15, 16]. Since the HMR used for creating the 2-year risk model was based on low-energy collimators, the model was adjusted to HMR values for low-energy collimators using the standardisation method developed by Nakajima et al. and Verschure et al. [22, 23]. The patients were stratified into three groups based on their 2-year cardiac mortality risk: low-risk (< 4%), intermediate-risk (4–12%), and high-risk groups (> 12%), similar to a previous study [16].
ADHERE and GWTG-HR risk scores
The ADHERE and GWTG-HF risk scores were calculated on admission. We classified patients into five groups based on whether three parameters (blood urea nitrogen, systolic blood pressure, and serum creatinine) were above or below specific cut-off values according to the ADHERE risk tree [7]. In addition, as reported in an earlier study, the GWTG-HF risk score was calculated by summing points assigned to the values of seven predictors, which are as follows: age, systolic blood pressure, heart rate, blood urea nitrogen, sodium, chronic obstructive pulmonary disease, and race [8].
Endpoints
After discharge, all patients were followed up in the HF unit at our centre at least once every 1 or 2 months. The primary endpoint was cardiac death, including PFD, SCD, and death due to acute myocardial infarction. PFD was defined as death resulting from deterioration of congestive HF with progression of congestive symptoms. SCD was defined as witnessed cardiac arrest or death within 1 h of the onset of acute symptoms, or unexpected, unwitnessed death of a patient known to have been well within the previous 24 h [10]. The secondary endpoints were all-cause death, unplanned hospitalisation for worsening HF (WHF), a composite of cardiac death and WHF, and a composite of all-cause mortality and WHF. The data on these events were obtained by physicians directly from their patients at the hospital in an outpatient setting or by dedicated coordinators and investigators via mail or telephone interviews with patients or their families.
Statistical analysis
Data are presented as medians and interquartile ranges of 25–75% for continuous variables and as percentages for categorical variables. The Kruskal–Wallis rank sum test and the chi-squared test were used to compare the differences in continuous and categorical variables, respectively. The prognostic value of the baseline characteristics was assessed using Cox proportional hazards regression analysis. A multivariate Cox model for the endpoints was adjusted for a total of eight characteristics (sex, hypertension, coronary artery disease, diabetes mellitus, atrial fibrillation, haemoglobin at admission, eGFR at admission, and B-type natriuretic peptide [BNP] level at admission), which were thought to be clinically important or were previously demonstrated to have prognostic significance. Age, LVEF, and NYHA functional class were not included in the multivariate Cox model because they were used in the 2-year MIBG risk model. The BNP level was log10 transformed prior to its inclusion in the Cox model. The event-free survival rate was calculated using the Kaplan–Meier method, and differences in survival rates were compared between groups using the log-rank test. The predictive values of the 2-year MIBG risk model and the ADHERE and GWTG-HF risk scores for the endpoints were evaluated using receiver operating characteristic (ROC) curve analysis, and the results were expressed in terms of the area under the curve (AUC) and its 95% confidence interval. Statistical significance was set at p < 0.05. Statistical analysis was performed using a standard statistical program package (MedCalc® Statistical Software version 20.009, MedCalc Software Ltd, Ostend, Belgium).
Results
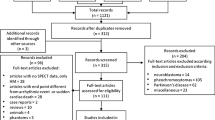
A total of 516 patients were enrolled in the study. After excluding 109 patients who met the exclusion criteria, the final cohort for analysis consisted of 407 patients (Fig. 1). Median days between admission and cardiac MIBG imaging were 15 (interquartile range: 12–20) days.
Baseline characteristics
The baseline characteristics of the 407 patients are summarised in Table 1. The patients were stratified into three groups based on their 2-year cardiac mortality risk: low-risk (n = 142), intermediate-risk (n = 172), and high-risk groups (n = 93). Patients in the high-risk group were older, had a higher NYHA class, had a higher prevalence of coronary artery disease, and were more likely to have a history of prior HF admission than those in the low-risk group. In addition, the proportion of patients with HF with reduced ejection fraction, LV end-diastolic dimension, serum creatinine and blood urea nitrogen levels, and the use of loop diuretics and cardiac resynchronization therapy were higher in the high-risk group. Body mass index, the proportion of patients with HF with preserved ejection fraction, systolic and diastolic blood pressures, heart rate, LVEF, haemoglobin level, serum sodium level, and eGFR were lower in the high-risk group. There were also significant differences in the aetiology of HF and the prevalence of atrial fibrillation among the three groups. No patients were taking sacubitril/valsartan because the drug had not yet been approved for clinical use in Japan.
Prognostic analysis
Over a median follow-up of 1039 days, 135 patients died. Of these, 61 patients died from cardiac causes (PFD, n = 34; SCD, n = 27), and 74 from non-cardiac causes (pneumonia, n = 18; cancer, n = 13; infection/sepsis, n = 9; old age, n = 8; renal failure, n = 5; stroke, n = 4; gastrointestinal bleeding, n = 4; other causes of death, n = 13). No patient died due to an acute myocardial infarction. WHF occurred in 120 patients.
Univariate and multivariate Cox proportional hazard analyses for the primary and secondary endpoints are shown in Table 2. Multivariate Cox analysis demonstrated that the 2-year mortality risk estimated by the MIBG-based risk model was independently associated not only with cardiac death but also with all secondary endpoints when used as a continuous variable. Moreover, in the multivariate Cox analysis, the 2-year mortality risk estimated by the MIBG-based risk model was shown to have an independent association with all endpoints, even when used as a categorical variable. Kaplan–Meier analysis showed that patients in the high-risk group had a significantly higher risk for all endpoints (Fig. 2).
Kaplan–Meier estimates of freedom from cardiac death (a), all-cause death (b), unplanned hospitalisation for worsening heart failure (c), cardiac death or unplanned hospitalisation for worsening heart failure (d), and all-cause death or unplanned hospitalisation for worsening heart failure (e), with patients stratified into low-risk, intermediate-risk, and high-risk groups according to the cardiac MIBG-based risk model
Actual cardiac mortality in each risk group
The median 2-year cardiac mortality rates estimated by the MIBG-based risk model were almost the same as those actually observed in each risk group; 3% vs. 4% for the low-risk group; 7% vs. 9% for the intermediate-risk group; and 20% vs. 23% for the high-risk group (Fig. 3).
The 2-year cardiac death rates estimated by the MIBG-based risk model (box plots) and actual cardiac mortality (green dots) in each risk group. The central line of the box plot denotes the median, the lower and upper lines denote the 25th and 75th percentiles, and the whiskers denote the value ranges
Comparison with ADHERE and GWTG-HF risk score
ROC curve analysis of the 2-year follow-up period showed that the 2-year MIBG-based risk model had higher predictive values, not only for cardiac death but also for all secondary endpoints, than the ADHERE and GWTG-HF risk scores (Fig. 4).
The receiver operating characteristic curve analysis for the prediction of cardiac death (a), all-cause death (b), unplanned hospitalization for worsening heart failure (c), cardiac death or unplanned hospitalization for worsening heart failure (d), and all-cause death or unplanned hospitalization for worsening heart failure (e), using the risk scores. AUC, area under the curve; GWTG-HF, Get With The Guidelines-Heart Failure; ADHERE, Acute Decompensated Heart Failure National Registry
Discussion
In this study, the 2-year MIBG-based cardiac mortality risk model was shown to predict the risk of cardiac death in patients who were admitted for ADHF. The model also predicted the risk of other endpoints including all-cause death, WHF, a composite of cardiac death and WHF, and a composite of all-cause death and WHF, regardless of whether the risk value was used as a continuous or categorical variable. Moreover, as shown in the ROC curve analysis, the 2-year MIBG-based risk model predicted all endpoints with greater accuracy than the ADHERE and GWTG-HF risk scores. To the best of our knowledge, this is the first report to demonstrate the usefulness of the MIBG-based risk model for the prediction of long-term clinical outcomes in patients with ADHF.
Cardiac 123I-MIBG imaging has been the gold standard for the assessment of CSNA. HMR represents the distribution of neurons and function of the uptake-1 pathway, while the washout rate reflects the retention of norepinephrine by sympathetic neurons [24]. These parameters in cardiac MIBG imaging have been shown to provide prognostic information on patients with CHF [10,11,12,13, 15, 16, 21]. In this study, we demonstrated the usefulness of cardiac MIBG imaging in predicting the prognosis of patients with ADHF [14]. Although the HMR is known to be affected by the difference in collimator types, it has been shown that this limitation can be overcome by the cross-calibration method, which enables comparison between differing national and international data [22, 23]. A simple four-parameter risk model, including cardiac MIBG HMR and easily obtainable clinical parameters such as age, LVEF, and NYHA functional class, was recently developed and validated for the prediction of 2-year cardiac mortality risk in patients with CHF [15, 16]. Our findings expand on these earlier reports by demonstrating that the 2-year cardiac MIBG risk model can also be used for risk stratification of patients admitted with ADHF, after stabilisation of HF symptoms.
Although there have been an increasing number of risk prediction models for patients with HF, there is still no established model which can be used for accurate risk stratification of patients with ADHF. In addition, the existing risk models for ADHF that primarily assess in-hospital mortality are often complex, need variables that are not readily available, and are uniformly underutilised [5]. Recently, it has been demonstrated that the ADHERE and GWTG-HF risk scores, which are simple risk assessment tools to predict in-hospital mortality of patients with ADHF, are also useful for predicting mortality and readmission rates ≤ 180 days after discharge. However, the utility of these risk scores for the prediction of long-term post-discharge clinical outcomes in patients with ADHF has not yet been reported. Our results indicate that the 2-year cardiac MIBG risk model has a superior predictive value for long-term prognosis in patients with ADHF than the ADHERE and GWTG-HF risk scores. Accurately predicting long-term prognosis can be of benefit even for patients with poor prognosis who are already on maximal HF therapy because non-pharmacological interventions including transitional care and a multidisciplinary approach might improve their clinical outcomes [25, 26], and palliative care can improve their quality of life and assist them in defining their goals of care [27]. Although the precise reason for the better prognostic accuracy of the 2-year cardiac MIBG risk model, compared to the other two risk scores, in predicting cardiac mortality remains unknown from our study, it might be explained in part by the inclusion of variables directly related to cardiac function in the 2-year cardiac MIBG risk model: LVEF and cardiac MIBG late HMR. LVEF is well known to be an important predictor of mortality in patients with HF [5], and LV systolic function is included in some established risk models of long-term mortality in patients with CHF [28, 29]. Moreover, the results of cardiac MIBG imaging have been shown to be an independent predictor of cardiac events which include cardiac death [10, 11].
Although there is an increasing focus on prevention of readmission after HF hospitalisation, prediction of HF rehospitalisation remains poorly understood and its prediction by risk models has been only moderately successful [4, 5]. Our results demonstrated that the 2-year cardiac MIBG risk model has a high predictive value for endpoints including WHF, and emphasised the usefulness of this novel risk model in patients with ADHF. Higher age and higher NYHA functional class at discharge has been shown to predict the risk of HF rehospitalisation [30]. The higher risk of HF rehospitalisation is also associated with poor renal function, which has been previously shown to influence CSNA [31]. Furthermore, cardiac MIBG uptake is reported to be affected by age in patients with HF [32], and the results of cardiac MIBG imaging itself are reported to be a potent predictor of HF hospitalisation [13]. These associations might explain, at least in part, the higher predictive power of the 2-year cardiac MIBG risk model for HF rehospitalisation compared to the ADHERE and GWTG-HF risk scores. Considering the deleterious impact of HF rehospitalisation on its prognosis and its considerable financial burden to the healthcare system [33, 34], further studies are needed to develop precise risk prediction models for HF readmission in patients with ADHF.
Limitations
This study had several limitations. First, the small and empirically chosen sample size was a major limitation. Second, as this was a single-centre study, possible ethnic differences should be considered when attempting to generalise our results to non-Japanese populations. Third, no study patient received sacubitril/valsartan during the study period, which could affect MIBG uptake and clinical outcomes. Fourth, serial 123I-MIBG imaging has been shown to have a greater prognostic value than a one-time MIBG scan in patients with CHF [35]. Moreover, improvement in LVEF is known to be predictive of a better prognosis in patients with HF [36]. Therefore, it remains to be elucidated whether longitudinal changes in the mortality risk estimated by the MIBG-based risk model also predict prognosis in patients with HF. Fifth, HF decompensation increases CSNA, and it seems that it takes at least a few weeks for the stabilisation of the results of cardiac MIBG imaging [37]. However, little is known about the time course of cardiac 123I-MIBG uptake before and after HF decompensation. Although we performed cardiac MIBG imaging about 2 weeks after admission and the 2-year cardiac mortality predicted by the MIBG-based risk model was nearly identical to that actually observed in each risk group, appropriateness of the use of the 2-year cardiac MIBG risk model in patients with ADHF needs further verification. Sixth, the ADHERE and GWTG-HF risk scores were developed for patients admitted with ADHF to predict in-hospital mortality but not to predict long-term prognosis [7, 8]. Therefore, suitable cut-off values or points assigned to the values of predictors might be different from the original scores for the prediction of post-discharge outcome. Lastly, future studies should clarify whether clinical decision-making using the cardiac MIBG risk model leads to better clinical outcomes in patients with HF.
Conclusion
In this study, the 2-year MIBG-based cardiac mortality risk model was shown to be useful for predicting post-discharge clinical outcomes in patients with ADHF. Larger multicentre studies are needed to further evaluate the usefulness of this model for the prognostication of patients with ADHF.
Data availability
All data generated or analysed during this study are included in this report.
References
Chang PP, Wruck LM, Shahar E, Rossi JS, Loehr LR, Russell SD, et al. Trends in hospitalizations and survival of acute decompensated heart failure in four us communities (2005–2014): ARIC Study Community Surveillance. Circulation. 2018;138:12–24. https://doi.org/10.1161/CIRCULATIONAHA.117.027551.
Rocha BML, Menezes FL. Acute decompensated heart failure (ADHF): a comprehensive contemporary review on preventing early readmissions and postdischarge death. Int J Cardiol. 2016;223:1035–44. https://doi.org/10.1016/j.ijcard.2016.07.259.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. https://doi.org/10.1093/eurheartj/ehw128.
Ouwerkerk W, Voors AA, Zwinderman AH. Factors influencing the predictive power of models for predicting mortality and/or heart failure hospitalization in patients with heart failure. JACC Heart Fail. 2014;2:429–36. https://doi.org/10.1016/j.jchf.2014.04.006.
Rahimi K, Bennett D, Conrad N, Williams TM, Basu J, Dwight J, et al. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail. 2014;2:440–6. https://doi.org/10.1016/j.jchf.2014.04.008.
Miro O, Rossello X, Platz E, Masip J, Gualandro DM, Peacock WF, et al. Risk stratification scores for patients with acute heart failure in the Emergency Department: a systematic review. Eur Heart J Acute Cardiovasc Care. 2020;9:375–98. https://doi.org/10.1177/2048872620930889.
Fonarow GC, Adams KF, Jr., Abraham WT, Yancy CW, Boscardin WJ; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–80. https://doi.org/10.1001/jama.293.5.572.
Peterson PN, Rumsfeld JS, Liang L, Albert NM, Hernandez AF, Peterson ED, et al. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes. 2010;3:25–32. https://doi.org/10.1161/CIRCOUTCOMES.109.854877.
Win S, Hussain I, Hebl VB, Dunlay SM, Redfield MM. Inpatient mortality risk scores and postdischarge events in hospitalized heart failure patients: a community-based study. Circ Heart Fail. 2017;10. https://doi.org/10.1161/CIRCHEARTFAILURE.117.003926.
Tamaki S, Yamada T, Okuyama Y, Morita T, Sanada S, Tsukamoto Y, et al. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J Am Coll Cardiol. 2009;53:426–35. https://doi.org/10.1016/j.jacc.2008.10.025.
Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55:2212–21. https://doi.org/10.1016/j.jacc.2010.01.014.
Kasama S, Toyama T, Kaneko Y, Iwasaki T, Sumino H, Kumakura H, et al. Relationship between late ventricular potentials and myocardial 123I-metaiodobenzylguanidine scintigraphy in patients with dilated cardiomyopathy with mild to moderate heart failure: results of a prospective study of sudden death events. Eur J Nucl Med Mol Imaging. 2012;39:1056–64. https://doi.org/10.1007/s00259-012-2092-1.
Parker MW, Sood N, Ahlberg AW, Jacobson AF, Heller GV, Lundbye JB. Relationship between quantitative cardiac neuronal imaging with 123I-meta-iodobenzylguanidine and hospitalization in patients with heart failure. Eur J Nucl Med Mol Imaging. 2014;41:1666–72. https://doi.org/10.1007/s00259-014-2819-2.
Seo M, Yamada T, Tamaki S, Watanabe T, Morita T, Furukawa Y, et al. Prognostic significance of cardiac I-123-metaiodobenzylguanidine imaging in patients with reduced, mid-range, and preserved left ventricular ejection fraction admitted for acute decompensated heart failure: a prospective study in Osaka Prefectural Acute Heart Failure Registry (OPAR). Eur Heart J Cardiovasc Imaging. 2021;22:58–66. https://doi.org/10.1093/ehjci/jeaa025.
Nakajima K, Nakata T, Matsuo S, Jacobson AF. Creation of mortality risk charts using 123I meta-iodobenzylguanidine heart-to-mediastinum ratio in patients with heart failure: 2- and 5-year risk models. Eur Heart J Cardiovasc Imaging. 2016;17:1138–45. https://doi.org/10.1093/ehjci/jev322.
Nakajima K, Nakata T, Doi T, Kadokami T, Matsuo S, Konno T, et al. Validation of 2-year 123I-meta-iodobenzylguanidine-based cardiac mortality risk model in chronic heart failure. Eur Heart J Cardiovasc Imaging. 2018;19:749–56. https://doi.org/10.1093/ehjci/jey016.
McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–6. https://doi.org/10.1056/NEJM197112232852601.
Kondo T, Yamada T, Tamaki S, Morita T, Furukawa Y, Iwasaki Y, et al. Serial change in serum chloride during hospitalization could predict heart failure death in acute decompensated heart failure patients. Circ J. 2018;82:1041–50. https://doi.org/10.1253/circj.CJ-17-0938.
Tamaki S, Mano T, Sakata Y, Ohtani T, Takeda Y, Kamimura D, et al. Interleukin-16 promotes cardiac fibrosis and myocardial stiffening in heart failure with preserved ejection fraction. PLoS ONE. 2013;8: e68893. https://doi.org/10.1371/journal.pone.0068893.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92. https://doi.org/10.1053/j.ajkd.2008.12.034.
Ogita H, Shimonagata T, Fukunami M, Kumagai K, Yamada T, Asano Y, et al. Prognostic significance of cardiac 123I metaiodobenzylguanidine imaging for mortality and morbidity in patients with chronic heart failure: a prospective study. Heart. 2001;86:656–60. https://doi.org/10.1136/heart.86.6.656.
Nakajima K, Okuda K, Yoshimura M, Matsuo S, Wakabayashi H, Imanishi Y, et al. Multicenter cross-calibration of I-123 metaiodobenzylguanidine heart-to-mediastinum ratios to overcome camera-collimator variations. J Nucl Cardiol. 2014;21:970–8. https://doi.org/10.1007/s12350-014-9916-2.
Verschure DO, Poel E, Nakajima K, Okuda K, van Eck-Smit BLF, Somsen GA, et al. A European myocardial 123I-mIBG cross-calibration phantom study. J Nucl Cardiol. 2018;25:1191–7. https://doi.org/10.1007/s12350-017-0782-6.
van der Veen BJ, Al Younis I, de Roos A, Stokkel MP. Assessment of global cardiac I-123 MIBG uptake and washout using volumetric quantification of SPECT acquisitions. J Nucl Cardiol. 2012;19:752–62. https://doi.org/10.1007/s12350-012-9539-4.
Albert NM, Barnason S, Deswal A, Hernandez A, Kociol R, Lee E, et al. Transitions of care in heart failure: a scientific statement from the American Heart Association. Circ Heart Fail. 2015;8:384–409. https://doi.org/10.1161/HHF.0000000000000006.
Kamiya K, Sato Y, Takahashi T, Tsuchihashi-Makaya M, Kotooka N, Ikegame T, et al. Multidisciplinary cardiac rehabilitation and long-term prognosis in patients with heart failure. Circ Heart Fail. 2020;13: e006798. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006798.
Diop MS, Rudolph JL, Zimmerman KM, Richter MA, Skarf LM. Palliative care interventions for patients with heart failure: a systematic review and meta-analysis. J Palliat Med. 2017;20:84–92. https://doi.org/10.1089/jpm.2016.0330.
Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. https://doi.org/10.1093/eurheartj/ehi555.
Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–33. https://doi.org/10.1161/CIRCULATIONAHA.105.584102.
Giamouzis G, Kalogeropoulos A, Georgiopoulou V, Laskar S, Smith AL, Dunbar S, et al. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. J Card Fail. 2011;17:54–75. https://doi.org/10.1016/j.cardfail.2010.08.010.
Kurata C, Uehara A, Sugi T, Ishikawa A, Fujita K, Yonemura K, et al. Cardiac autonomic neuropathy in patients with chronic renal failure on hemodialysis. Nephron. 2000;84:312–9. https://doi.org/10.1159/000045605.
Rengo G, Pagano G, Vitale DF, Formisano R, Komici K, Petraglia L, et al. Impact of aging on cardiac sympathetic innervation measured by 123I-mIBG imaging in patients with systolic heart failure. Eur J Nucl Med Mol Imaging. 2016;43:2392–400. https://doi.org/10.1007/s00259-016-3432-3.
Fernandez-Gasso L, Hernando-Arizaleta L, Palomar-Rodriguez JA, Abellan-Perez MV, Pascual-Figal DA. Trends, causes and timing of 30-day readmissions after hospitalization for heart failure: 11-year population-based analysis with linked data. Int J Cardiol. 2017;248:246–51. https://doi.org/10.1016/j.ijcard.2017.07.094.
Kitakata H, Kohno T, Kohsaka S, Shiraishi Y, Parizo JT, Niimi N, et al. Prognostic implications of early and midrange readmissions after acute heart failure hospitalizations: a report from a Japanese multicenter registry. J Am Heart Assoc. 2020;9: e014949. https://doi.org/10.1161/JAHA.119.014949.
Kasama S, Toyama T, Sumino H, Nakazawa M, Matsumoto N, Sato Y, et al. Prognostic value of serial cardiac 123I-MIBG imaging in patients with stabilized chronic heart failure and reduced left ventricular ejection fraction. J Nucl Med. 2008;49:907–14. https://doi.org/10.2967/jnumed.107.047548.
Cintron G, Johnson G, Francis G, Cobb F, Cohn JN. Prognostic significance of serial changes in left ventricular ejection fraction in patients with congestive heart failure. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI17–23.
Kasama S, Toyama T, Funada R, Takama N, Koitabashi N, Ichikawa S, et al. Effects of adding intravenous nicorandil to standard therapy on cardiac sympathetic nerve activity and myocyte dysfunction in patients with acute decompensated heart failure. Eur J Nucl Med Mol Imaging. 2015;42:761–70. https://doi.org/10.1007/s00259-015-2990-0.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Shunsuke Tamaki. The first draft of the manuscript was written by Shunsuke Tamaki, Takahisa Yamada, Tetsuya Watanabe, and Masatake Fukunami, and all the authors commented on earlier versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. The Institutional Ethics Committee approved the study protocol.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Patients signed informed consent for publication of their data.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was presented in part as a poster presentation at the ESC congress 2019 in Paris, France.
This article is part of the Topical Collection on Cardiology.
Rights and permissions
About this article
Cite this article
Tamaki, S., Yamada, T., Watanabe, T. et al. Usefulness of the 2-year iodine-123 metaiodobenzylguanidine-based risk model for post-discharge risk stratification of patients with acute decompensated heart failure. Eur J Nucl Med Mol Imaging 49, 1906–1917 (2022). https://doi.org/10.1007/s00259-021-05663-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05663-y