Abstract
Purpose
Nicorandil, an adenosine triphosphate-sensitive potassium channel opener, improves cardiac sympathetic nerve activity (CSNA) in ischemic heart disease or chronic heart failure. However, its effects on CSNA and myocyte dysfunction in acute heart failure (AHF) remain unclear. We investigated the effects of adding intravenous nicorandil to standard therapy on CSNA and myocyte dysfunction in AHF.
Methods
We selected 70 patients with mild to moderate nonischemic AHF who were treated with standard conventional therapy soon after admission. Thirty-five patients were assigned to additionally receive intravenous nicorandil (4–12 mg/h; group A), whereas the remaining patients continued their current drug regimen (group B). Delayed total defect score (TDS), delayed heart to mediastinum count (H/M) ratio, and washout rate (WR) were determined by 123I-metaiodobenzylguanidine (MIBG) scintigraphy within 3 days of admission and 4 weeks later. High sensitivity troponin T (hs-TnT) level was also measured at the same time points.
Results
After treatment, MIBG scintigraphic parameters significantly improved in both groups. However, the extent of the changes in these parameters in group A significantly exceeded the extent of the changes in group B [TDS −11.3 ± 4.3 in group A vs −4.0 ± 6.0 in group B (p < 0.01); H/M ratio 0.31 ± 0.16 vs 0.14 ± 0.16 (p < 0.01); WR −13.8 ± 7.8 % vs −6.1 ± 8.9 % (p < 0.01)]. The hs-TnT level decreased significantly from 0.052 ± 0.043 to 0.041 ± 0.033 ng/ml (p < 0.05) in group A, but showed no significant change in group B. Moreover, in both groups, no relationships between the extent of changes in MIBG parameters and hs-TnT level were observed.
Conclusion
Adding intravenous nicorandil to standard therapy provides additional benefits for CSNA and myocyte dysfunction over conventional therapy alone in AHF patients. Furthermore, the mechanisms of improvement in CSNA and myocyte dysfunction after nicorandil treatment in AHF patients were distinct.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sympathetic nervous system activation is a cardinal pathophysiological abnormality associated with heart failure in humans [1]. Therefore, plasma norepinephrine concentrations affect the prognosis of patients with heart failure [2]. Myocardial imaging with 123I-metaiodobenzylguanidine (MIBG), an analogue of norepinephrine, is useful for detecting cardiac sympathetic nerve activity (CSNA) abnormalities in patients with chronic heart failure (CHF) [3, 4]. Furthermore, CSNA evaluated by cardiac 123I-MIBG scintigraphy has useful prognostic value in CHF [4–6]. Plasma concentrations of troponin T (TnT), a marker of myocyte injury [7], can also be used for risk stratification of patients with CHF [8, 9] or acute heart failure (AHF) [10, 11]. This biomarker has well-known broad implications in prognosis, treatment selection, development of new treatments, and understanding of the underlying mechanisms [12].
As a drug with both nitrate-like and adenosine triphosphate (ATP)-sensitive potassium channel-activating properties [13], nicorandil [N-(2-hydroxyethyl)-nicotinamide nitrate, Chugai, Tokyo, Japan] has been reported to reduce the incidence of cardiac events in patients with ischemic heart disease [14, 15] or CHF [16]. It has been reported that CSNA is modulated by the activation of ATP-sensitive potassium channels [17], suggesting that nicorandil can improve CSNA by activating ATP-sensitive potassium channels in patients with CHF [16]. Moreover, this agent exerts protective effects against myocyte injury in experimental animal models of heart failure [18].
Accordingly, this study was performed to determine whether nicorandil treatment improves CSNA, as evaluated by 123I-MIBG scintigraphy, and affects myocyte function in patients with AHF, and to evaluate the relationship between CSNA and myocyte dysfunction after nicorandil treatment.
Materials and methods
Patient population and study protocol
Between June 2008 and May 2010, 198 patients were admitted to our institutions with a first episode of decompensated AHF with a left ventricular (LV) ejection fraction (EF) <45 %. Patients were excluded from the study for the following reasons: cardiogenic shock or hypotension (defined as systolic blood pressure <80 mmHg; 22 patients), acute coronary syndrome including acute myocardial infarction (29 patients), severe renal or liver dysfunction (18 patients), and need for mechanical support (mechanical ventilation, intra-aortic balloon pumping, LV assist device, or cardiac resynchronization therapy; 21 patients). None of the patients had a history of HF (Fig. 1).
Soon after admission, standard therapy including a vasodilator and/or diuretics was started. Furthermore, if necessary, low-dose dopamine or dobutamine was started for blood pressure maintenance. In this prospective randomized study (open-label, 1:1 ratio), after hemodynamic stability was established, 108 patients were randomized to receive intravenous nicorandil (4–12 mg/h) in addition to standard therapy (group A; n = 54) or to continue their established drug regimen (group B; n = 54) (Fig. 1). In case of high (>150 mmHg) or low (<90 mmHg) systolic blood pressure, the doses of dopamine/dobutamine or nicorandil were adjusted to maintain a constant blood pressure. Nicorandil was continuously infused for > 3 days (mean 5.9 days; Table 1) in group A. The design was a prospective study, and all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
During or after intravenous treatments for AHF, patients received oral diuretics, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and/or beta-blockers. In the present study, no patient was treated with oral nicorandil therapy, because we evaluated the effects of adding intravenous nicorandil to standard therapy on CSNA and myocyte dysfunction in patients with AHF. In group A, one patient experienced a cerebral infarction after admission, and in group B, three patients died of progression of congestive heart failure. All the remaining patients underwent stress perfusion scintigraphy or coronary angiography; 15 patients in group A and 12 patients in group B were excluded because of coronary artery disease (Fig. 1).
We performed 123I-MIBG scintigraphy and echocardiography within 3 days of admission and 4 weeks later (mean 4.1 weeks). High sensitivity troponin T (hs-TnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations were measured before treatment (soon after admission) and at 7 days and 4 weeks after admission.
Three patients from group A and four patients from group B were excluded because of incomplete follow-up examinations. Thus, 70 of 77 patients enrolled in the trial completed the entire protocol (group A, n = 35; group B, n = 35) (Fig. 1).
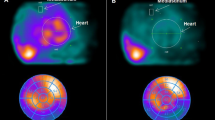
Cardiac 123I-MIBG scintigraphy
The 123I-MIBG imaging method used has been described previously [19, 20]. In brief, 123I-MIBG was obtained from a commercial source (FUJIFILM RI Pharma Co. Ltd, Tokyo, Japan). Patients were injected intravenously with 123I-MIBG (111 MBq) while in a supine position. At 15 min and at 4 h after injection, static data were acquired in the anterior view with a single-head gamma camera (Millennium MPR, GE Medical Systems, Waukesha, WI, USA) equipped with a low-energy, general-purpose, parallel-hole collimator. Static images on a 128×128 matrix were collected for 5 min with a 20 % window centered on 159 keV, corresponding to the 123I photopeak. After the static planar images were acquired, single photon emission computed tomography (SPECT) images were obtained. The camera was rotated over 180° from the 45° right anterior oblique position to the 45° left posterior oblique position in 32 views with an acquisition time of 40 s per view. Scans were acquired in a 64×64 matrix, and the images were reconstructed by a filtered backprojection method.
The heart to mediastinum count (H/M) ratio was determined from the anterior planar delayed 123I-MIBG image. The washout rate (WR) was calculated from early and delayed planar images. Regional tracer uptake was assessed semiquantitatively using a 5-point scoring system (0 = normal to 4 = no uptake) in 17 segments on the delayed SPECT image as recommended by the American Heart Association [21]. The total defect score (TDS) was calculated as the sum of all defect scores. At our laboratory, the normal range for the delayed TDS is 6–10, the delayed H/M ratio range is from 2.18 to 2.70, and the normal WR range is from 20 to 30 %, as previously reported [19, 20].
Echocardiography
Echocardiography was performed using the standard method in a blinded manner. Two independent and experienced echocardiographers with no knowledge of the study performed all measurements. LV end-diastolic volume (EDV), LV end-systolic volume (ESV), and LVEF were calculated using the 2-D biplane method [22].
Plasma hs-TnT and NT-proBNP concentrations
Blood was sampled before treatment (soon after admission) and at 7 days and 4 weeks after admission. Serum levels of hs-TnT and NT-proBNP were measured by electrochemiluminescence immunoassay (ECLIA) on the Cobas 6000 analyzer (Roche Diagnostics Ltd.).
Statistical analysis
The analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA) or SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) software. Numerical results were expressed as the mean ± SD. In all analyses, p < 0.05 was considered statistically significant.
Categorical data were compared between the two groups using two-sided chi-square tests, and differences between continuous variables were evaluated using the unpaired t test (Table 1). In patients who underwent 123I-MIBG scintigraphic and echocardiographic assessments, changes from baseline were evaluated within each treatment group using paired t tests and between the two groups using two-way analysis of variance (ANOVA; Fig. 2 and Table 2). For validation, a two-way repeated measures ANOVA was used to examine the changes in the NT-proBNP and hs-TnT levels from before to after treatment (Fig. 3). Furthermore, to evaluate the contribution of the degree of change in WR (i.e., delta-WR), univariate and stepwise multivariate analyses were used to examine the variable of interest (Table 3).
Results
Clinical characteristics
There were no significant differences in clinical characteristics or cardiac medications including intravenous catecholamine infusion between the two groups. At baseline, the TDS, H/M ratio, WR, LVEDV, LVESV, LVEF, NT-proBNP level, and hs-TnT level were similar between the two groups (Table 1).
Comparison of cardiac 123I-MIBG scintigraphic findings at baseline and after treatment
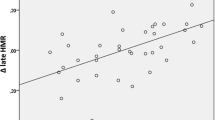
Figure 2 and Table 2 summarize the TDSs, H/M ratios, and WRs. In both groups, TDS significantly decreased after 4 weeks relative to that at baseline. However, the delta-TDS in group A was significantly lower than that in group B. In both groups, the H/M ratio significantly increased after 4 weeks compared with that at baseline. However, the delta-H/M ratio was significantly higher in group A than in group B. Finally, in both groups, the WR significantly decreased after 4 weeks relative to that at baseline. However, the delta-WR in group A was significantly lower than that in group B.
Comparison of echocardiographic findings at baseline and after treatment
Table 2 also provides a summary of the LVEDV, LVESV, and LVEF. In both groups, after 4 weeks, LVEDV and LVESV significantly decreased and LVEF significantly increased relative to baseline. Changes in LVEDV and LVESV were significantly greater in group A than in group B. The change in LVEF tended to be more favorable in group A than in group B, although not significantly.
Evaluation of factors predicting increased delta-WR
Table 3 shows the results of the univariate and multivariate analyses to assess factors predicting an increase in delta-WR. In the univariate analysis, age, non-beta-blocker treatment, and non-intravenous nicorandil treatment were predictive factors. The non-hypertensive etiology, non-ACE inhibitor treatment, non-ARB treatment, non-mineralocorticoid receptor antagonist treatment, and nicorandil dose in this study tended to be predictive factors, but these factors were not statistically significant. The stepwise multivariate analysis identified non-beta-blocker treatment and non-intravenous nicorandil treatment as significant independent predictors of increasing delta-WR in the AHF patients.
Comparison of plasma NT-proBNP and hs-TnT concentrations at baseline and after treatment
Figure 3 summarizes the plasma NT-proBNP and hs-TnT concentrations. In group A, plasma NT-proBNP concentrations significantly decreased after 7 days and after 4 weeks of treatment. However, in group B, these parameters significantly decreased only at 4 weeks. The hs-TnT concentrations in group A significantly decreased after 4 weeks relative to those at baseline. By contrast, in group B, baseline and posttreatment hs-TnT concentrations did not differ significantly.
Relationship between plasma hs-TnT concentrations and 123I-MIBG scintigraphic findings before and after treatment
The extent of changes in the 123I-MIBG scintigraphic parameters was not related to the extent of changes in the hs-TnT level in group A (TDS, r = −0.020, p = 0.913; H/M ratio, r = 0.121, p = 0.493; and WR, r = −0.204, p = 0.242) or in group B (TDS, r = 0.053, p = 0.764; H/M ratio, r = 0.158, p = 0.366; and WR, r = −0.150, p = 0.392).
Discussion
Patients in this study were stratified into a group that received intravenous nicorandil in addition to standard therapy and a group that received conventional therapy alone. Improvements in 123I-MIBG scintigraphic and echocardiographic parameters were observed in both groups, with more favorable changes occurring in the group receiving nicorandil. Stepwise multivariate analyses showed that non-intravenous nicorandil treatment had independent and significant predictors of increasing delta-WR in AHF patients. The hs-TnT concentrations significantly decreased in the group receiving nicorandil, but did not change significantly in the group receiving conventional therapy alone. Moreover, the extent of changes in MIBG parameters and hs-TnT level were not related in either group.
Nicorandil exerts a vasodilatory effect mainly on the systemic veins, as do conventional nitrates, but it also dilates arteries, including peripheral arteries, by opening ATP-sensitive potassium channels [13]. Previous reports have indicated that intravenous administration of nicorandil in the acute phase improves cardiac output, reduces pulmonary pressure, and modulates hemodynamic parameters in patients with AHF [23]. Several potential mechanisms have been proposed for the cardioprotective effects of nicorandil: (1) reduction in pre- and afterload [24], (2) improved myocardial perfusion [25], (3) prevention of Ca2+ overload by opening ATP-sensitive potassium channels [26], and (4) free radical scavenging and neutrophil-modulating properties [27]. Moreover, nicorandil has a pharmacological preconditioning effect [28], and this effect has been reported to provide cardioprotection against ischemia. Therefore, there have been many reports of favorable effects for patients with ischemic heart disease. We previously reported that intravenous nicorandil improves CSNA in patients with acute coronary syndrome [29]. However, little is known about the effects of intravenous administration of nicorandil on CSNA evaluated by 123I-MIBG scintigraphy in patients with nonischemic AHF. In our study patients, TDS, H/M ratio, and WR determined by cardiac 123I-MIBG scintigraphy were all significantly improved after addition of intravenous nicorandil to standard therapy compared with the standard therapy alone.
Furthermore, in this study, the stepwise multivariate analyses revealed that the non-intravenous nicorandil treatment had independent and significant predictors of increasing delta-WR in AHF patients. Given our previously reported observation that delta-WR is the best currently available prognostic indicator of heart failure [6], our findings demonstrated for the first time that intravenous nicorandil treatment may be the available agent for improving CSNA and for preventing cardiac events of patients with AHF.
On the other hand, in nonischemic HF patients, the exact mechanisms by which nicorandil exerts a cardioprotective effect remain unknown. Neglia et al. [30] reported that myocardial blood flow is severely depressed in the whole heart in HF patients with nonischemic cardiomyopathy. Therefore, it seems plausible that nicorandil may have ischemic preconditioning-like effects that improve microvascular circulation during the acute phase, as myocardial flow is depressed during this period; this assumption may account for the better outcome, as was the case in the prior study [23].
123I-MIBG is an analogue of the adrenergic neuron-blocking agent guanethidine, which is thought to utilize the same myocardial uptake and release mechanisms as norepinephrine [31]. Therefore, cardiac 123I-MIBG imaging is a useful tool for detecting abnormalities of the myocardial adrenergic nervous system in CHF patients [3, 4]. Furthermore, many reports have suggested that the HF treatments can improve CSNA on the basis of cardiac 123I-MIBG scintigraphic findings [19, 20, 32–34]. However, limited 123I-MIBG scintigraphy data exist on the effects of intravenous nicorandil therapy on CSNA in AHF patients. In this study, we examined whether adding intravenous nicorandil to standard therapy improved 123I-MIBG scintigraphic parameters in our AHF patients. We found that the group receiving additional nicorandil showed improvement compared with the group receiving conventional therapy alone. Given our previously reported observation that the extent of changes in 123I-MIBG scintigraphic parameters is the best currently available prognostic indicator for HF patients [6], our findings demonstrated for the first time that adding intravenous nicorandil to standard therapy may improve the prognosis of AHF patients.
It has been reported that in the failing heart release of norepinephrine is enhanced, whereas its uptake is prevented [35]. Kang et al. [36] demonstrated that norepinephrine release and uptake are modulated by the activation of ATP-sensitive potassium channels in experimental rat models. As nicorandil is well known to activate ATP-sensitive potassium channels and has cardioprotective effects [13], it may attenuate CSNA. Therefore, we hypothesize that the addition of nicorandil therapy can improve CSNA in AHF patients. However, further study will be required to confirm this hypothesis.
The measurement of circulating cardiac TnT levels plays a fundamental role in the diagnosis and management of acute coronary syndromes [37, 38]. In addition to its role in ischemic heart disease, accumulating data provide evidence of the importance of TnT level measurement in AHF patients [10, 11]. The extent of changes in hs-TnT level has been shown to be associated with long-term LV function and prognosis in patients with HF caused by nonischemic cardiomyopathy [39]. For this reason, increasing efforts have been directed toward pharmacologically decreasing hs-TnT levels following HF. This study found that adding intravenous nicorandil to standard therapy significantly decreased hs-TnT levels compared to conventional therapy.
Adding intravenous nicorandil to standard therapy improved not only cardiac 123I-MIBG scintigraphic findings, but also myocyte dysfunction (i.e., decreasing plasma hs-TnT level) in AHF patients. However, the extent of changes in MIBG parameters was not related to the change in hs-TnT concentrations in these patients. Therefore, the mechanisms of the improvement in CSNA and the improvement in myocyte dysfunction after nicorandil treatment in AHF patients were distinct. We need to clarify the related mechanisms of improvement in myocyte dysfunction after nicorandil treatment in the future.
In this study, TDS was calculated from SPECT imaging, whereas H/M ratio and WR were determined from anterior planar imaging. In general, for evaluation of 123I-MIBG imaging, both H/M ratio and WR are frequently used and clinically useful [4, 5, 32] compared with TDS. Moreover, it is known that dilated LV volume affects defect score because of the partial volume effect [40]. However, in this study, there were significant correlations between TDS and H/M ratio (baseline r = −0.739, p < 0.001, and 4 weeks r = −0.751, p < 0.001) or WR (baseline r = 0.865, p < 0.001, and 4 weeks r = 0.884, p < 0.001). Therefore, we believe that TDS in this study can be useful for evaluation of CSNA as well as H/M ratio and WR.
Study limitations
The small number of AHF patients in this study limited the statistical power, caused by a prospective, single-center trial. The quantitative 123I-MIBG parameters differ between institutions and between instruments, because the choice of collimator influences the value of the H/M ratio and WR. For these reasons, cardiac 123I-MIBG has yet to achieve broad clinical acceptance; thus, few multicenter trials using the imaging modality have been conducted. However, it has been reported that the correction method can standardize the 123I-MIBG imaging among various gamma cameras and various collimators [41]. In the future, we need to examine the effects of adding intravenous nicorandil to standard therapy on CSNA by a prospective, multicenter trial with larger numbers of patients, using the previously reported correction method [41].
Conclusion
123I-MIBG scintigraphic and echocardiographic parameters improved in both groups, but more significantly in the group receiving intravenous nicorandil in addition to standard therapy. The hs-TnT concentrations significantly decreased in the group receiving nicorandil, whereas no significant change was observed in the group receiving conventional therapy alone. Moreover, the extent of changes in MIBG parameters was not related to the extent of changes in the hs-TnT levels in both groups. These findings indicate that adding intravenous nicorandil to standard therapy may provide additional benefits for CSNA and myocyte dysfunction over conventional therapy alone in AHF patients. Furthermore, the mechanisms of improvement in CSNA and myocyte dysfunction after nicorandil treatment were distinct.
References
Thomas JA, Marks BH. Plasma norepinephrine in congestive heart failure. Am J Cardiol 1978;41(2):233–43.
Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984;311(13):819–23.
Henderson EB, Kahn JK, Corbett JR, Jansen DE, Pippin JJ, Kulkarni P, et al. Abnormal I-123 metaiodobenzylguanidine myocardial washout and distribution may reflect myocardial adrenergic derangement in patients with congestive cardiomyopathy. Circulation 1988;78(5 Pt 1):1192–9.
Merlet P, Valette H, Dubois-Randé JL, Moyse D, Duboc D, Dove P, et al. Prognostic value of cardiac metaiodobenzylguanidine imaging in patients with heart failure. J Nucl Med 1992;33(4):471–7.
Nakata T, Nakajima K, Yamashina S, Yamada T, Momose M, Kasama S, et al. A pooled analysis of multicenter cohort studies of (123)I-mIBG imaging of sympathetic innervation for assessment of long-term prognosis in heart failure. JACC Cardiovasc Imaging 2013;6(7):772–84.
Kasama S, Toyama T, Sumino H, Nakazawa M, Matsumoto N, Sato Y, et al. Prognostic value of serial cardiac 123I-MIBG imaging in patients with stabilized chronic heart failure and reduced left ventricular ejection fraction. J Nucl Med 2008;49(6):907–14.
Sato Y, Kita T, Takatsu Y, Kimura T. Biochemical markers of myocyte injury in heart failure. Heart 2004;90(10):1110–3.
Perna ER, Macin SM, Canella JP, Augier N, Stival JL, Cialzeta JR, et al. Ongoing myocardial injury in stable severe heart failure: value of cardiac troponin T monitoring for high-risk patient identification. Circulation 2004;110(16):2376–82.
Sato Y, Yamada T, Taniguchi R, Nagai K, Makiyama T, Okada H, et al. Persistently increased serum concentrations of cardiac troponin t in patients with idiopathic dilated cardiomyopathy are predictive of adverse outcomes. Circulation 2001;103(3):369–74.
Peacock 4th WF, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008;358(20):2117–26.
Sakhuja R, Green S, Oestreicher EM, Sluss PM, Lee-Lewandrowski E, Lewandrowski KB, et al. Amino-terminal pro-brain natriuretic peptide, brain natriuretic peptide, and troponin T for prediction of mortality in acute heart failure. Clin Chem 2007;53(3):412–20.
Del Carlo CH, O’Connor CM. Cardiac troponins in congestive heart failure. Am Heart J 1999;138(4 Pt 1):646–53.
Taira N. Nicorandil as a hybrid between nitrates and potassium channel activators. Am J Cardiol 1989;63(21):18J–24.
The IONA Study Group. Effect of nicorandil on coronary events in patients with stable angina: the Impact of Nicorandil in Angina (IONA) randomised trial. Lancet 2002;359(9314):1269–75.
Ishii H, Ichimiya S, Kanashiro M, Amano T, Imai K, Murohara T, et al. Impact of a single intravenous administration of nicorandil before reperfusion in patients with ST-segment-elevation myocardial infarction. Circulation 2005;112(9):1284–8.
Kasama S, Toyama T, Iwasaki T, Sumino H, Kumakura H, Minami K, et al. Effects of oral nicorandil therapy on sympathetic nerve activity and cardiac events in patients with chronic heart failure: subanalysis of our previous report using propensity score matching. Eur J Nucl Med Mol Imaging 2014;41(1):144–54.
Lee TM, Lin MS, Chang NC. Effect of pravastatin on sympathetic reinnervation in postinfarcted rats. Am J Physiol Heart Circ Physiol 2007;293(6):H3617–26.
Sanbe A, Marunouchi T, Yamauchi J, Tanonaka K, Nishigori H, Tanoue A. Cardioprotective effect of nicorandil, a mitochondrial ATP-sensitive potassium channel opener, prolongs survival in HSPB5 R120G transgenic mice. PLoS One 2011;6(4):e18922.
Kasama S, Toyama T, Sumino H, Kumakura H, Takayama Y, Minami K, et al. Effects of mineralocorticoid receptor antagonist spironolactone on cardiac sympathetic nerve activity and prognosis in patients with chronic heart failure. Int J Cardiol 2013;167:244–9.
Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, et al. Effects of candesartan on cardiac sympathetic nerve activity in patients with congestive heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol 2005;45(5):661–7.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105(4):539–42.
Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2(5):358–67.
Ishihara S, Koga T, Kaseda S, Nyuta E, Haga Y, Fujishima S, et al. Effects of intravenous nicorandil on the mid-term prognosis of patients with acute heart failure syndrome. Circ J 2012;76(5):1169–76.
Krumenacker M, Roland E. Clinical profile of nicorandil: an overview of its hemodynamic properties and therapeutic efficacy. J Cardiovasc Pharmacol 1992;20 Suppl 3:S93–102.
Yoneyama F, Satoh K, Taira N. Nicorandil increases coronary blood flow predominantly by K-channel opening mechanism. Cardiovasc Drugs Ther 1990;4(4):1119–26.
Lopez JR, Jahangir R, Jahangir A, Shen WK, Terzic A. Potassium channel openers prevent potassium-induced calcium loading of cardiac cells: possible implications in cardioplegia. J Thorac Cardiovasc Surg 1996;112(3):820–31.
Mizumura T, Nithipatikom K, Gross GJ. Effects of nicorandil and glyceryl trinitrate on infarct size, adenosine release, and neutrophil infiltration in the dog. Cardiovasc Res 1995;29(4):482–9.
Matsubara T, Minatoguchi S, Matsuo H, Hayakawa K, Segawa T, Matsuno Y, et al. Three minute, but not one minute, ischemia and nicorandil have a preconditioning effect in patients with coronary artery disease. J Am Coll Cardiol 2000;35(2):345–51.
Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, et al. Effects of nicorandil on cardiac sympathetic nerve activity after reperfusion therapy in patients with first anterior acute myocardial infarction. Eur J Nucl Med Mol Imaging 2005;32(3):322–8.
Neglia D, Michelassi C, Trivieri MG, Sambuceti G, Giorgetti A, Pratali L, et al. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation 2002;105(2):186–93.
Wieland DM, Wu J, Brown LE, Mangner TJ, Swanson DP, Beierwaltes WH. Radiolabeled adrenergic neuron-blocking agents: adrenomedullary imaging with [131I]iodobenzylguanidine. J Nucl Med 1980;21(4):349–53.
Takeishi Y, Atsumi H, Fujiwara S, Takahashi K, Tomoike H. ACE inhibition reduces cardiac iodine-123-MIBG release in heart failure. J Nucl Med 1997;38(7):1085–9.
Toyama T, Hoshizaki H, Seki R, Isobe N, Adachi H, Naito S, et al. Efficacy of carvedilol treatment on cardiac function and cardiac sympathetic nerve activity in patients with dilated cardiomyopathy: comparison with metoprolol therapy. J Nucl Med 2003;44(10):1604–11.
Yamazaki J, Muto H, Kabano T, Yamashina S, Nanjo S, Inoue A. Evaluation of beta-blocker therapy in patients with dilated cardiomyopathy–clinical meaning of iodine 123-metaiodobenzylguanidine myocardial single-photon emission computed tomography. Am Heart J 2001;141(4):645–52.
Burgdorf C, Dendorfer A, Kurz T, Schömig E, Stölting I, Schütte F, et al. Role of neuronal KATP channels and extraneuronal monoamine transporter on norepinephrine overflow in a model of myocardial low flow ischemia. J Pharmacol Exp Ther 2004;309(1):42–8.
Kang CS, Chen CC, Lin CC, Chang NC, Lee TM. Effect of ATP-sensitive potassium channel agonists on sympathetic hyperinnervation in postinfarcted rat hearts. Am J Physiol Heart Circ Physiol 2009;296(6):H1949–59.
Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction, Jaffe AS, Apple FS, et al. Universal definition of myocardial infarction. Circulation 2007;116(22):2634–53.
Pollack Jr CV, Braunwald E. 2007 update to the ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: implications for emergency department practice. Ann Emerg Med 2008;51(5):591–606.
Sato Y, Fujiwara H, Takatsu Y. Cardiac troponin and heart failure in the era of high-sensitivity assays. J Cardiol 2012;60(3):160–7.
Nakajima K, Okuda K, Nyström K, Richter J, Minarik D, Wakabayashi H, et al. Improved quantification of small hearts for gated myocardial perfusion imaging. Eur J Nucl Med Mol Imaging 2013;40(8):1163–70.
Matsuo S, Nakajima K, Okuda K, Kawano M, Ishikawa T, Hosoya T, et al. Standardization of the heart-to-mediastinum ratio of 123I-labelled-metaiodobenzylguanidine uptake using the dual energy window method: feasibility of correction with different camera-collimator combinations. Eur J Nucl Med Mol Imaging 2009;36(4):560–6.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasama, S., Toyama, T., Funada, R. et al. Effects of adding intravenous nicorandil to standard therapy on cardiac sympathetic nerve activity and myocyte dysfunction in patients with acute decompensated heart failure. Eur J Nucl Med Mol Imaging 42, 761–770 (2015). https://doi.org/10.1007/s00259-015-2990-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-2990-0