Abstract
The management of patients with rheumatoid arthritis (RA) has rapidly evolved with the development of newer disease-modifying drugs and the recognition that long-term damage can be mitigated by an earlier and more-informed use of these medications. Historically, radiographs were the mainstay of imaging in RA patients, but radiographic joint narrowing and erosions are late and insensitive findings in the disease. MRI (with intravenous contrast agent) and ultrasound (with power Doppler interrogation) of the hands and wrists are able to demonstrate erosions earlier and with greater sensitivity than radiographs. More importantly, these imaging studies also depict synovitis and active soft-tissue inflammation, which represents a precursor to structural damage. Additionally, MRI can show inflammation within the bones (osteitis), which is proving to be the most important prognosticator of an aggressive disease course. Part I of this review discusses the imaging techniques, pitfalls, definitions, and comparative studies of MRI and ultrasound for identifying and quantifying erosions, synovitis, and osteitis. Part II will demonstrate how these imaging findings influence the clinical management of RA patients throughout their disease course, from presentation through clinical remission.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disorder that targets synovium and affects multiple organ systems. Advanced disease results in irreversible structural damage to joints, which is associated with disability, decreased quality of life, and early mortality [1, 2]. The prevalence in different populations and ethnic groups varies from 0.1% to 5% [3], with worldwide estimates of 1 in 400 people affected [4].
In Part I of this series I review the specifics of performing and interpreting magnetic resonance imaging (MRI) and ultrasound in the hands and wrists of arthritis patients, addressing pitfalls and controversies along the way. The discussion emphasizes three findings: erosions, osteitis, and synovitis. The review also compares and contrasts the accuracy and relative advantages and disadvantages of each modality. Arthritis in large joints, conditions in the axial skeleton, or juvenile-onset disease are not specifically covered, although some of the same concepts apply in those conditions. Part II will illustrate how MRI and ultrasound findings can influence the clinical management of RA at several time points in the disease course: at presentation, during treatment, and at remission.
A fair question is why limit this discussion to imaging of the hands and wrists, given that RA is a systemic disease affecting many joints and organs. First, the hands and wrists are a common high-yield target region in RA with many joints, bones, and synovial surfaces in one region. Although identifying and quantifying cartilage loss is easier in large joints such as the knee [5], erosions are easier to detect in small bones compared with large ones. Synovitis identified in the hands seems to be a reasonable surrogate for the total synovitis burden shown by whole-body MRI examinations [6]. Historically, when radiographs were the primary imaging modality in RA patients, investigators noted more involvement of the foot compared with hand joints in the first 3 years of disease [7], with erosions often appearing earlier in the feet [8]. However, an MRI study of RA patients with symptoms for a mean of 5 months found only rare instances of synovitis or erosions in the feet without these features in the hand or wrist; conversely, 20% of patients had erosions in the wrist or metacarpophalangeal (MCP) joints without erosions in the metatarsophalangeal joints [9]. Overall then, the hand and wrist provide a window into the workings of RA throughout the body; working from this assumption, researchers have created and validated scoring systems and atlases of disease based on imaging of the hand and wrist.
One final question is why a review like the current one is relevant for radiologists. Currently, an overwhelming amount of research being done on the impact of advanced imaging in RA is conducted outside of the USA (primarily in Western Europe, Scandinavia, Japan, and Oceania.) Furthermore, as can be appreciated by scanning the references in this review, most research in this field is published in the rheumatology, not the radiology, literature. The result is that many practicing radiologists have not been exposed to the most up-to-date information on this topic. Thus, a comprehensive review of imaging findings and their implications will help the radiologist performing MRI or ultrasound to provide relevant information that improves the management of RA patients.
Imaging techniques
Ultrasound should be performed using a linear transducer with the highest possible frequency to obtain high-resolution images of the relatively superficial bones and joints. For each joint, the examiner should evaluate each of the bone surfaces (radial, ulnar, dorsal, and volar) that are accessible, recognizing that the visibility of the radial and ulnar portions of the mid-carpal bones and central MCP joints are blocked by the adjacent bones. The synovial membranes and joint contents should be insonated from both the volar and dorsal sides. Graded compression allows distinction of joint fluid from non-compressible synovitis. Power Doppler examination needs to be able to show low levels of flow using a 9-MHz or higher transducer [10]. For the power Doppler examination, the filter should be set for low velocity flow and then the color gain adjusted until noise appears. The gain is then slowly turned down until only flow signal (if present) is shown [11].
The biggest question regarding ultrasound is how many joints to examine. Evidence does not point to an ideal number. Investigations have included as many as 78 separate joints in single patients to evaluate response to drug treatment. Although the ultrasound scores obtained with this method correlate with clinical and laboratory measures of disease activity, the average time for each ultrasound examination is greater than 1 h [12]. Clearly, such a comprehensive approach is not practical for routine clinical use. Others have suggested more limited examinations [13, 14], with as few as seven joints (the dominant wrist, second and third MCP, index and long finger proximal interphalangeal, and unilateral second and fifth metatarsophalangeal joints) constituting a complete study [15].
An MRI examination can be done unilaterally (typically of the dominant or most symptomatic limb) or can include both sides [16]. In either case, the radiofrequency coil diameter should closely match the size of the scanned limb to maximize the signal-to-noise ratio, while at the same time providing the necessary coverage and patient comfort. Typical, commercially available dedicated wrist coils can cover from the distal radio-ulnar joints through the carpometacarpal joints, or from the carpometacarpal joints through the MCP joints, but not both. Flexible surface coils 160–200 mm in length can be wrapped around the limb and provide coverage from the distal radio-ulnar joint through the MCP joints. Alternatively, a whole-volume knee coil can be used, as described in the following paragraph, to provide the equivalent coverage for scanning both sides simultaneously. If one side is imaged, supine positioning with the arm at the side is usually comfortable, but it places the hand near the periphery of the bore where the static field is relatively heterogeneous, and requires a coil that can be positioned next to the patient’s body. Alternatively, the arm can be elevated above the head (usually with the patient prone) allowing the hand and wrist to be placed in a whole volume coil near the magnet isocenter. Sometimes called the “superman” position, in reality lying with the shoulder straight overhead (like the caped crusader) quickly causes severe pain in the shoulder in almost all patients [17], but especially those who may have arthritis in multiple joints. I find that placing as many pillows as possible under the chest to elevate the upper body permits the patient to forward flex at the shoulder and is much more tolerable.
The evaluation of certain findings, such as the progression of erosions, is equivalent if based on MRI examination of one or both sides [18]. However, symmetric synovitis—one of the cardinal features of RA that influences both early diagnosis and prognosis—requires bilateral, contrast-enhanced examination [19,20,21,22,23]. Contrast-enhanced MRI of each side can be performed on separate days [21]; however, this approach is logistically awkward. Alternatively, both hands and wrists can be imaged simultaneously in a supine or prone patient, with both arms raised and immobilized above the head [24], but patients find this position quite uncomfortable [9]. My preferred technique is to allow the patient to lay decubitus, with the shoulders, elbows, hips, and knees comfortably flexed (Fig. 1). The palms are opposed in front and slightly cranial to the patient’s face within a knee coil. Padding and pillows between the patient’s back and the side of the bore, between the knees, and around the hands in the coil allows even patients with multiple painful joints to comfortably remain motionless for the duration of the 20- to 25-min examination. At my institution, we have imaged several hundred RA patients using this “fetal-praying” position with success.
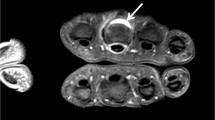
Decubitus patient positioning allowing simultaneous MRI of both hands and wrists. a-c Photographs of a volunteer lying on her side, with shoulders, elbows, hips, and knees flexed. The palms are placed together slightly cranial to the head in an extremity coil (top of the coil removed for demonstration purposes). Note padding and pillows between limbs, and between patient and scanner. d Sagittal scout gradient-echo-recalled image shows uniform signal reception from the distal radio-ulnar through the metacarpophalangeal joints bilaterally. Vitamin E capsule (arrow) is used to denote the right side
The recommended MRI protocol for routine evaluation of an RA patient consists of fat-suppressed T2-weighted (or short tau inversion recovery [STIR]) sequences and T1-weighted pre- and post-intravenous contrast-enhanced sequences (typically using fat suppression after contrast agent administration) in the coronal and transverse planes. A 2D spin-echo or fast-spin-echo sequence in each plane can be used for the T1-weighted images, or a near-isotropic, 3D spoiled gradient-echo-recalled acquisition in one plane can be reconstructed into the other plane [25]. The result should be three sets of images that, interpreted together, can distinguish erosions, osteitis, effusion, synovitis, and tenosynovitis (Fig. 2).
Typical MRI examination in a 56-year-old woman who had had seropositive rheumatoid arthritis for 3 years. A tryptic of transverse series: a T1-weighted, b fat-suppressed T2-weighted, and c intravenous contrast-enhanced fat-suppressed T1-weighted images through the metacarpophalangeal joints showing third metacarpal head erosions (black arrows), metacarpophalangeal synovitis (long white arrows), and flexor tenosynovitis (short white arrows). The left side is displayed inferiorly. d Coronal contrast-enhanced fat-suppressed T1-weighted image of the left side shows additional severe synovitis (long arrow) and erosions in the carpus (short arrows). e Postero-anterior radiograph of the left wrist confirms erosions in the third metacarpal head and the first metacarpal base (arrows), but underestimates the extent of erosions in the wrist. There is no joint space narrowing
Much has been written and debated about the specific requirements for an adequate MRI examination in RA patients. Specific issues include whether intravenous contrast agent is necessary (and if so, is there value in altering the dose, or in scanning during dynamic contrast agent administration), whether T2-weighted or STIR images are necessary in addition to T1-weighted and post-contrast images, and whether low-field scanners can provide the same information as high-field systems. I will address all these questions below (see Additional imaging issues). But the answers are nuanced and largely depend on what specific findings are most important in a given scenario. Therefore, I want to fully describe each imaging finding before returning to these technique questions.
Structural damage, bone inflammation, and soft-tissue inflammation are the critical imaging findings that influence diagnosis, management, prognosis, and follow-up in RA patients. The radiologist needs a reliable way of identifying and rating the extent and severity of each finding for meaningful comparisons over time. In the literature, methods used for scoring radiographic, MRI, and ultrasound findings are primarily used for clinical and drug trials; although these have importance in large populations, they are seldom invoked in the management of an individual patient [26]. Outcome Measures in Rheumatology (OMERACT), a multi-institution study group, is tasked with developing and testing evaluation systems for use in MRI and ultrasound. The OMERACT Rheumatoid Arthritis MRI Scoring (RAMRIS) system is a semiquantitative method that assigns points for various MRI findings [25]. Although the actual assigning of scores is impractical for routine clinical use (see Part II of this review), I find the RAMRIS definitions useful to apply when clinically interpreting a study (without assigning scores), and I discuss them below. The OMERACT working group has developed an analogous scoring system for ultrasound, to which I also refer.
Structural damage
Structural damage represents (mostly) irreversible damage done by the rheumatoid inflammatory process. The main target areas are bone (manifest as erosions), articular cartilage (joint space narrowing), and tendon (rupture). Long-term loss of function seems to be correlated with structural damage [27]. Historically, erosions and joint narrowing were often lumped together, because both are visible on radiographs. MRI and ultrasound are much more sensitive than radiographs for erosions, which are the most relevant feature of structural damage in the hands and wrists.
Erosions
Erosions indicate focal bone destruction, reflecting cumulative damage in RA. Development of erosions is associated with premature mortality, disability, and decreased quality of life [28]. Although less than half of patients have erosions at presentation, erosions develop in most RA patients over time. Less than 2% of patients who meet the 1987 American College of Rheumatology classification criteria for RA (covered in depth in Part II) for 5 or more years truly have non-erosive disease (no erosions visible even by CT, which is considered the most sensitive test and de facto gold standard) [29]. There are two proposed mechanisms of erosion development. The commonly accepted explanation is that activated synovitis (pannus) interacts with articular cartilage producing collagenase, leading to direct destruction of cortical bone, which exposes the underlying marrow to invasion by B-cells and plasma cells, and which also activates osteoblasts inducing new bone formation [26, 30]. This “outside-in” model is supported by data showing that synovitis demonstrated by MRI or ultrasound is associated with future development and progression of erosions [31,32,33,34,35,36]. A second theory suggests that some erosions develop from inflammation inside the bone working outward, in keeping with the observation that even some large bone erosions do not communicate with the articular surface [37]; this model finds support in the research done on osteitis (described below), which reveals that osteitis is the strongest prognosticator of erosive disease in all clinical phases of RA.
Although most patients do not have radiographic erosions at the time of initial diagnosis, almost half do by 1 year and the rate of progression is fastest in the first 2 years of the disease [38, 39]. Radiographically, erosions appear as focal discontinuities in the subchondral bone plate or articular cortex, with or without destruction visible in the underlying trabeculae. The cortical disruption is easiest to recognize when seen in profile; en face erosions appear as focal radiolucent lytic lesions. The traditional way of quantifying radiographic erosions is to grade their subjective visibility or size for a given articular surface and then to total the observations over the number of affected bones as a composite score [40, 41], a method that has high inter- and intra-observer agreement [42]. Computer-aided detection has been suggested to improve recognition and quantification of erosions [43], but is not in widespread use.
Early researchers noted that MRI images of the hands and wrists in patients with RA frequently showed erosions that were not visible radiographically [44, 45]. The reasons for this increased sensitivity are two-fold. Like all tomographic examinations, MRI is free from superimposition of out-of-plane structures. Additionally, MRI uses contrast agent differences in the bone marrow to identify disease, and so is not reliant solely on spatial resolution to identify erosions [46]. The RAMRIS definition of an erosion—a sharply-defined juxta-articular bone lesion that is visible in two orthogonal planes, and which disrupts the bone cortex in at least one (Fig. 3)—shows moderate inter-observer correlation (intraclass correlation coefficients of 0.46–0.85, better for MCP than for wrist joints) with smallest detectable differences of 24–42% [25, 47]. The two-plane criterion is important to ensure that normal contour variations—foramina, nutrient vessels, and ligament attachments that are present in normal, asymptomatic subjects [48, 49]—are not misinterpreted as pathological. Lesion enhancement with intravenous contrast agent is not part of the definition. For OMERACT/RAMRIS grading, individual erosions are assigned a score from 0 to 10 by estimating in 10% increments (0 = no erosion, 1 = 1–10%, 2 = 11–20%, through 10 = 91–100%, with a score of 10 also assigned if joint fusion is present) how large the erosion is compared with a standard assessed bone volume. This comparison volume is defined for long bones such as metacarpals and phalanges as the distal or proximal 1 cm; for carpal bones, it is the entire bone [25, 50]. Compared with CT, MRI is slightly less sensitive, but 87% of erosions seen in the wrist, and 82% of the erosions seen in the MCP joints with the two modalities are concordant and comparable in size [51, 52]. Conversely, MRI is much more sensitive than radiographs (Fig. 2), showing up to seven times more erosions in early arthritis [53, 54]. Fewer than half of the erosions detected by baseline MRI ever become visible radiographically. Estimates range from 21% at 1 year [55] to 41% at 7 years [56], with a median lag of 2 years for those that eventually appear on radiographs [57]. In the MCP joints, erosions on MRI that destroy less than 20% of the bone area are consistently invisible on radiographs [58], but even erosions 1 cm in diameter visible on MRI may be radiographically occult [37].
Wrist erosions in a 51-year-old woman with seropositive rheumatoid arthritis. a Transverse T1-weighted and b coronal fat-suppressed T2-weighted images show subchondral marrow-replacing bone lesions in the trapezoid and capitate (arrows), visible in orthogonal planes and destroying the cortex in at least one. Additional erosions are present in other carpal bones and the distal ulna
It is important to recognize that erosions are not specific to RA, nor are they even pathognomonic of arthritis. MRI examination may demonstrate small erosions in the hands and wrists of some healthy controls (in approximately 2% of the bones) [59] and erosions in normal subjects increase in prevalence with advancing age [60].
The OMERACT consensus ultrasound definition of an erosion has high inter- and intra-observer agreement, and is similar to that for MRI: discontinuity of the intra-articular bone cortex visible in orthogonal planes (Fig. 4) [61, 62]. Compared with CT as the gold standard, ultrasound has a 44% sensitivity for erosions in the MCP joints in RA patients, but detects 95% of the lesions with more than 20% bone loss on CT [63]. The sensitivity of ultrasound is decreased in areas where bone surfaces are not easily accessible, including the radial and ulnar surfaces of the central carpal bones and metacarpal heads [63, 64]. False-positives are 29% with ultrasound compared with micro-CT [65]. One common pitfall is mistaking the normal depression on the dorsal metacarpal head for an erosion [64]. Nevertheless, ultrasound is much more sensitive than radiography for erosion detection, showing approximately 6.5 times as many MCP lesions in early RA, and 3 times as many in established RA [62]. Most studies have found that ultrasound reveals fewer erosions in both baseline and follow-up studies of RA patients compared with MRI [66, 67], although one study of a small number of RA patients found no significant difference in the detection of wrist erosions, and an advantage of ultrasound over MRI for MCP erosions [68].
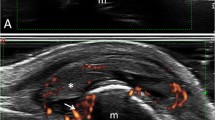
Long finger proximal interphalangeal joint erosions in a 66-year-old woman with both rheumatoid arthritis and gout. a Sagittal and b transverse ultrasound images show disruption of the highly echogenic bone cortex of the proximal phalanx head in two planes (long arrows). A smaller erosion is present in the middle phalanx base (short arrow). c Sagittal Doppler examination shows associated hyperemia
In general, the natural history of erosive disease is that it either remains stable or progresses; thus, it is not surprising that more and larger erosions are found in patient groups with longer-standing disease [29, 69]. The typical RA patient experiences the fastest progression of erosions in the initial 2 years after diagnosis [39] and then their evolution will slow or stop. There is general agreement among experts that repair of erosions can occur [70, 71], albeit rarely. Radiographically, erosion healing in RA patients treated with disease-modifying anti-rheumatic drugs (especially biologic agents) occurs in 7–11% of patients [72, 73] and in approximately 4% of affected joints [74]. MRI studies demonstrate decreased size in a minority (17%) of erosions in RA patients managed with these agents at 1 year, with partial repopulation of the affected bone by marrow fat at 6 years [75, 76]. However, in individual patients, if there is any partial erosion repair, progression and development of new erosions typically occur simultaneously [73].
Joint space narrowing
Joint space narrowing (JSN) is a hold-over term from radiographs indicating that the distance between the visible subchondral bone plate of two articulating bones is less than normal, reflecting loss of the radiolucent articular cartilage. Some linguistic purists object to the phrase, arguing that there is no empty “space” in the gap between the bones. Nevertheless, other anatomical regions in the body—from the space of Retzius to the space of Poirier—are similarly named, despite having tissue contents, and JSN is an accepted descriptor in rheumatological practice. In research studies on RA patients, erosions and JSN in a set number of hand and wrist joints are often tabulated together on radiographs to assign a single (modified Sharp) score [40, 41]. Although useful as one measure of the total visible burden of structural damage, these scores are not used in clinical practice. Furthermore, JSN and erosions represent different unique pathophysiology, cartilage and bone destruction respectively, and data suggest that investigating each component separately may benefit both patients and research. Indeed, clinically, irreversible disability seems to correlate more strongly with JSN than with erosions for patients in remission [77].
In larger joints like the knees, the normal articular cartilage is several millimeters thick and can be assessed relatively easily with morphological cross-sectional imaging techniques. However, in the hands and wrists, the thinness and highly curved contour of the cartilage makes direct visualization and assessment more difficult with MRI and ultrasound compared with high-quality radiographs. Several investigators have demonstrated that it is possible to semiquantitatively assess the thickness of the hyaline cartilage in the hands and wrists with relatively high inter-observer agreement (at least using 3-T MRI scanners with a multichannel receiver coil and 1 mm thick, 3D acquisitions) and that these assessments correlate with radiographic JSN [78,79,80,81]. An MRI scoring system for direct cartilage loss has been validated [82, 83], but has not been formally incorporated into RAMRIS, and is not currently used clinically. Investigational techniques such as delayed gadolinium-enhanced MRI imaging of cartilage and T2 mapping, which reflect the loss of cartilage proteoglycans, have been applied to the MCP joints in RA patients. Interestingly, these studies seem to show that proteoglycan loss is strongly associated with bone inflammation (osteitis), less strongly associated with synovitis, and not necessarily correlated with erosions [84, 85]. This observation supports the notion that bone and cartilage destruction may reflect different pathophysiologies, and that more than one pathway may lead to articular cartilage loss.
Tendon rupture
Tendon rupture in the hand and wrist occurs spontaneously in at least 1% of patients with long-standing RA, although the incidence is likely underestimated [86]. Nevertheless, loss of tendon function has a profound negative impact on hand function, and is difficult to manage surgically once rupture has occurred, especially if multiple tendons are affected. Finger extensor tendons are more commonly torn compared with flexors [87, 88]. The cause can be attritional, owing to the tendons rubbing along the rough surfaces of the eroded carpal bones or distal ulna. Alternatively, proliferative tenosynovitis can directly invade or compress tendons in their sheaths leading to ischemia in addition to ultrastructural changes in the tendon collagen [88,89,90]. Whether de novo inflammation within a tendon occurs in RA is uncertain; if tenosynovitis need not precede tendinitis, it would represent another potential “inside-out” mechanism of damage in RA.
Surprisingly, clinical evaluation for tendon tears in rheumatoid wrists and hands is relatively poor because rupture is insidious and often painless, and the physical signs may be masked by more profound limitations from the joint disease and malalignments. The MRI signs of tendon rupture are the same throughout the body, whether in the hands, rotator cuff, or Achilles’ tendon: disruption of some or all of the fibers, with variable fibrotic tissue and fluid in the tendon gap. The OMERACT group is currently working on consensus ultrasound definitions and an ultrasound scoring system for tendon disease in the hands of rheumatoid patients, and there is relatively high inter- and intra-observer agreement of the ultrasound findings within the wrist and finger tendons [91, 92]. Compared with findings at surgery, the reported sensitivity of MRI for full-thickness extensor tendon tears in patients with longstanding RA varies from 17 to 90% and for ultrasound is 67%, but both imaging techniques have a lower sensitivity for partial tears [86, 89].
Bone inflammation
Osteitis is defined as a region within bone marrow that shows ill-defined MRI signal intensity that is “edematous,” i.e., hypointense on short TR/TE sequences and hyperintense on T2-weighted and STIR sequences, compared with skeletal muscle (Fig. 5) [25]. For research purposes, a score of 1–3 is assigned based on what percentage of the bone (0–100%, in 33% increments) shows the signal changes. Osteitis can coexist with sharply marginated erosions in the same bone, or can manifest in bones without erosions. Although some authors still refer to this finding as “bone marrow edema,” in RA it does not represent simply increased fluid in the marrow. Rather the abnormal bone marrow enhances after intravenous contrast agent administration, and histological studies demonstrate inflammatory cellular infiltrates consisting of macrophages, plasma cells, T-cells, and B-cells [93,94,95]. Thus, the term osteitis, meaning true bone inflammation, is more correct. Of the common imaging modalities, only MRI is able to show osteitis. Radiographs and ultrasound cannot show alterations within the marrow elements, which is a major limitation of ultrasound in the evaluation of RA patients. Likewise, standard CT is insensitive to marrow edema or inflammation, although in the future, dual-energy CT may be able to detect and potentially quantify osteitis. Preliminary pilot studies have investigated this application in patients with gout [96] and RA [97].
Wrist osteitis, synovitis, and tenosynovitis in RA. Same patient as Fig. 3. Transverse a T1-weighted, b fat-suppressed T2-weighted, and c contrast-enhanced fat-suppressed T1-weighted images show an ill-defined reticulated marrow “edema” pattern with contrast enhancement, preserving the trabeculae in the scaphoid (S) and pisiform (P) bones, representing osteitis. The dorsal synovium and flexor tenosynovium in the carpal tunnel are severely thickened and enhancing. Note that the erosion in the triquetrum (black arrow) enhances, whereas those in the lunate (white arrows) do not; enhancement is not part of the definition of erosions
At least three associations make osteitis one of the most important clinical findings in RA. First, unlike erosions (and as will be seen, synovitis) osteitis is fairly specific for inflammatory arthritis: with the exception of one small nonblinded study [48], osteitis is not found in healthy, asymptomatic control subjects [59]. Second, osteitis is a marker for patients who manifest a particularly aggressive, severe disease phenotype. In early RA (less than 2 years’ duration), patients with osteitis in the hand or wrist have higher degrees of positivity in all other imaging, serological, and genetic indicators of disease, and are more likely to have positive serological markers compared with those without the MRI finding [98, 99]. Third, because it does portend an aggressive course, osteitis is the single strongest independent predictor (of all imaging, immunological, and clinical variables) of future negative outcomes—especially the development and progression of structural disease [100,101,102,103]. Interestingly, future erosions often develop in the same bones that previously showed osteitis [100], supporting osteitis as a precursor lesion for at least some erosions [95]. One mechanism for this “inside-out” development of erosions is the release of cytokines by the intraosseous inflammatory cells, which then stimulate osteoclasts to resorb bone [101, 104].
Soft-tissue inflammation
Synovitis
Synovitis, defined as inflammation of the lining of the joints, is one of the hallmark findings in RA. Although very early in the disease (in the first 2 months of symptoms), synovitis is more common in the foot metatarsophalangeal joints than in the MCP joints, by 6 months 90% of RA patients have MRI evidence of synovitis in the MCP and/or wrist joints [105]. The clinical assessment of synovitis is relatively insensitive, both at presentation and especially when assessing for remission [106,107,108,109]. The examiner palpates individual joints and records whether each is subjectively swollen, tender, or both. A clinical disease activity score is then assigned by counting the number of affected joints [110, 111].
Early studies with intravenous contrast-enhanced MRI in patients with established RA showed that both focal and diffuse enhancement occurs in the synovium [112]. Furthermore, in patients with early arthritis, dynamic contrast-enhanced MRI shows early synovial enhancement compared with control subjects [113]. The OMERACT definition of synovitis is the presence of synovium that is both thicker than normal (the normal synovial membrane is barely perceptible) and that shows “above normal enhancement” after intravenous contrast agent administration (Figs. 2, 5) [25]. The phrase denoting enhancement greater than normal implies that the normal synovial membrane shows some enhancement. One study of healthy control subjects found minimal enhancement confined only to the prestyloid recess in nonthickened synovium [114], which would not meet the consensus definition of synovitis. A second study depicted only minimal early synovial enhancement on dynamic, contrast-enhanced MRI images in less than 10% of MCP and wrist joints in control subjects [59]. Of note, most of the patients who showed enhancing synovium in this study actually had elevated C-reactive protein levels, suggesting systemic inflammation, despite their “healthy” label. The degree of enhancement in both of these studies would be scored as 0–1 in the semiquantitative RAMRIS system, where the amount of synovitis is subjectively assigned a rating from 0 (none) to 3 (severe) [25]; in my experience, most RA patients with active synovitis show enhancing synovium that is unequivocally brighter and thicker than normal in multiple compartments. Additionally, the cut-off for the presence/absence of synovitis in most published research studies is a RAMRIS score of 2 or higher.
Magnetic resonance imaging and clinical evidence for synovitis are strongly correlated and concordant in approximately 60% of joints [106, 107]. However, MRI appears to be much more sensitive. In a cohort of RA patients with a median disease duration of 5 months, 64% of the patients whose MRI studies showed bilateral synovitis without erosions or osteitis had no tender or swollen joints on physical examination [107]. Another study showed that MRI revealed ten times the number of MCP joints with synovitis in patients with active RA compared with clinical examination. In 105 total wrist and hand joints, synovitis was found on MRI, but not by clinical examination; the converse (clinical examination positive, MRI negative) was true for only two joints [106].
The consensus ultrasound definition of synovitis is intra-articular tissue that is typically (but not always) hypoechoic compared with subcutaneous fat, and that is nondisplaceable and poorly compressible (Fig. 6) [61]. Lack of compressibility is important to distinguish synovitis from simple joint fluid. Synovitis may or may not exhibit signal with power Doppler interrogation, and in research studies the incidence of synovitis on grayscale and Doppler images is often analyzed separately. Experimentally, administration of an intravascular ultrasound contrast agent that contains micro-air bubbles may enhance the Doppler signal in inflamed synovium [115]. Although it is tempting to consider Doppler signal as an indication of active disease, the sensitivity of this finding is uncertain. Biopsies of Doppler-positive synovium in patients with mainly long-standing RA have shown histological evidence for synovitis and immunohistochemical staining for increased vascularity [116]. However, in the same study, biopsy of Doppler-negative areas also often showed pathological synovitis (i.e., a negative Doppler examination does not rule out active synovitis). Power Doppler ultrasound is more sensitive and specific than physical examination for detecting synovitis in the MCP joints (with contrast-enhanced MRI as the standard) [11], and a systematic review of 23 peer-reviewed articles confirms high inter- and intra-observer agreement among highly trained operators for interpretation of the Doppler images [117]. But at least in patients with early arthritis, baseline examination with MRI detects more synovitis than Doppler ultrasound, especially in the wrist and MCP joints [16]. Unlike on MRI, it may be difficult at times to distinguish synovium from adjacent cartilage sonographically. Other ultrasound pitfalls include motion and electronic noise, which can result in spurious Doppler signals, and over-compression, which can obscure them [64].
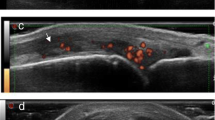
Second MCP synovitis in a 58-year-old woman with RA. a Sagittal grayscale ultrasound shows tissue with medium echogenicity in the dorsal recess of the MCP (asterisk). This material was not compressible. b Power Doppler image shows severe hyperemia of the tissue. The apparent Doppler signals that map to the bone cortex and subchondral bone are artefactual
As synovitis is a measure of disease activity, a reliable and reproducible method of quantifying it is highly desirable to apply to follow-up examinations. Essentially, there are three possible ways of accomplishing this task using MRI. The current de facto standard is the semiquantitative method of rating the synovitis in each joint on a subjective 0–3 scale, and adding the scores for multiple joints [25]. When observers apply this method after intense training and with the use of a standardized atlas [50], interobserver reliability is good-to-moderate (though worse than that reported for erosions and osteitis); the smallest detectable change is estimated to be 26–35%, which may not be sensitive enough for all patients [118,119,120,121]. Experimental studies frequently use two observers for each examination because of the inherent variability [105]. The training requirements, interpretation time, and need for double reading make the RAMRIS synovitis score impractical for routine clinical use [122,123,124]. One proposed way to decrease observer times for semiquantitative rating is to create coronal, maximum intensity projections from dynamic or static contrast-enhanced images that provide a visual overview of the involved joints (and tendon sheaths) [125, 126]. Although this technique usually involves first subtracting precontrast images, it can also be done without subtraction, allowing a time saving by eliminating the need to acquire a matching precontrast sequence (Fig. 7).
Polyarticular synovitis in a 29-year-old woman with seronegative RA. Coronal maximum intensity projection made from a set of contrast-enhanced fat-suppressed T1-weighted images gives an overview of the amount, distribution, and severity of the synovitis in the intercarpal, MCP, and thumb’s interphalangeal joints
A second approach to quantifying synovitis on MRI is to sum the volume of enhancing synovium in a given joint or region. The volume of MR-determined synovitis does correlate with later progression rates of erosions, but volume determination requires manual outlining of regions on dozens of transverse images [32], which is again not practical for clinical use. The procedure can be simplified by only analyzing three transverse images and using computer-assisted manual segmentation; volumes calculated in this way show good inter-observer agreement and strong correlation with RAMRIS synovitis scores, but even for three images, the manual outlining still takes longer than assigning semiquantitative scores [127]. Automated software algorithms have also been developed to estimate synovitis volumes on MRI. Although these result in measurements that also correlate with RAMRIS scores [81], the programs are currently proprietary and not available for individual patient assessment.
A final approach is to administer intravenous contrast agent dynamically and analyze the enhancement kinetics (e.g., the time to enhancement onset, the rate of early enhancement, or the relative amounts of enhancement). In the knee joint, the overall enhancement rate 30–60 s after contrast agent administration does correlate with histological findings of active synovitis, but extensive regional variation and heterogeneity of inflammation limit correlations of smaller synovial sections [128]. This spatial variation affects the use of dynamic contrast analysis in the hands and wrists, where results vary depending on the precise placement of regions of interest [129, 130]. Again, automated software that analyzes the enhancement kinetics of every voxel has been developed to reduce this variability [131, 132], but in reality, results derived from dynamic contrast analysis do not seem to have an advantage over those that are based on static images made 2–5 min after contrast agent administration [124].
Quantifying synovitis for temporal comparison in individual patients is even more challenging with ultrasound. Reproducibly calculating synovial volumes is not possible on ultrasound images and it is difficult to compare the degree of synovitis during scanning. These factors make ultrasound less well suited compared with MRI for sequential examinations in RA patients. Currently, the only way to quantify the synovitis burden in a patient using ultrasound is to assign a subjective severity rating to each joint based on its gray-scale or power Doppler appearance and then sum these ratings over a standard number of joints [133]. The ideal number of joints to evaluate is not clear—various studies have investigated from 6 to 44 separate joints in single patients [108, 134]. Assessing more joints has the potential to detect smaller changes over time (although this has not been proved), but increases the examination time to a point where it would be prohibitive to perform for clinical purposes.
Tenosynovitis
Tenosynovitis, inflammation in the lining of the tendon sheaths, is an important ancillary finding in RA, and may appear before synovitis [135]. A small amount of fluid in the tendon sheaths can occur in healthy subjects. An abnormal amount of fluid in the hands or wrist is typically defined by either distension of a sheath greater than 1 mm or fluid diameter greater than the tendon diameter in a sheath on a T2-weighted MRI sequence [89]. As is the case for synovitis in a joint, intravenous contrast agent enhancement is needed to distinguish a tendon sheath effusion from tenosynovitis (Fig. 8). The same MRI criteria apply: tenosynovitis is tissue thicker than the normal tenosynovial membrane [136, 137]. The ultrasound criteria for tenosynovitis are also analogous to the consensus definition of synovitis. Tenosynovitis is characterized by hypoechoic or anechoic thickened tissue within a tendon sheath, visible in two perpendicular planes, with or without fluid in the sheath, and with or without Doppler signal [61]. Applying this definition results in good-to-excellent inter- and intra-observer agreement with trained examiners [92]. MRI appears to be more sensitive than ultrasound for detecting tenosynovitis, at least in patients presenting early in the course of disease [16].
Tenosynovitis in a 50-year-old man with RA. a Transverse fat-suppressed T2-weighted image shows high signal material in the carpal tunnel (asterisk) and extensor carpi ulnaris tendon sheath (arrow). b Contrast-enhanced fat-suppressed T1-weighted image shows enhancing tenosynovitis in the extensor carpi ulnaris sheath (arrow). In the flexor tendon compartment, the synovium is of normal thickness and the contents of the tendon sheaths do not enhance (asterisk), representing a simple effusion. Active synovitis is present in the distal radio-ulnar joint
The prevalence of tenosynovitis varies at different stages of disease. Overall, approximately 20% of a heterogeneous group of RA patients with different disease duration had evidence of tenosynovitis on ultrasound [92]. The extensor carpi ulnaris tendon sheath is most frequently involved at 1 and 6 years’ disease duration [75]. But in early arthritis, flexor tenosynovitis is more common than extensor tenosynovitis [138]. The reason for this discrepancy is unclear. It may be that flexor tendon sheath disease is transient by nature, or perhaps that flexor tenosynovitis has a better response to treatment than other inflamed sites.
Additional imaging issues
Before proceeding to explain how the imaging findings can influence clinical management over the disease course of RA, it is useful to address some common imaging questions and controversies.
Is intravenous contrast necessary for MRI?
In a word, yes. Injecting (gadolinium-based) intravenous contrast agent does add cost, time, and discomfort to the examination, may pose a logistic challenge in some practices, and carries a small risk of allergic reactions and nephrogenic systemic sclerosis. But it greatly enhances the assessment of disease activity. In patients with early arthritis, the sensitivity and specificity for identifying synovitis and tenosynovitis are lower when using T2-weighted sequences alone compared with contrast-enhanced images, although the same is not true for detecting erosions or osteitis [139,140,141,142]. Using T2-weighted images alone tends to overestimate synovitis and tenosynovitis, because simple effusions may not be distinguishable from inflamed tissues (Fig. 8) [17]. And unenhanced T1-weighted images cannot distinguish active synovitis from fibrotic pannus in burned-out disease [105]. In one study, synovitis was evident in 50% more of the early arthritis patients who eventually developed RA on contrast-enhanced MRI studies compared with unenhanced scans (even on a 3-T scanner), with contrast-enhanced images identifying nearly twice as many patients with tenosynovitis [143]. There is one caveat, however: although not in widespread use clinically or part of the OMERACT methodology, preliminary data suggest that diffusion-weighted sequences might be able to distinguish fluid from synovitis without the need for intravenous contrast agent. Specifically, the measured apparent diffusion coefficient is lower in joint contents corresponding to enhancing synovitis compared with regions where unenhanced effusions are present [144, 145].
How much contrast agent should be given?
Contrast enhancement is frequently less conspicuous on low-field magnets (0.2 T). Doubling the “typical” dose of gadolinium-based contrast agent (from 0.1 mmol/kg body weight to 0.2 mmol) can increase the assigned synovitis score on low-field scanners [146], but most studies performed with a standard dose of contrast agent show similar scores when compared with high-field MRI [147,148,149]. In fact, “half-dose” (0.05 mmol/kg of body weight) contrast agent results in diagnostically equivalent images for scoring synovitis and tenosynovitis compared with full dose at 0.2 T [150] and 3 T [151]. In short, it should not be necessary for most practices to change their contrast agent dosing schedules for RA patients, regardless of the equipment used, and a standard 0.1-mmol/kg dose of any gadolinium-based agent should suffice.
Is dynamic administration of contrast agent needed?
Without automated software capable of separately analyzing every synovial voxel, dynamic enhancement studies require the user to manually select a region of interest to investigate, which has been shown to be an important source of variation [129, 130]. Most objective measures of enhancement kinetics (such as the rate of early enhancement and the relative amount of overall enhancement) do correlate strongly with clinical measures of disease activity in early arthritis; however, the same is true for RAMRIS synovitis scores, which are determined on static, post-contrast images [152]. Although calculating measures of enhancement kinetics has been suggested as a useful tool for following treated RA patients, the sensitivity to change appears to be similar to that for parameters based on static contrast enhancement [31, 153, 154]. For clinical assessment, simply administering contrast agent and immediately obtaining transverse and coronal sequences lasting 4–5 min each probably provides adequate information. Is there a delay that is too long? Although theoretically, contrast agent could “leak” from inflamed synovium into a joint effusion over time and falsely elevate the apparent amount of enhancing tissue, even in the knee joint, where synovitis and effusion often coexist, the border between the two remains defined for at least 11 min after intravenous contrast agent administration [155].
Are T2-weighted or STIR sequences necessary?
Here the answer is maybe. In early and established RA, contrast-enhanced images are equivalent to T2-weighted images for identifying osteitis [156]. However, eliminating T2-weighted images would result in missing unenhanced findings such as effusions, ganglia, and possibly rheumatoid nodules and burned-out fibrotic synovitis. Additionally, erosions need not enhance. In an RA cohort that incompletely responded to methotrexate, T2-weighted images demonstrated some erosions that were concordantly shown on CT images, but that were not always visible on contrast-enhanced images (although the converse was also true) [157]. Presumably, though, these erosions would be detected on T1-weighted images.
Is a low-field, dedicated extremity magnet sufficient?
This question is harder to answer, and the response probably depends on the indication for the study and what finding is most important in a given patient. Most studies looking at the predictive value of MRI findings have been based on high-field (1.5-T or greater) scanners [122]. There are several 0.2-T dedicated extremity machines available. Manufacturers are actively marketing these to rheumatologists, who can site them in the office or clinic and then offer limited MRI examinations as an adjunct to their clinical assessments. Advantages of these dedicated extremity scanners include reduced initial and operating costs, fewer issues with siting (including shielding, need for cryogens, space, and weight), less risk from projectiles and implanted devices, and better acceptance by some patients (because of the less painful and constrictive position, low noise, and eliminated claustrophobia concerns) [158]. Offsetting disadvantages include the lower signal-to-noise ratio (because of the lower static magnetic field), a smaller useable field of view (typically limited to either a unilateral wrist or hand examination, but not both), and longer acquisition times (which may prohibit dynamic contrast enhancement studies). The lower magnet field (and higher heterogeneity) compared with high-field magnets means that chemical (frequency-selective) fat suppression is unreliable on these machines. Although STIR sequences can be used to suppress fat signal, STIR sequences have inherently lower signal-to-noise ratios and efficiency (number of slices obtainable per TR period) compared with fast spin-echo sequences, and cannot be used following contrast agent administration because gadolinium works by shortening T1, which decreases the signal on a STIR sequence.
Several studies have compared results using dedicated 0.2-T or 0.25-T scanners with systems using 1-T or higher magnetic fields. In general, when intravenous contrast agent is used, studies performed on low-field machines are able to identify approximately 90% of the synovitis seen on high-field examinations, with comparable estimates of synovial volume involved [137, 147, 148], although the low-field studies may require two separate scans for the same anatomical coverage [158]. Agreement between low- and high-field MRI examinations is lower when analyzing parameters of dynamic enhancement [148]. Additionally, when contrast agent is not used, sensitivity for synovitis is lower for examinations performed on low-field compared with high-field systems [139]. Erosion detection using low-field equipment that is optimized for spatial resolution is also similar to that achieved with high-field scanners [137, 147, 159], and erosions are detectable on a 0.2-T scanner even when contrast agent is not used or on scanners that can only image two MCP joints [160, 161]. However, disagreements are common between low- and high-field examinations investigating osteitis, with lower sensitivity for this finding at the lower field strengths [147, 159].
An American College of Rheumatology task force critically examined the literature on dedicated extremity MRI studies in RA patients, and found few data supporting their clinical utility. The small number of studies comparing different magnet systems often did not use optimized techniques, introducing potential bias that could falsely elevate the apparent accuracy of the extremity scanners [122]. There is one commercially available high-field dedicated extremity magnet. One study investigating patients with established RA found excellent agreement comparing contrast-enhanced examinations on this system compared with a whole-body, high-field system for RAMRIS scoring of synovitis, osteitis, and erosions. The extremity scanner studies used thinner sections (2 mm vs 3 mm for the whole-body scanner) and a STIR sequence in lieu of a fat-suppressed fast spin echo sequence. The examination times were longer but most patients found being positioned in a chair for the dedicated extremity scan more comfortable than being examined with the arm raised above the head. However, the field-of-view only allowed examination of either the MCP joints or the wrist, but not both, in a single scan [149].
Summary
Three major imaging findings in the hands and wrists—synovitis, osteitis, and erosions—have specific definitions, and together provide information that complements clinical evaluation and radiographs in the evaluation of rheumatoid arthritis patients. Synovitis indicates active disease. Both MRI (with intravenous contrast agent) and ultrasound (with power Doppler interrogation) are more sensitive than clinical examination for detecting synovitis. MRI is probably more sensitive than ultrasound, and there are more potential ways to reliably quantify the amount or severity of synovitis with MRI, making it an ideal candidate for sequentially evaluating patients during treatment and in clinical remission. Osteitis represents inflammation in bone and indicates high-risk disease with the potential for rapid, progressive structural damage. Osteitis can only be visualized by MRI (and evidence suggests that high-field systems might be more sensitive); it is occult on ultrasound, radiographs, and conventional CT studies. Erosions are a manifestation of cumulative damage by inflammation—potentially both from the synovium and from inside the bone. Both ultrasound and MRI are much more sensitive than radiographs for detecting erosions.
Part II of this review will discuss how these cardinal MRI and ultrasound findings, together with clinical and laboratory features, can influence the management of patients with RA. Scenarios discussed include the use of advanced imaging at initial presentation, during treatment to evaluate response and remission, and possibly to individualize management based on specific imaging findings.
Abbreviations
- JSN:
-
Joint space narrowing
- MCP:
-
Metacarpophalangeal
- OMERACT:
-
Outcome Measures in Rheumatology
- RA:
-
Rheumatoid arthritis
- RAMRIS:
-
Rheumatoid Arthritis MRI Scoring
References
Katz PP. The impact of rheumatoid arthritis on life activities. Arthritis Care Res. 1995;8(4):272–8.
Pincus T, Callahan LF, Sale WG, Brooks AL, Payne LE, Vaughn WK. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27(8):864–72.
Hochberg MC, Spector TD. Epidemiology of rheumatoid arthritis: update. Epidemiol Rev. 1990;12:247–52.
Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1316–22.
Van der Heijde D. Erosions versus joint space narrowing in rheumatoid arthritis: what do we know? Ann Rheum Dis. 2011;70(Suppl 1):i116–8.
Kamishima T, Fujieda Y, Atsumi T, Mimura R, Koike T, Terae S, et al. Contrast-enhanced whole-body joint MRI in patients with unclassified arthritis who develop early rheumatoid arthritis within 2 years: feasibility study and correlation with MRI findings of the hands. AJR Am J Roentgenol. 2010;195(4):W287–92.
Van der Heijde DM, van Leeuwen MA, van Riel PL, Koster AM, van’t Hof MA, van Rijswijk MH, et al. Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum. 1992;35(1):26–34.
Brook A, Corbett M. Radiographic changes in early rheumatoid disease. Ann Rheum Dis. 1977;36(1):71–3.
Boutry N, Larde A, Lapegue F, Solau-Gervais E, Flipo RM, Cotten A. Magnetic resonance imaging appearance of the hands and feet in patients with early rheumatoid arthritis. J Rheumatol. 2003;30(4):671–9.
Grassi W, Gaywood I, Pande I, Filippucci E. From DAS 28 to SAS 1. Clin Exp Rheumatol. 2012;30(5):649–51.
Szkudlarek M, Court-Payen M, Strandberg C, Klarlund M, Klausen T, Ostergaard M. Power Doppler ultrasonography for assessment of synovitis in the metacarpophalangeal joints of patients with rheumatoid arthritis: a comparison with dynamic magnetic resonance imaging. Arthritis Rheum. 2001;44(9):2018–23.
Hammer HB, Sveinsson M, Kongtorp AK, Kvien TK. A 78-joints ultrasonographic assessment is associated with clinical assessments and is highly responsive to improvement in a longitudinal study of patients with rheumatoid arthritis starting adalimumab treatment. Ann Rheum Dis. 2010;69(7):1349–51.
Naredo E, Valor L, De la Torre I, Martinez-Barrio J, Hinojosa M, Aramburu F, et al. Ultrasound joint inflammation in rheumatoid arthritis in clinical remission: how many and which joints should be assessed? Arthritis Care Res (Hoboken). 2013;65(4):512–7.
Dougados M, Jousse-Joulin S, Mistretta F, d’Agostino MA, Backhaus M, Bentin J, et al. Evaluation of several ultrasonography scoring systems for synovitis and comparison to clinical examination: results from a prospective multicentre study of rheumatoid arthritis. Ann Rheum Dis. 2010;69(5):828–33.
Backhaus M, Ohrndorf S, Kellner H, Strunk J, Backhaus TM, Hartung W, et al. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: a pilot project. Arthritis Rheum. 2009;61(9):1194–201.
Navalho M, Resende C, Rodrigues AM, Pereira da Silva JA, Fonseca JE, Campos J, et al. Bilateral evaluation of the hand and wrist in untreated early inflammatory arthritis: a comparative study of ultrasonography and magnetic resonance imaging. J Rheumatol. 2013;40(8):1282–92.
Boesen M, Ostergaard M, Cimmino MA, Kubassova O, Jensen KE, Bliddal H. MRI quantification of rheumatoid arthritis: current knowledge and future perspectives. Eur J Radiol. 2009;71(2):189–96.
Ejbjerg BJ, Vestergaard A, Jacobsen S, Thomsen HS, Ostergaard M. The smallest detectable difference and sensitivity to change of magnetic resonance imaging and radiographic scoring of structural joint damage in rheumatoid arthritis finger, wrist, and toe joints: a comparison of the OMERACT rheumatoid arthritis magnetic resonance imaging score applied to different joint combinations and the Sharp/van der Heijde radiographic score. Arthritis Rheum. 2005;52(8):2300–6.
Tamai M, Kawakami A, Uetani M, Takao S, Arima K, Iwamoto N, et al. A prediction rule for disease outcome in patients with undifferentiated arthritis using magnetic resonance imaging of the wrists and finger joints and serologic autoantibodies. Arthritis Rheum. 2009;61(6):772–8.
Tamai M, Kawakami A, Uetani M, Takao S, Rashid H, Tanaka F, et al. Early prediction of rheumatoid arthritis by serological variables and magnetic resonance imaging of the wrists and finger joints: results from prospective clinical examination. Ann Rheum Dis. 2006;65(1):134–5.
Sugimoto H, Takeda A, Hyodoh K. Early-stage rheumatoid arthritis: prospective study of the effectiveness of MR imaging for diagnosis. Radiology. 2000;216(2):569–75.
Tamai M, Kita J, Nakashima Y, Suzuki T, Horai Y, Okada A, et al. Combination of MRI-detected bone marrow oedema with 2010 rheumatoid arthritis classification criteria improves the diagnostic probability of early rheumatoid arthritis. Ann Rheum Dis. 2014;73(12):2219–20.
Ji L, Li G, Xu Y, Zhou W, Zhang Z. Early prediction of rheumatoid arthritis by magnetic resonance imaging in the absence of anti-cyclic citrullinated peptide antibodies and radiographic erosions in undifferentiated inflammatory arthritis patients: a prospective study. Int J Rheum Dis. 2015;18(8):859–65.
Boutry N, Hachulla E, Flipo RM, Cortet B, Cotten A. MR imaging findings in hands in early rheumatoid arthritis: comparison with those in systemic lupus erythematosus and primary Sjogren syndrome. Radiology. 2005;236(2):593–600.
Ostergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30(6):1385–6.
Farrant JM, Grainger AJ, O’Connor PJ. Advanced imaging in rheumatoid arthritis. II. Erosions. Skeletal Radiol. 2007;36(5):381–9.
Kirwan JR. Conceptual issues in scoring radiographic progression in rheumatoid arthritis. J Rheumatol. 1999;26(3):720–5.
Ollier WE, Harrison B, Symmons D. What is the natural history of rheumatoid arthritis? Best Pract Res Clin Rheumatol. 2001;15(1):27–48.
Amaya-Amaya J, Calixto OJ, Saade-Lemus S, Calvo-Paramo E, Mantilla RD, Rojas-Villarraga A, et al. Does non-erosive rheumatoid arthritis exist? A cross-sectional analysis and a systematic literature review. Semin Arthritis Rheum. 2015;44(5):489–98.
Jimenez-Boj E, Redlich K, Turk B, Hanslik-Schnabel B, Wanivenhaus A, Chott A, et al. Interaction between synovial inflammatory tissue and bone marrow in rheumatoid arthritis. J Immunol. 2005;175(4):2579–88.
Huang J, Stewart N, Crabbe J, Robinson E, McLean L, Yeoman S, et al. A 1-year follow-up study of dynamic magnetic resonance imaging in early rheumatoid arthritis reveals synovitis to be increased in shared epitope-positive patients and predictive of erosions at 1 year. Rheumatology (Oxford). 2000;39(4):407–16.
Ostergaard M, Hansen M, Stoltenberg M, Gideon P, Klarlund M, Jensen KE, et al. Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42(5):918–29.
McQueen FM, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan PL, et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Ann Rheum Dis. 1999;58(3):156–63.
Boyesen P, Haavardsholm EA, Ostergaard M, van der Heijde D, Sesseng S, Kvien TK. MRI in early rheumatoid arthritis: synovitis and bone marrow oedema are independent predictors of subsequent radiographic progression. Ann Rheum Dis. 2011;70(3):428–33.
Dougados M, Devauchelle-Pensec V, Ferlet JF, Jousse-Joulin S, D’Agostino MA, Backhaus M, et al. The ability of synovitis to predict structural damage in rheumatoid arthritis: a comparative study between clinical examination and ultrasound. Ann Rheum Dis. 2013;72(5):665–71.
Conaghan PG, O’Connor P, McGonagle D, Astin P, Wakefield RJ, Gibbon WW, et al. Elucidation of the relationship between synovitis and bone damage: a randomized magnetic resonance imaging study of individual joints in patients with early rheumatoid arthritis. Arthritis Rheum. 2003;48(1):64–71.
Tehranzadeh J, Ashikyan O, Dascalos J, Dennehey C. MRI of large intraosseous lesions in patients with inflammatory arthritis. AJR Am J Roentgenol. 2004;183(5):1453–63.
Fex E, Jonsson K, Johnson U, Eberhardt K. Development of radiographic damage during the first 5-6 yr of rheumatoid arthritis. A prospective follow-up study of a Swedish cohort. Br J Rheumatol. 1996;35(11):1106–15.
Van der Heijde DM. Joint erosions and patients with early rheumatoid arthritis. Br J Rheumatol. 1995;34(Suppl 2):74–8.
Sharp JT, Young DY, Bluhm GB, Brook A, Brower AC, Corbett M, et al. How many joints in the hands and wrists should be included in a score of radiologic abnormalities used to assess rheumatoid arthritis? Arthritis Rheum. 1985;28(12):1326–35.
Van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27(1):261–3.
Genant HK, Jiang Y, Peterfy C, Lu Y, Redei J, Countryman PJ. Assessment of rheumatoid arthritis using a modified scoring method on digitized and original radiographs. Arthritis Rheum. 1998;41(9):1583–90.
Langs G, Peloschek P, Bischof H, Kainberger F. Model-based erosion spotting and visualization in rheumatoid arthritis. Acad Radiol. 2007;14(10):1179–88.
Beltran J, Caudill JL, Herman LA, Kantor SM, Hudson PN, Noto AM, et al. Rheumatoid arthritis: MR imaging manifestations. Radiology. 1987;165(1):153–7.
Gilkeson G, Polisson R, Sinclair H, Vogler J, Rice J, Caldwell D, et al. Early detection of carpal erosions in patients with rheumatoid arthritis: a pilot study of magnetic resonance imaging. J Rheumatol. 1988;15(9):1361–6.
Yocum DE, Conaghan PG, Olech E, Peterfy CG. Response: office-based low-field extremity magnetic resonance imaging: is the glass half empty or half full? Arthritis Rheum. 2006;54(4):1048–50.
McQueen F, Lassere M, Edmonds J, Conaghan P, Peterfy C, Bird P, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Summary of OMERACT 6 MR imaging module. J Rheumatol. 2003;30(6):1387–92.
Robertson PL, Page PJ, McColl GJ. Inflammatory arthritis-like and other MR findings in wrists of asymptomatic subjects. Skeletal Radiol. 2006;35(10):754–64.
McQueen F, Ostergaard M, Peterfy C, Lassere M, Ejbjerg B, Bird P, et al. Pitfalls in scoring MR images of rheumatoid arthritis wrist and metacarpophalangeal joints. Ann Rheum Dis. 2005;64(Suppl 1):i48–55.
Ostergaard M, Edmonds J, McQueen F, Peterfy C, Lassere M, Ejbjerg B, et al. An introduction to the EULAR-OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis. 2005;64(Suppl 1):i3–7.
Dohn UM, Ejbjerg BJ, Hasselquist M, Narvestad E, Court-Payen M, Szkudlarek M, et al. Rheumatoid arthritis bone erosion volumes on CT and MRI: reliability and correlations with erosion scores on CT. MRI and radiography Ann Rheum Dis. 2007;66(10):1388–92.
Perry D, Stewart N, Benton N, Robinson E, Yeoman S, Crabbe J, et al. Detection of erosions in the rheumatoid hand; a comparative study of multidetector computerized tomography versus magnetic resonance scanning. J Rheumatol. 2005;32(2):256–67.
Klarlund M, Ostergaard M, Jensen KE, Madsen JL, Skjodt H, Lorenzen I. Magnetic resonance imaging, radiography, and scintigraphy of the finger joints: one year follow up of patients with early arthritis. The TIRA Group. Ann Rheum Dis. 2000;59(7):521–8.
McQueen FM, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan PL, et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals a high prevalence of erosions at four months after symptom onset. Ann Rheum Dis. 1998;57(6):350–6.
McQueen FM, Benton N, Crabbe J, Robinson E, Yeoman S, McLean L, et al. What is the fate of erosions in early rheumatoid arthritis? Tracking individual lesions using x rays and magnetic resonance imaging over the first two years of disease. Ann Rheum Dis. 2001;60(9):859–68.
Scheel AK, Hermann KG, Ohrndorf S, Werner C, Schirmer C, Detert J, et al. Prospective 7 year follow up imaging study comparing radiography, ultrasonography, and magnetic resonance imaging in rheumatoid arthritis finger joints. Ann Rheum Dis. 2006;65(5):595–600.
Ostergaard M, Hansen M, Stoltenberg M, Jensen KE, Szkudlarek M, Pedersen-Zbinden B, et al. New radiographic bone erosions in the wrists of patients with rheumatoid arthritis are detectable with magnetic resonance imaging a median of two years earlier. Arthritis Rheum. 2003;48(8):2128–31.
Ejbjerg BJ, Vestergaard A, Jacobsen S, Thomsen H, Ostergaard M. Conventional radiography requires a MRI-estimated bone volume loss of 20% to 30% to allow certain detection of bone erosions in rheumatoid arthritis metacarpophalangeal joints. Arthritis Res Ther. 2006;8(3):R59.
Ejbjerg B, Narvestad E, Rostrup E, Szkudlarek M, Jacobsen S, Thomsen HS, et al. Magnetic resonance imaging of wrist and finger joints in healthy subjects occasionally shows changes resembling erosions and synovitis as seen in rheumatoid arthritis. Arthritis Rheum. 2004;50(4):1097–106.
Olech E, Crues JV 3rd, Yocum DE, Merrill JT. Bone marrow edema is the most specific finding for rheumatoid arthritis (RA) on noncontrast magnetic resonance imaging of the hands and wrists: a comparison of patients with RA and healthy controls. J Rheumatol. 2010;37(2):265–74.
Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D’Agostino MA, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32(12):2485–7.
Wakefield RJ, Gibbon WW, Conaghan PG, O’Connor P, McGonagle D, Pease C, et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum. 2000;43(12):2762–70.
Dohn UM, Terslev L, Szkudlarek M, Hansen MS, Hetland ML, Hansen A, et al. Detection, scoring and volume assessment of bone erosions by ultrasonography in rheumatoid arthritis: comparison with CT. Ann Rheum Dis. 2013;72(4):530–4.
Rowbotham EL, Grainger AJ. Rheumatoid arthritis: ultrasound versus MRI. AJR Am J Roentgenol. 2011;197(3):541–6.
Finzel S, Ohrndorf S, Englbrecht M, Stach C, Messerschmidt J, Schett G, et al. A detailed comparative study of high-resolution ultrasound and micro-computed tomography for detection of arthritic bone erosions. Arthritis Rheum. 2011;63(5):1231–6.
Szkudlarek M, Klarlund M, Narvestad E, Court-Payen M, Strandberg C, Jensen KE, et al. Ultrasonography of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis: a comparison with magnetic resonance imaging, conventional radiography and clinical examination. Arthritis Res Ther. 2006;8(2):R52.
Hoving JL, Buchbinder R, Hall S, Lawler G, Coombs P, McNealy S, et al. A comparison of magnetic resonance imaging, sonography, and radiography of the hand in patients with early rheumatoid arthritis. J Rheumatol. 2004;31(4):663–75.
Magnani M, Salizzoni E, Mule R, Fusconi M, Meliconi R, Galletti S. Ultrasonography detection of early bone erosions in the metacarpophalangeal joints of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2004;22(6):743–8.
Zayat AS, Ellegaard K, Conaghan PG, Terslev L, Hensor EM, Freeston JE, et al. The specificity of ultrasound-detected bone erosions for rheumatoid arthritis. Ann Rheum Dis. 2015;74(5):897–903.
Sharp JT, Van Der Heijde D, Boers M, Boonen A, Bruynesteyn K, Emery P, et al. Repair of erosions in rheumatoid arthritis does occur. Results from 2 studies by the OMERACT subcommittee on healing of erosions. J Rheumatol. 2003;30(5):1102–7.
Van der Heijde D, Landewe R, Boonen A, Einstein S, Herborn G, Rau R, et al. Expert agreement confirms that negative changes in hand and foot radiographs are a surrogate for repair in patients with rheumatoid arthritis. Arthritis Res Ther. 2007;9(4):R62.
Ideguchi H, Ohno S, Hattori H, Senuma A, Ishigatsubo Y. Bone erosions in rheumatoid arthritis can be repaired through reduction in disease activity with conventional disease-modifying antirheumatic drugs. Arthritis Res Ther. 2006;8(3):R76.
Van der Linden MP, Boja R, Klarenbeek NB, Huizinga TW, van der Heijde DM, van der Helm-van Mil AH. Repair of joint erosions in rheumatoid arthritis: prevalence and patient characteristics in a large inception cohort. Ann Rheum Dis. 2010;69(4):727–9.
Lukas C, van der Heijde D, Fatenajad S, Landewe R. Repair of erosions occurs almost exclusively in damaged joints without swelling. Ann Rheum Dis. 2010;69(5):851–5.
Stewart NR, Crabbe JP, McQueen FM. Magnetic resonance imaging of the wrist in rheumatoid arthritis: demonstration of progression between 1 and 6 years. Skeletal Radiol. 2004;33(12):704–11.
Lisbona M, Maymo J, Solano A, Almirall M, Navallas M, Ares J, et al. Repair of erosions in patients with rheumatoid arthritis treated with etanercept: magnetic resonance imaging findings after 1 year of follow-up. Scand J Rheumatol. 2013;42(6):437–44.
Aletaha D, Funovits J, Smolen JS. Physical disability in rheumatoid arthritis is associated with cartilage damage rather than bone destruction. Ann Rheum Dis. 2011;70(5):733–9.
McQueen F, Clarke A, McHaffie A, Reeves Q, Williams M, Robinson E, et al. Assessment of cartilage loss at the wrist in rheumatoid arthritis using a new MRI scoring system. Ann Rheum Dis. 2010;69(11):1971–5.
Ostergaard M, Boyesen P, Eshed I, Gandjbakhch F, Lillegraven S, Bird P, et al. Development and preliminary validation of a magnetic resonance imaging joint space narrowing score for use in rheumatoid arthritis: potential adjunct to the OMERACT RA MRI scoring system. J Rheumatol. 2011;38(9):2045–50.
McQueen FM, McHaffie A, Clarke A, Lee AC, Reeves Q, Curteis B, et al. MRI osteitis predicts cartilage damage at the wrist in RA: a three-year prospective 3T MRI study examining cartilage damage. Arthritis Res Ther. 2014;16(1):R33.
Yang H, Rivoire J, Hoppe M, Srikhum W, Imboden J, Link TM, et al. Computer-aided and manual quantifications of MRI synovitis, bone marrow edema-like lesions, erosion and cartilage loss in rheumatoid arthritis of the wrist. Skeletal Radiol. 2015;44(4):539–47.
Glinatsi D, Lillegraven S, Haavardsholm EA, Eshed I, Conaghan PG, Peterfy C, et al. Validation of the OMERACT magnetic resonance imaging joint space narrowing score for the wrist in a multireader longitudinal trial. J Rheumatol. 2015;42(12):2480–5.
Ostergaard M, Bird P, Gandjbakhch F, Eshed I, Haugen IK, Haavardsholm EA, et al. The OMERACT MRI in arthritis working group—update on status and future research priorities. J Rheumatol. 2015;42(12):2470–2.
Schleich C, Muller-Lutz A, Sewerin P, Ostendorf B, Buchbender C, Schneider M, et al. Intra-individual assessment of inflammatory severity and cartilage composition of finger joints in rheumatoid arthritis. Skeletal Radiol. 2015;44(4):513–8.
Herz B, Albrecht A, Englbrecht M, Welsch GH, Uder M, Renner N, et al. Osteitis and synovitis, but not bone erosion, is associated with proteoglycan loss and microstructure damage in the cartilage of patients with rheumatoid arthritis. Ann Rheum Dis. 2014;73(6):1101–6.
Swen WA, Jacobs JW, Hubach PC, Klasens JH, Algra PR, Bijlsma JW. Comparison of sonography and magnetic resonance imaging for the diagnosis of partial tears of finger extensor tendons in rheumatoid arthritis. Rheumatology (Oxford). 2000;39(1):55–62.
Valeri G, Ferrara C, Ercolani P, De Nigris E, Giovagnoni A. Tendon involvement in rheumatoid arthritis of the wrist: MRI findings. Skeletal Radiol. 2001;30(3):138–43.
Ertel AN, Millender LH, Nalebuff E, McKay D, Leslie B. Flexor tendon ruptures in patients with rheumatoid arthritis. J Hand Surg Am. 1988;13(6):860–6.
Rubens DJ, Blebea JS, Totterman SM, Hooper MM. Rheumatoid arthritis: evaluation of wrist extensor tendons with clinical examination versus MR imaging—a preliminary report. Radiology. 1993;187(3):831–8.
Neurath MF, Stofft E. Ultrastructural causes of rupture of hand tendons in patients with rheumatoid arthritis. A transmission and scanning electron microscopic study. Scand J Plast Reconstr Surg Hand Surg. 1993;27(1):59–65.
Bruyn GA, Hanova P, Iagnocco A, d’Agostino MA, Moller I, Terslev L, et al. Ultrasound definition of tendon damage in patients with rheumatoid arthritis. Results of a OMERACT consensus-based ultrasound score focussing on the diagnostic reliability. Ann Rheum Dis. 2014;73(11):1929–34.
Bruyn GA, Moller I, Garrido J, Bong D, d’Agostino MA, Iagnocco A, et al. Reliability testing of tendon disease using two different scanning methods in patients with rheumatoid arthritis. Rheumatology (Oxford). 2012;51(9):1655–61.
Jimenez-Boj E, Nobauer-Huhmann I, Hanslik-Schnabel B, Dorotka R, Wanivenhaus AH, Kainberger F, et al. Bone erosions and bone marrow edema as defined by magnetic resonance imaging reflect true bone marrow inflammation in rheumatoid arthritis. Arthritis Rheum. 2007;56(4):1118–24.
Dalbeth N, Smith T, Gray S, Doyle A, Antill P, Lobo M, et al. Cellular characterisation of magnetic resonance imaging bone oedema in rheumatoid arthritis; implications for pathogenesis of erosive disease. Ann Rheum Dis. 2009;68(2):279–82.
McQueen FM, Gao A, Ostergaard M, King A, Shalley G, Robinson E, et al. High-grade MRI bone oedema is common within the surgical field in rheumatoid arthritis patients undergoing joint replacement and is associated with osteitis in subchondral bone. Ann Rheum Dis. 2007;66(12):1581–7.
Diekhoff T, Scheel M, Hermann S, Mews J, Hamm B, Hermann KA. Osteitis: a retrospective feasibility study comparing single-source dual-energy CT to MRI in selected patients with suspected acute gout. Skeletal Radiol. 2017;46(2):185–90.
Jans L, De Kock I, Herregods N, Verstraete K, Van den Bosch F, Carron P, et al. Dual-energy CT: a new imaging modality for bone marrow oedema in rheumatoid arthritis. Ann Rheum Dis. 2018;77(6):958–60.
Tamai M, Kawakami A, Uetani M, Takao S, Tanaka F, Fujikawa K, et al. Bone edema determined by magnetic resonance imaging reflects severe disease status in patients with early-stage rheumatoid arthritis. J Rheumatol. 2007;34(11):2154–7.
Tamai M, Kawakami A, Uetani M, Takao S, Tanaka F, Nakamura H, et al. The presence of anti-cyclic citrullinated peptide antibody is associated with magnetic resonance imaging detection of bone marrow oedema in early stage rheumatoid arthritis. Ann Rheum Dis. 2006;65(1):133–4.
McQueen FM, Benton N, Perry D, Crabbe J, Robinson E, Yeoman S, et al. Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48(7):1814–27.
McQueen FM, Ostendorf B. What is MRI bone oedema in rheumatoid arthritis and why does it matter? Arthritis Res Ther. 2006;8(6):222.
Haavardsholm EA, Boyesen P, Ostergaard M, Schildvold A, Kvien TK. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression. Ann Rheum Dis. 2008;67(6):794–800.
Hetland ML, Ejbjerg B, Horslev-Petersen K, Jacobsen S, Vestergaard A, Jurik AG, et al. MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis. Results from a 2-year randomised controlled trial (CIMESTRA). Ann Rheum Dis. 2009;68(3):384–90.
Schett G, Firestein GS. Mr outside and Mr inside: classic and alternative views on the pathogenesis of rheumatoid arthritis. Ann Rheum Dis. 2010;69(5):787–9.
McQueen FM. The MRI view of synovitis and tenosynovitis in inflammatory arthritis: implications for diagnosis and management. Ann N Y Acad Sci. 2009;1154:21–34.
Goupille P, Roulot B, Akoka S, Avimadje AM, Garaud P, Naccache L, et al. Magnetic resonance imaging: a valuable method for the detection of synovial inflammation in rheumatoid arthritis. J Rheumatol. 2001;28(1):35–40.
Tamai M, Kawakami A, Iwamoto N, Kawashiri SY, Fujikawa K, Aramaki T, et al. Comparative study of the detection of joint injury in early-stage rheumatoid arthritis by magnetic resonance imaging of the wrist and finger joints and physical examination. Arthritis Care Res (Hoboken). 2011;63(3):436–9.
Ben Abdelghani K, Miladi S, Souabni L, Kassab S, Chekili S, Laatar A, et al. Role of ultrasound in assessing remission in rheumatoid arthritis. Diagn Interv Imaging. 2015;96(1):3–10.
Chew LC, Chandra Mohan P, Chan LP, Fong KY, Thumboo J. Use of magnetic resonance imaging in detecting subclinical synovitis in rheumatoid arthritis and correlation of imaging findings with interleukin-18 levels. Int J Rheum Dis. 2016;19(8):790–8.
Aletaha D, Smolen J. The simplified disease activity index (SDAI) and the clinical disease activity index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S100–8.
Aletaha D, Ward MM, Machold KP, Nell VP, Stamm T, Smolen JS. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum. 2005;52(9):2625–36.
Yanagawa A, Takano K, Nishioka K, Shimada J, Mizushima Y, Ashida H. Clinical staging and gadolinium-DTPA enhanced images of the wrist in rheumatoid arthritis. J Rheumatol. 1993;20(5):781–4.
Klarlund M, Ostergaard M, Rostrup E, Skjodt H, Lorenzen I. Dynamic magnetic resonance imaging of the metacarpophalangeal joints in rheumatoid arthritis, early unclassified polyarthritis, and healthy controls. Scand J Rheumatol. 2000;29(2):108–15.
Partik B, Rand T, Pretterklieber ML, Voracek M, Hoermann M, Helbich TH. Patterns of gadopentetate-enhanced MR imaging of radiocarpal joints of healthy subjects. AJR Am J Roentgenol. 2002;179(1):193–7.
Magarelli N, Guglielmi G, Di Matteo L, Tartaro A, Mattei PA, Bonomo L. Diagnostic utility of an echo-contrast agent in patients with synovitis using power Doppler ultrasound: a preliminary study with comparison to contrast-enhanced MRI. Eur Radiol. 2001;11(6):1039–46.
Andersen M, Ellegaard K, Hebsgaard JB, Christensen R, Torp-Pedersen S, Kvist PH, et al. Ultrasound colour Doppler is associated with synovial pathology in biopsies from hand joints in rheumatoid arthritis patients: a cross-sectional study. Ann Rheum Dis. 2014;73(4):678–83.
Cheung PP, Dougados M, Gossec L. Reliability of ultrasonography to detect synovitis in rheumatoid arthritis: a systematic literature review of 35 studies (1,415 patients). Arthritis Care Res (Hoboken). 2010;62(3):323–34.
Haavardsholm EA, Ostergaard M, Ejbjerg BJ, Kvan NP, Uhlig TA, Lilleas FG, et al. Reliability and sensitivity to change of the OMERACT rheumatoid arthritis magnetic resonance imaging score in a multireader, longitudinal setting. Arthritis Rheum. 2005;52(12):3860–7.
Ostergaard M, Klarlund M, Lassere M, Conaghan P, Peterfy C, McQueen F, et al. Interreader agreement in the assessment of magnetic resonance images of rheumatoid arthritis wrist and finger joints—an international multicenter study. J Rheumatol. 2001;28(5):1143–50.
Conaghan P, Lassere M, Ostergaard M, Peterfy C, McQueen F, O’Connor P, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Exercise 4: an international multicenter longitudinal study using the RA-MRI score. J Rheumatol. 2003;30(6):1376–9.
Lassere M, McQueen F, Ostergaard M, Conaghan P, Shnier R, Peterfy C, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Exercise 3: an international multicenter reliability study using the RA-MRI score. J Rheumatol. 2003;30(6):1366–75.
American College of Rheumatology Extremity Magnetic Resonance Imaging Task Force. Extremity magnetic resonance imaging in rheumatoid arthritis: report of the American College of Rheumatology Extremity Magnetic Resonance Imaging Task Force. Arthritis Rheum. 2006;54(4):1034–47.
Cyteval C, Miquel A, Hoa D, Daures JP, Mariette X, Combe B. Rheumatoid arthritis of the hand: monitoring with a simplified MR imaging scoring method--preliminary assessment. Radiology. 2010;256(3):863–9.
Boesen M, Kubassova O, Bouert R, Axelsen MB, Ostergaard M, Cimmino MA, et al. Correlation between computer-aided dynamic gadolinium-enhanced MRI assessment of inflammation and semi-quantitative synovitis and bone marrow oedema scores of the wrist in patients with rheumatoid arthritis—a cohort study. Rheumatology (Oxford). 2012;51(1):134–43.
Karlo C, Zanetti M, Stolzmann P, Steurer-Dober I, Brunner F, Hodler J, et al. Synovitis maps for the assessment of inflammatory diseases of the hand. Eur Radiol. 2011;21(7):1499–508.
Mori G, Tokunaga D, Takahashi KA, Hojo T, Fujiwara H, Arai Y, et al. Maximum intensity projection as a tool to diagnose early rheumatoid arthritis. Mod Rheumatol. 2008;18(3):247–51.
Chand AS, McHaffie A, Clarke AW, Reeves Q, Tan YM, Dalbeth N, et al. Quantifying synovitis in rheumatoid arthritis using computer-assisted manual segmentation with 3 Tesla MRI scanning. J Magn Reson Imaging. 2011;33(5):1106–13.
Ostergaard M, Stoltenberg M, Lovgreen-Nielsen P, Volck B, Sonne-Holm S, Lorenzen I. Quantification of synovitis by MRI: correlation between dynamic and static gadolinium-enhanced magnetic resonance imaging and microscopic and macroscopic signs of synovial inflammation. Magn Reson Imaging. 1998;16(7):743–54.
McQueen FM, Crabbe J, Stewart N. Dynamic gadolinium-enhanced magnetic resonance imaging of the wrist in patients with rheumatoid arthritis: comment on the article by Cimmino et al. Arthritis Rheum. 2004;50(2):674–5; author reply 5-6.
Boesen M, Kubassova O, Parodi M, Bliddal H, Innocenti S, Garlaschi G, et al. Comparison of the manual and computer-aided techniques for evaluation of wrist synovitis using dynamic contrast-enhanced MRI on a dedicated scanner. Eur J Radiol. 2011;77(2):202–6.
Boesen M, Kubassova O, Cimmino MA, Ostergaard M, Taylor P, Danneskiold-Samsoe B, et al. Dynamic contrast enhanced MRI can monitor the very early inflammatory treatment response upon intra-articular steroid injection in the knee joint: a case report with review of the literature. Arthritis. 2011;2011:578252.
Kubassova O, Boesen M, Cimmino MA, Bliddal H. A computer-aided detection system for rheumatoid arthritis MRI data interpretation and quantification of synovial activity. Eur J Radiol. 2010;74(3):e67–72.
Zufferey P, Brulhart L, Tamborrini G, Finckh A, Scherer A, Moller B, et al. Ultrasound evaluation of synovitis in RA: correlation with clinical disease activity and sensitivity to change in an observational cohort study. Joint Bone Spine. 2014;81(3):222–7.
Mandl P, Naredo E, Wakefield RJ, Conaghan PG, D’Agostino MA, Force OUT. A systematic literature review analysis of ultrasound joint count and scoring systems to assess synovitis in rheumatoid arthritis according to the OMERACT filter. J Rheumatol. 2011;38(9):2055–62.
Kleyer A, Krieter M, Oliveira I, Faustini F, Simon D, Kaemmerer N, et al. High prevalence of tenosynovial inflammation before onset of rheumatoid arthritis and its link to progression to RA-A combined MRI/CT study. Semin Arthritis Rheum. 2016;46(2):143–50.
Haavardsholm EA, Ostergaard M, Ejbjerg BJ, Kvan NP, Kvien TK. Introduction of a novel magnetic resonance imaging tenosynovitis score for rheumatoid arthritis: reliability in a multireader longitudinal study. Ann Rheum Dis. 2007;66(9):1216–20.
Schirmer C, Scheel AK, Althoff CE, Schink T, Eshed I, Lembcke A, et al. Diagnostic quality and scoring of synovitis, tenosynovitis and erosions in low-field MRI of patients with rheumatoid arthritis: a comparison with conventional MRI. Ann Rheum Dis. 2007;66(4):522–9.
Eshed I, Feist E, Althoff CE, Hamm B, Konen E, Burmester GR, et al. Tenosynovitis of the flexor tendons of the hand detected by MRI: an early indicator of rheumatoid arthritis. Rheumatology (Oxford). 2009;48(8):887–91.
Ostergaard M, Conaghan PG, O’Connor P, Szkudlarek M, Klarlund M, Emery P, et al. Reducing invasiveness, duration, and cost of magnetic resonance imaging in rheumatoid arthritis by omitting intravenous contrast injection -- does it change the assessment of inflammatory and destructive joint changes by the OMERACT RAMRIS? J Rheumatol. 2009;36(8):1806–10.
Tamai M, Kawakami A, Uetani M, Fukushima A, Arima K, Fujikawa K, et al. Magnetic resonance imaging (MRI) detection of synovitis and bone lesions of the wrists and finger joints in early-stage rheumatoid arthritis: comparison of the accuracy of plain MRI-based findings and gadolinium-diethylenetriamine pentaacetic acid-enhanced MRI-based findings. Mod Rheumatol. 2012;22(5):654–8.
Tehranzadeh J, Ashikyan O, Anavim A, Tramma S. Enhanced MR imaging of tenosynovitis of hand and wrist in inflammatory arthritis. Skeletal Radiol. 2006;35(11):814–22.
Stomp W, Krabben A, van der Heijde D, Huizinga TW, Bloem JL, Ostergaard M, et al. Aiming for a simpler early arthritis MRI protocol: can Gd contrast administration be eliminated? Eur Radiol. 2015;25(5):1520–7.
Aoki T, Yamashita Y, Saito K, Tanaka Y, Korogi Y. Diagnosis of early-stage rheumatoid arthritis: usefulness of unenhanced and gadolinium-enhanced MR images at 3 T. Clin Imaging. 2013;37(2):348–53.
Qu J, Lei X, Zhan Y, Li H, Zhang Y. Assessing synovitis and bone erosion with apparent diffusion coefficient in early stage of rheumatoid arthritis. J Comput Assist Tomogr. 2017;41(5):833–8.
Li X, Liu X, Du X, Ye Z. Diffusion-weighted MR imaging for assessing synovitis of wrist and hand in patients with rheumatoid arthritis: a feasibility study. Magn Reson Imaging. 2014;32(4):350–3.
Eshed I, Althoff CE, Schink T, Scheel AK, Schirmer C, Backhaus M, et al. Low-field MRI for assessing synovitis in patients with rheumatoid arthritis. Impact of Gd-DTPA dose on synovitis scoring. Scand J Rheumatol. 2006;35(4):277–82.
Ejbjerg BJ, Narvestad E, Jacobsen S, Thomsen HS, Ostergaard M. Optimised, low cost, low field dedicated extremity MRI is highly specific and sensitive for synovitis and bone erosions in rheumatoid arthritis wrist and finger joints: comparison with conventional high field MRI and radiography. Ann Rheum Dis. 2005;64(9):1280–7.
Lee RK, Griffith JF, Wang DF, Shi L, Yeung DK, Li EK, et al. Dynamic contrast-enhanced imaging of the wrist in rheumatoid arthritis: dedicated low-field (0.25-T) versus high-field (3.0-T) MRI. Skeletal Radiol. 2015;44(8):1095–101.
Naraghi AM, White LM, Patel C, Tomlinson G, Keystone EC. Comparison of 1.0-T extremity MR and 1.5-T conventional high-field-strength MR in patients with rheumatoid arthritis. Radiology. 2009;251(3):829–37.
Bonel HM, Schneider P, Seemann MD, Huegli R, Srivastav S, Lodemann KP, et al. MR imaging of the wrist in rheumatoid arthritis using gadobenate dimeglumine. Skeletal Radiol. 2001;30(1):15–24.
Schueller-Weidekamm C, Lodemann KP, Grisar J, Schueller G, Weber M, Kainberger F, et al. Contrast-enhanced MR imaging of hand and finger joints in patients with early rheumatoid arthritis: do we really need a full dose of gadobenate dimeglumine for assessing synovial enhancement at 3 T? Radiology. 2013;268(1):161–9.
Navalho M, Resende C, Rodrigues AM, Gaspar A, Fonseca JE, Canhao H, et al. Dynamic contrast-enhanced 3-T magnetic resonance imaging: a method for quantifying disease activity in early polyarthritis. Skeletal Radiol. 2012;41(1):51–9.
Cimmino MA, Innocenti S, Livrone F, Magnaguagno F, Silvestri E, Garlaschi G. Dynamic gadolinium-enhanced magnetic resonance imaging of the wrist in patients with rheumatoid arthritis can discriminate active from inactive disease. Arthritis Rheum. 2003;48(5):1207–13.
Tam LS, Griffith JF, Yu AB, Li TK, Li EK. Rapid improvement in rheumatoid arthritis patients on combination of methotrexate and infliximab: clinical and magnetic resonance imaging evaluation. Clin Rheumatol. 2007;26(6):941–6.
Ostergaard M, Klarlund M. Importance of timing of post-contrast MRI in rheumatoid arthritis: what happens during the first 60 minutes after IV gadolinium-DTPA? Ann Rheum Dis. 2001;60(11):1050–4.
Stomp W, Krabben A, van der Heijde D, Huizinga TW, Bloem JL, van der Helm-van Mil AH, et al. Aiming for a shorter rheumatoid arthritis MRI protocol: can contrast-enhanced MRI replace T2 for the detection of bone marrow oedema? Eur Radiol. 2014;24(10):2614–22.
Yao L, Magalnick M, Wilson M, Lipsky P, Goldbach-Mansky R. Periarticular bone findings in rheumatoid arthritis: T2-weighted versus contrast-enhanced T1-weighted MRI. AJR Am J Roentgenol. 2006;187(2):358–63.
Savnik A, Malmskov H, Thomsen HS, Bretlau T, Graff LB, Nielsen H, et al. MRI of the arthritic small joints: comparison of extremity MRI (0.2 T) vs high-field MRI (1.5 T). Eur Radiol. 2001;11(6):1030–8.
Bird P, Ejbjerg B, Lassere M, Ostergaard M, McQueen F, Peterfy C, et al. A multireader reliability study comparing conventional high-field magnetic resonance imaging with extremity low-field MRI in rheumatoid arthritis. J Rheumatol. 2007;34(4):854–6.
Crues JV, Shellock FG, Dardashti S, James TW, Troum OM. Identification of wrist and metacarpophalangeal joint erosions using a portable magnetic resonance imaging system compared to conventional radiographs. J Rheumatol. 2004;31(4):676–85.
Taouli B, Zaim S, Peterfy CG, Lynch JA, Stork A, Guermazi A, et al. Rheumatoid arthritis of the hand and wrist: comparison of three imaging techniques. AJR Am J Roentgenol. 2004;182(4):937–43.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author declares that he has no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rubin, D.A. MRI and ultrasound of the hands and wrists in rheumatoid arthritis. I. Imaging findings. Skeletal Radiol 48, 677–695 (2019). https://doi.org/10.1007/s00256-019-03179-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-019-03179-z