Abstract
Purpose
To evaluate whether intravenous gadolinium (Gd) contrast administration can be eliminated when evaluating synovitis and tenosynovitis in early arthritis patients, thereby decreasing imaging time, cost, and invasiveness.
Materials and Methods
Wrist MRIs of 93 early arthritis patients were evaluated by two readers for synovitis of the radioulnar, radiocarpal, and intercarpal joints, according to the Rheumatoid Arthritis MRI Scoring method (RAMRIS), and for tenosynovitis in ten compartments. Scores of MRI images without Gd contrast enhancement were compared to scores obtained when evaluating all, including contrast-enhanced, MRI images as reference. Subsequently, a literature review and pooled analysis of data from the present and two previous studies were performed.
Results
At the individual joint/tendon level, sensitivity to detect synovitis without Gd contrast was 91 % and 72 % for the two readers, respectively, with a specificity of 51 % and 81 %. For tenosynovitis, the sensitivity was 67 % and 54 %, respectively, with a specificity of 87 % and 91 %. Pooled data analysis revealed an overall sensitivity of 81 % and specificity of 50 % for evaluation of synovitis. Variations in tenosynovitis scoring systems hindered pooled analyses.
Conclusion
Eliminating Gd contrast administration resulted in low specificity for synovitis and low sensitivity for tenosynovitis, indicating that Gd contrast administration remains essential for an optimal assessment.
Key Points
• Eliminating gadolinium contrast administration results in low specificity for synovitis
• For tenosynovitis, sensitivity is low without gadolinium contrast administration
• Gadolinium contrast administration remains essential for evaluating synovitis and tenosynovitis in early arthritis
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance imaging (MRI) is increasingly used in research of rheumatoid arthritis (RA) and in clinical practice. MRI has high sensitivity to depict local inflammation in the form of synovitis, tenosynovitis, and bone marrow oedema [1]. The MRI protocol is standardized in the Outcome Measures in Rheumatology (OMERACT) Rheumatoid Arthritis MRI scoring (RAMRIS) method, which is a standardized scoring system for the assessment of synovitis, bone marrow oedema, and erosions [2]. OMERACT recommended MRI sequences include non-contrast-enhanced T1-weighted images which are primarily used to assess erosions, T2-weighted, fat saturated images (T2), or short tau inversion recovery (STIR) images to evaluate bone marrow oedema [2], and post-gadolinium (Gd) contrast T1-weighted images (T1Gd) used in combination with non-contrast T1-weighted images for evaluation of synovitis and tenosynovitis [2–4].
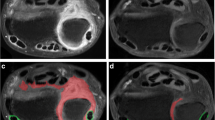
The use of intravenous Gd contrast has drawbacks; it is an invasive procedure, carries a small risk of nephrogenic systemic fibrosis, is costly, and prolongs the required imaging time. When Gd contrast administration can be eliminated, MRI becomes more patient-friendly and accessibility is increased. We hypothesized that based on the high signal intensity of both synovitis and tenosynovitis on T2 and T1Gd images (illustrated in Fig. 1), and despite the high signal intensity of synovium and fluid, it is possible to evaluate synovitis and tenosynovitis on T2 instead of T1Gd.
Example of synovitis as visualized by T2-weighted and T1-weighted postcontrast sequences. (a) T2-weighted coronal image before Gd administration, and (b) corresponding T1-weighted coronal image after Gd administration. Synovitis of the radioulnar, radiocarpal and intercarpal joints is clearly visible on both sequences
The objective of this study was to determine whether Gd contrast administration could be eliminated from the MRI protocol when assessing synovitis and tenosynovitis. This was achieved by a study of 93 early arthritis patients, a literature review, and an analysis of pooled data from the current study and two previous studies.
Material and methods
Patients
Between July 2011 and April 2012, MRI was performed on 93 early arthritis patients at the first visit of the hospital’s Early Arthritis Clinic. The Early Arthritis Clinic is a population-based inception cohort that includes patients with confirmed arthritis and symptoms for <2 years [5]. These patients were part of a larger group in whom MRI was performed and results on which have been previously reported [1, 6, 7]; the current study concerns a subgroup of consecutive patients in which an extra axial T2-weighted sequence of the wrist was obtained. All patients provided informed consent and the study was approved by the institutional review board.
MRI
MRI of the wrist was performed within 2 weeks after inclusion, of the most painful side, or in case of completely symmetric symptoms, of the dominant side. The presence of clinical arthritis at physical examination of the wrist was not a prerequisite. MRI was performed on an MSK-extreme 1.5 T extremity MRI system (GE, Wisconsin, USA) using a 100-mm quadrature volume transmit and receive coil. The patient was positioned in a chair beside the scanner, with the hand fixed inside the coil with cushions.
The following sequences were acquired before contrast administration: T1-weighted FSE sequence in the coronal plane (TR/TE 650/17 ms; acquisition matrix 388 × 88; ETL2); T2-weighted FSE sequence with frequency selective fat saturation in the coronal and axial plane (TR/TE 3000/61.8 ms; acquisition matrix, 300x224, ETL7).
After intravenous administration of Gd contrast (gadoteric acid, Guerbet, Paris, France, standard dose of 0.1 mmol/kg) the following sequences were obtained: T1-weighted FSE sequence with frequency selective fat saturation in the coronal plane (TR/TE 650/17 ms, acquisition matrix 364 × 224, ETL2), T1-weighted FSE sequence with frequency selective fat saturation in the axial plane (TR/TE 570/7 ms; acquisition matrix 320x192; ETL2).
Field-of-view was 100 mm. Coronal sequences had 18 slices with a slice thickness of 2 mm and a slice gap of 0.2 mm. All axial sequences had 20 slices with a slice thickness of 3 mm and a slice gap of 0.3 mm. Total imaging time was approximately 25 min.
The MRIs were scored by two readers independently (research fellows with 2 and 3 years of experience with RA MRI assessment, respectively), blinded to clinical data. Previously documented within-reader intraclass correlation coefficients for the total RAMRIS score were 0.98 and 0.83, and between-reader interclass correlation coefficient was 0.82 [1]. All datasets were scored twice, one time using all acquired images (Gdset), and another time using only unenhanced images (T2set). The order of examinations was randomized between assessments and there was an interval of at least 2 months between assessments. Images were scored for synovitis according to RAMRIS on a 0–3 scale for the radioulnar, radiocarpal, and the combined intercarpal and carpometacarpal joints [2]. Tenosynovitis was evaluated in 10 tendons/compartments on a 0–3 scale as described by Haavardsholm et al. [4].
Literature review and pooled data analysis
For the literature review PubMed was searched with a broad search strategy using the search term (“gadolinium” OR “contrast” OR “enhancement”) AND (“synovitis” OR “arthritis” OR “tenosynovitis”) AND (“MRI” OR “MR” OR “magnetic resonance”). Available literature up to November 2013 was searched. This yielded 1,035 results. Abstracts were screened and we selected studies that reported on findings for Gd contrast-enhanced images compared to findings for images obtained without Gd contrast in MRI of joints of the hand of adult patients with any type of arthritis. For relevant studies (n = 3) full-text articles were obtained. Furthermore, references of obtained full-text articles were screened for further relevant studies, which did not yield any additional studies. Of the three studies that were found, two were relevant for synovitis and one for tenosynovitis.
For synovitis we performed a pooled data analysis; raw data were obtained from the literature [8] or obtained via personal communication [9] and combined to determine overall test characteristics. For tenosynovitis, due to different scoring systems used, we could not perform a pooled data analysis.
Reference standard and statistics
Gd-enhanced image scores were the reference standard. Comparisons were made for the two readers independently. To determine whether the same absolute scores were obtained by both methods, scores were compared with weighted kappa statistic and intraclass correlation coefficient (ICC) for absolute agreement. Bland-Altman plots were used to visualize the level of agreement. Furthermore, the sensitivity and specificity were calculated at both the joint/tendon level (with results combined for all assessed joints/tendons) and at the patient level (total synovitis and tenosynovitis scores), with scores ≥1 considered positive at both joint/tendon and patient levels.
Results
Data from 92 patients were analyzed, as one MRI was excluded because of severe artefacts caused by a metallic foreign body. Patient characteristics are listed in Table 1. Both readers were able to assess all joints using both image sets. Based on reader 1 scores for Gdset (the reference standard), MRI synovitis was present in 162 joints (59 %) and 81 patients (88 %); tenosynovitis was present in 153 tendon compartments (17 %) and 52 patients (57 %).
Agreement for total synovitis and tenosynovitis scores
For total scores within each patient, Bland-Altman plots showed acceptable levels of agreement (Fig. 2). For tenosynovitis there was a tendency towards more variation with higher scores (heteroscedasticity), especially for reader 2. There was little systematic bias for both readers between the sets with and without Gd contrast, reader 1 had slightly higher scores on the T2set, while reader 2 had virtually zero difference on average. ICCs between the T2set (without contrast) and Gdset (with Gd contrast) images were 0.75 (95 %CI 0.54 – 0.86) and 0.82 (95 %CI 0.74 – 0.88) for synovitis for the two readers, respectively, and 0.72 (95 %CI 0.60 – 0.81) and 0.57 (95 %CI 0.42 – 0.70) for tenosynovitis, respectively, indicating moderate to good agreement for total synovitis and tenosynovitis scores.
Bland-Altman plots of assessment of synovitis and tenosynovitis with and without Gd enhancement. Plots for total scores for synovitis (upper row) and tenosynovitis (lower row) for reader 1 (left) and reader 2 (right). The differences (T2set - Gdset) between paired measurements are plotted against the means of the two measurements. The middle line in each graph shows the systematic bias between the two measurement methods. The observation that the line is located around 0 indicates that systematic bias was low. The upper and lower lines show the ±95 % limits of agreement. For tenosynovitis, variation increases with higher scores for reader 2
Test characteristics at the patient level
When evaluating the presence of synovitis at the patient level without Gd contrast (the T2set), the sensitivity was 96 % and 78 %, respectively, for the two readers and the specificity was 36 % and 71 %. When tenosynovitis was assessed using the T2set, the sensitivity was 89 % and 71 % and the specificity was 40 % and 68 % (Table 2).
Agreement for individual joint/tendon scores
Subsequent analyses were performed at the joint level with Gdset images as reference. Weighted Kappa’s for agreement of synovitis scores in individual joints based on T2set and Gdset were 0.65 (95 %CI 0.49 – 0.81) and 0.71 (95 %CI 0.63 – 0.80) for the two readers, indicating good agreement. For tenosynovitis corresponding values were 0.52 (95 %CI 0.36 – 0.68) and 0.46 (95 %CI 0.33 – 0.60), indicating moderate agreement.
Test characteristics at the joint/tendon level
The sensitivity to detect synovitis without Gd contrast was 91 % and 72 %, respectively, for the 2 readers, and the specificity was 51 % and 81 %. Similarly, for tenosynovitis the sensitivity was 67 % and 54 %, and the specificity was 87 % and 91 % for the two readers (Table 2).
Large discrepancies in scores in individual joints/tendons
Differences of >1 point between T2set and Gdset scores in individual joints or tendons were present in only 1.8 % of joints for synovitis and 0.3 – 0.5 % of tendons for tenosynovitis. These cases were reviewed for the cause of this discrepancy. For synovitis, in all cases areas of high signal on T2 showed no enhancement on T1Gd images, indicating false positive results on T2 due to effusion (Fig. 3). For tenosynovitis no clear explanation was found.
Example of large discrepancy in synovitis score between T1-weighted postcontrast and T2-weighted sequences. (a) T2-weighted coronal image before Gd administration, and (b) corresponding T1-weighted coronal image after Gd administration. Effusion in the radioulnar and radiocarpal joints results in high signal on T2-weighted images without enhancement on post-Gd images
Literature review and pooled data analysis
Table 3 lists all studies that were identified and results of each individual study; two studies evaluated synovitis and one other study assessed tenosynovitis with and without Gd contrast [8–10]. The tendency of the findings on the joint/tendon level was consistent across studies: low specificity for synovitis, low sensitivity for tenosynovitis. The only exception was assessment of synovitis at 0.2 T extremity MRI (as compared to 1.0 or 1.5 T for other studies), where the sensitivity was low [9]. Figure 4 shows the sensitivity and specificity for synovitis obtained with 1.0/1.5 T MRI in different studies. For synovitis, raw data of three studies were pooled at the joint level; the overall sensitivity to detect synovitis without Gd was 81 % and the overall specificity 50 % (Fig. 4, Table 4). For tenosynovitis pooling could not be performed due to differences in the scoring methods used.
Sensitivity and specificity of evaluation of synovitis without Gd contrast in separatestudies. Plot of sensitivity (A) and specificity (B) estimates when evaluatingsynovitis and tenosynovitis for individual joints and tendons. For studies withmultiple readers, point estimates for the first reader are shown as soliddiamonds and for the second reader as open diamonds. The solid linesrepresent 95 % CIs
Discussion
MRI is sensitive to detect inflammation, but is also time-consuming and costly. We investigated the consequences of eliminating Gd contrast administration for MRI of the wrist in a cohort of early arthritis patients and subsequently analyzed pooled data from this study and two previously published studies identified by a literature review. We observed that the sensitivity and specificity were markedly decreased when eliminating the post-Gd contrast sequences.
Gd administration adds to the cost and duration of the examination and increases patient discomfort. Furthermore it is contraindicated in patients with severe renal failure due to the risk of nephrogenic systemic fibrosis [11]. For assessment of bone marrow oedema and erosions no Gd contrast is necessary [8, 9]. However, based on our findings and the literature review, Gd contrast is necessary for optimal assessment of synovitis and tenosynovitis. Agreement for total synovitis and tenosynovitis scores, as tested by Bland-Altman plots and ICCs, was moderate to good with little systemic bias. Therefore when only total inflammation burden within the whole imaged area is of interest, a reasonably good indication may be obtained even without Gd contrast. However, test characteristics were unacceptably too low to determine the presence of inflammation either at the patient or individual joint/tendon level, and therefore, in most practical situations, Gd contrast administration cannot be eliminated.
An important advantage of using Gd contrast is the ability to differentiate between effusion and inflammation. In our population, this was apparent when we reviewed the largest discrepancies in scores, which for synovitis were all in joints with evident effusion. This was an uncommon occurrence, however, in these early arthritis patients. Nevertheless, even small, physiologic amounts of joint fluid may have given the false appearance of synovitis on T2 images, negatively affecting specificity. In patients with more advanced disease, this might pose a bigger problem as effusion is more prevalent in these patients, thus we expect reliability without Gd contrast in these patients to be even lower.
In juvenile idiopathic arthritis patients who underwent knee MRI, omitting Gd contrast administration decreased the reliability of synovial hypertrophy scores [12]. Sensitivity rather than specificity was mainly affected; a possible explanation might be the more common occurrence of joint effusion in the knee, from which synovitis is difficult to differentiate without contrast enhancement. Likewise, in patients with knee osteoarthritis, MRI adequately assessed effusion volume but did not adequately detect synovitis [13].
Although our results show that Gd contrast enhanced sequences cannot be simply left out of the MRI protocol, there might still be alternative options to avoid Gd contrast administration. The most promising option might be using diffusion-weighted imaging (DWI), which has successfully been applied in RA in a few small-scale studies [14, 15]. More advanced imaging with arterial spin-labelling could potentially even accurately quantify inflammation without using Gd contrast [16]. Furthermore, an alternative option to come to a shorter MRI protocol would be to assess BME on post-contrast series, an option we recently successfully explored [7].
A strength of our study is that we included patients at early disease stage when inflammation is usually limited and MRI may be of additional value in detecting it. Furthermore, we did not limit inclusion to a single diagnosis, which makes our results more widely applicable.
A limitation is that we only assessed wrist joints and not MCP joints. We chose this for time reasons, as we prioritized to acquire axial T2-weighted fat suppressed images in order to have optimal sequences for assessment of synovitis and especially tenosynovitis without contrast administration. Secondly, we only made cross-sectional comparisons, so the sensitivity to change, which is important for clinical trials, could not be assessed. However, as cross-sectional data alone documented that non-contrast enhanced sequences cannot replace contrast-enhanced sequences, longitudinal data are less relevant. Thirdly, we used the RAMRIS method, which has only been validated in RA. However, no method exists that has been validated across multiple rheumatologic conditions and the RAMRIS is the best available validated method to assess synovitis, thus we considered it appropriate to use the RAMRIS method. Likewise, we also used the best available method to assess tenosynovitis. Finally, our data were obtained in early arthritis patients with relatively low inflammation scores and may not be generalizable to patients with more advanced disease.
In conclusion, eliminating Gd contrast results in low specificity for synovitis and low sensitivity for tenosynovitis. Consequently, MRI without Gd contrast administration cannot be recommended for evaluation of synovitis and tenosynovitis in early arthritis patients.
References
Krabben A, Stomp W, Huizinga TWJ et al (2013) Concordance between inflammation at physical examination and on MRI in patients with early arthritis. Ann Rheum Dis. doi:10.1136/annrheumdis-2013-204005
Østergaard M, Peterfy C, Conaghan P et al (2003) OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol 30:1385–1386
Eshed I, Feist E, Althoff CE et al (2009) Tenosynovitis of the flexor tendons of the hand detected by MRI: an early indicator of rheumatoid arthritis. Rheumatology (Oxford) 48:887–891
Haavardsholm EA, Østergaard M, Ejbjerg BJ et al (2007) Introduction of a novel magnetic resonance imaging tenosynovitis score for rheumatoid arthritis: reliability in a multireader longitudinal study. Ann Rheum Dis 66:1216–1220
De Rooy DPC, van der Linden MPM, Knevel R et al (2011) Predicting arthritis outcomes–what can be learned from the Leiden Early Arthritis Clinic? Rheumatology (Oxford) 50:93–100
Stomp W, Krabben A, van der Heijde D et al (2014) Are rheumatoid arthritis patients discernible from other early arthritis patients using 1.5 T extremity magnetic resonance imaging? A large cross-sectional study. J Rheumatol 41:1630–1637. doi:10.3899/jrheum.131169
Stomp W, Krabben A, van der Heijde D et al (2014) Aiming for a shorter rheumatoid arthritis MRI protocol: can contrast-enhanced MRI replace T2 for the detection of bone marrow oedema? Eur Radiol 24:2614–2622. doi:10.1007/s00330-014-3272-0
Tamai M, Kawakami A, Uetani M et al (2012) Magnetic resonance imaging (MRI) detection of synovitis and bone lesions of the wrists and finger joints in early-stage rheumatoid arthritis: comparison of the accuracy of plain MRI-based findings and gadolinium-diethylenetriamine pentaacetic acid-enhanced MRI-based findings. Mod Rheumatol 22:654–658
Ostergaard M, Conaghan PG, O’Connor P et al (2009) Reducing invasiveness, duration, and cost of magnetic resonance imaging in rheumatoid arthritis by omitting intravenous contrast injection – Does it change the assessment of inflammatory and destructive joint changes by the OMERACT RAMRIS? J Rheumatol 36:1806–1810
Tehranzadeh J, Ashikyan O, Anavim A, Tramma S (2006) Enhanced MR imaging of tenosynovitis of hand and wrist in inflammatory arthritis. Skelet Radiol 35:814–822
Thomsen HS, Morcos SK, Almén T et al (2013) Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 23:307–318
Hemke R, Kuijpers TW, van den Berg JM et al (2013) The diagnostic accuracy of unenhanced MRI in the assessment of joint abnormalities in juvenile idiopathic arthritis. Eur Radiol 23:1998–2004. doi:10.1007/s00330-013-2770-9
Loeuille D, Sauliere N, Champigneulle J et al (2011) Comparing non-enhanced and enhanced sequences in the assessment of effusion and synovitis in knee OA: associations with clinical, macroscopic and microscopic features. Osteoarthr Cartil/OARS, Osteoarthr Res Soc. doi:10.1016/j.joca.2011.08.010
Li X, Liu X, Du X, Ye Z (2014) Diffusion-weighted MR imaging for assessing synovitis of wrist and hand in patients with rheumatoid arthritis: A feasibility study. Magn Reson Imaging 32:350–353. doi:10.1016/j.mri.2013.12.008
Jeromel M, Jevtič V, Serša I et al (2012) Quantification of synovitis in the cranio-cervical region: dynamic contrast enhanced and diffusion weighted magnetic resonance imaging in early rheumatoid arthritis–a feasibility follow up study. Eur J Radiol 81:3412–3419
Boss A, Martirosian P, Fritz J et al (2009) Magnetic resonance spin-labeling perfusion imaging of synovitis in inflammatory arthritis at 3.0 T. MAGMA 22:175–180
Acknowledgements
The scientific guarantor of this publication is Monique Reijnierse. Wouter Stomp has received speaker fees from GE Healthcare. Mikkel Østergaard has received consultancy/speaker fees and/or research support form Abbott/Abbvie, BMS, Boehringer-Ingelheim, Eli-Lilly, Centocor, GSK, Janssen, Merck, Mundipharma, Novo, Pfizer, Schering-Plough, Roche UCB, and Wyeth. This research was performed within the framework of CTMM, the Center for Translational Molecular Medicine (www.ctmm.nl), and the Dutch Arthritis Foundation, project TRACER (grant 04I-202). The research has also been funded by The European Community Seventh Framework Program FP7 Health-F2-2008-223404 (Masterswitch) as well as by a European grant from the Innovative medicine Initiative: BTCURE. This work of A. van der Helm-van Mil is supported by a Vidi-grant of the Netherlands Organisation for Scientific Research. The work of A. Krabben is supported by a grant of the Dutch Arthritis Foundation. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects in this study. Patients are participating in the Leiden Early Arthritis clinic, and multiple articles have been published about this cohort with ongoing inclusion, including two other studies on MRI results (Krabben A, Stomp W, Huizinga TWJ, et al. (2013) Concordance between inflammation at physical examination and on MRI in patients with early arthritis. Ann Rheum Dis. doi: 10.1136/annrheumdis-2013-204005 and Stomp W, Krabben A, van der Heijde D, et al. (2014) Aiming for a shorter rheumatoid arthritis MRI protocol: can contrast-enhanced MRI replace T2 for the detection of bone marrow oedema? Eur Radiol 24:2614–2622. doi: 10.1007/s00330-014-3272-0). Methodology: prospective cross sectional study performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stomp, W., Krabben, A., van der Heijde, D. et al. Aiming for a simpler early arthritis MRI protocol: can Gd contrast administration be eliminated?. Eur Radiol 25, 1520–1527 (2015). https://doi.org/10.1007/s00330-014-3522-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3522-1