Abstract
Ecological methods are becoming increasingly popular. One of these methods is plant biotization. In our paper, we focus on selection of Vaccinium corymbosum hairy root-inhabiting fungi for plant growth promotion in a single microorganism inoculation setup and then composed a multiorganismal inoculum enriched with a representative of another group of fungi, leaf endophytes. The hairy roots of V. corymbosum hosted 13 fungal taxa. In single inoculation of the plant with fungal strains, the most beneficial for plant growth were Oidiodendron maius and Phialocephala fortinii. Additional inoculation of the plants with three root symbiotic fungi (O. maius, Hymenoscyphus sp. and P. fortinii) and with the endophytic fungus Xylaria sp. increased plant height in laboratory experiments. On a semi-industrial scale, inoculation improved plant biomass and vitality. Therefore, the amendment of root-associated fungal communities with a mixture of ericoid mycorrhizal and endophytic fungi may represent an alternative to conventional fertilization and pesticide application in large-scale blueberry production.
Key points
• O. maius and P. fortinii significantly stimulated V. corymbosum growth in a single inoculation.
• Multimicroorganismal inoculum increased plant biomass and vitality.
• Blueberry biotization with ericoid and endophytic fungi is recommended.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Highbush blueberry (Vaccinium corymbosum L.) is a plant species native to North America. In recent years, the production of V. corymbosum has increased significantly in many regions of the world, including the USA, South America and Europe (Strik and Yarborough 2005, www.cbi.eu), and reached over 820,000 tons per year in 2019 (FAOSTAT 2019, www.fao.org). The global blueberry market is predicted to reach 4.5 billion USD by 2024 (www.reportlinker.com).
To gain market advantage on the market, farmers seek new and verified, existing plant production methods to reach higher crop yields and improved fruit quality. Ecological methods are becoming increasingly popular, particularly due to restricting legislation that limits traditional fertilization and pest control utilization. An environmentally friendly alternative method is plant biotization, a new biotechnological approach that improves plant production. It relies on inoculating plants with appropriately selected symbiotic microorganisms, which promote plant growth and stress tolerance (Gianinazzi et al. 2003; Gollotte et al. 2009).
In intensive berry production, plant nutrition and protection are provided mainly by fertilization and pesticides, respectively. These agrotechnical treatments may lead to disturbances in plant symbioses with their native microbiota and deleterious effects on the environment. It has been shown that plant fertilization with excess N and P can decrease mycorrhizal fungal growth (Treseder 2004; Wang et al. 2009) and antioxidant concentrations in fruits, resulting in decreased nutritional value of crops. Blueberries are one of the richest sources of antioxidants among fresh fruits and vegetables (Prior et al. 1998); therefore, agrotechnical procedures in the cultivation of blueberries should be carried out with special caution. To provide the highest antioxidant capacity in berry fruits, pesticide and fertilizer utilization should be minimized. Furthermore, new, more ecological methods in field management are needed. The key factor in management programs is to consider plant-microorganism interactions (Gianinazzi et al. 2002; Jeffries et al. 2003).
Blueberries, which are ericoid plants, are associated with specialized mycorrhizas known as ericoid mycorrhizas. Fungal species involved in this type of symbiosis include Rhizoscyphus ericae aggregate (Fehrer et al. 2019) (= Hymenoscyphus ericae aggregate (anamorph Scytalidum vaccinii) (Pearson and Read 1973)), Oidiodendron spp. (teleomorph Myxotrichum and Byssoascus) (Jansa and Vosátka 2000) and Cadophora sp. (Bizabani and Dames 2015). Recently, it has been shown that roots, leaves and fruits of highbush blueberry are inhabited by representatives of another group, endophytic fungi, belonging to the following taxa: Anteaglonium sp. (Wu et al. 2021), Penicillium sp., Fusarium oxysporum (Hamim et al. 2017) and Angustimassarina, Dothidea, Fellozyma, Pseudohyphozyma, Hannaella coprosmae and Oberwinklerozyma straminea (Nguyen et al. 2021). Among endophytic bacteria, the most abundant genera hosted by blueberry plants are Pseudomonas, Pantoea, Burkholderia and Bacillus (Ortiz-Galeana et al. 2018). In addition, the above-enumerated taxa of endophytic fungi and bacteria and rhizosphere microorganisms can provide many benefits to the plant (Gaskins et al. 1985). Rhizosphere-inhabiting bacteria and fungi, Pseudomonas fluorescens, Pseudomonas corrugata, Bacillus pumilus, Gliocladium virens and Trichoderma harzianum have been studied as candidates with potential highbush blueberry growth-promoting properties (De Silva et al. 2000).
The majority of available reports concerning plant-fungal symbiosis and plant biotization focus on plant inoculation with a single microorganism, yielding very ambiguous results. In nature, almost all, if not all, plants are colonized not with one microorganism but with communities of microorganisms. These microorganisms play many diverse and important roles in host fitness and drive the functioning of microbial consortia introduced into the rhizosphere (Hu et al. 2016). The effect of multimicroorganismal inocula on plant growth has rarely been studied, but multimicroorganismal inocula usually provide many benefits for the plant (Remans et al. 2008; Liu et al. 2012; Ważny et al. 2018; Yu et al. 2020).
The following hypotheses were verified in this study: (i) fungi isolated from V. corymbosum hairy roots applied to plants in culture will improve rooting, growth rate and vitality; (ii) to optimize the plant growth-promoting effect, an appropriate combination of symbiotic microorganisms needs to be applied. Multiorganismal inocula will more effectively biotize plants than inoculation with single microorganisms. (iii) Efficient biotization with appropriately selected microorganisms can be performed not only in the early stages of plant development but also later, in older plants. Here, we applied the treatment to plants growing in pots for 2 years.
Materials and methods
Experimental design
Preparation of highbush blueberry biotization technology was carried out in three steps (Fig. 1). In the first step, ericoid fungi were isolated from the hairy roots of plants and identified. In the second step, a series of experiments in plant growth chambers were carried out to select fungal components for the combined inoculum to improve blueberry growth. In the last step, the efficiency of the selected inoculum was verified on a semi-industrial scale of blueberry production in a greenhouse (polytunnel). In the semi-industrial experiment, we tested the growth parameters of plants inoculated at the moment of transferring them from in vitro to peat substrate (4-week-old plants) and at the moment of transferring (2-year-old) plants cultivated in peat substrate from small to larger pots. In the second case, we wanted to answer a question very frequently asked by blueberry producers: can we effectively biotize older plants?
Fungal isolation and identification
Fungi were isolated from the hairy roots of the five plants derived from blueberry plantations where very good plant growth without any symptoms of pathogens was observed. Hairy roots were observed using light microscopy and fragments with visible mycelium coils in root cells were selected for fungal isolation. Selected hairy roots were surface sterilized for 50 s in 75% ethanol, followed by thorough washing in sterile distilled water (Turnau et al. 2007). The roots were cut into 3-mm pieces and placed on potato dextrose agar (PDA) medium. Samples were incubated in the dark at 26 °C and inspected every 1–2 days for 4 weeks. Cultures of emerging fungi were transferred to new PDA and incubated in the dark at 26 °C.
Pure cultures of fungi were identified based on morphological and anatomical features and using molecular methods (polymerase chain reaction (PCR) and sequencing of the internal transcribed spacer (ITS) rDNA sequence). DNA was extracted with the Genomic Mini AX Plant (A&A Biotechnology, Gdańsk, PL). The ITS rDNA region was amplified with ITS1F (Gardes and Bruns 1993) and ITS4 primers (White et al. 1990). PCR was performed with 10 ng DNA template, 1 µL of each of the primers at 10 pmol concentration for each sample and Maxima Hot Start Green PCR Master Mix (Thermo Scientific, Waltham, USA) according to the manufacturer’s protocol. Isopropanol and 0.3 M sodium acetate precipitation was used for PCR purification (http://openwetware.org). Sanger sequencing was performed by the Macrogen Laboratory (Amsterdam, NL). The ITS4 primer was used for reading sequences. The sequences were edited with Chromas software (www.technelysium.com.au) and compared with sequences available in NCBI (National Centre for Biotechnology Information; www.ncbi.nlm.nih.gov) using the BLASTn algorithm. Sequence data were deposited in the NCBI database under accession numbers OM729672–OM729684. Pure cultures of the four fungal species selected for V. corymbosum biotization technology were deposited in the culture collection of the Institute of Agricultural and Food Biotechnology (IAFB) in Poland belonging to the World Data Centre for Microorganisms (WDCM) under accession numbers KKP2073p (Xylaria sp.), KKP2075p (Phialocephala fortinii), KKP2076p (Hymenoscyphus sp.) and KKP2077p (Oidiodendron maius).
Substrate
Peat (Hollas, Pasłęk, PL) was used as a substrate for the pot experiments. Available P (Colwell 1963) and Kjeldahl N concentrations in the substrates were measured according to Wilke (2005). Briefly, to determine the concentration of N, the samples were dried at 40 °C for 12 h, transferred to glass tubes and digested with 98% H2SO4 at a temperature of 300 °C in the presence of sodium thiosulfate and the Kjeldahl catalyst. The solutions were distilled with water vapour. The concentration of nitrogen was determined using titration with 0.01 M HCl. To determine the concentration of P, a colorimetric method using a UV–Vis spectrophotometer (Bio Tek Epoch 2; BioTek, Winooski, VT, USA) with the external standard calibration method was used. To determine the potassium concentration, first, the water content in the samples (at 80 °C) was determined using a moisture analyser (Radwag MAC 50/1, Radom, PL), and then the concentration of K was measured using atomic absorption spectrometry (flame atomic absorption spectrometry) in emission mode, with a CSX 260 autosampler (Thermo Scientific, iCE 3000; Thermo Scientific, Waltham, MA, USA). Briefly, the samples were digested in 65% nitric acid (5 mL) for 2 h (room temperature—1 h, boiling point—1 h). After cooling, 1.65 mL of 30% H2O2 was added, and the suspension was heated until boiling. The suspension was centrifuged for 15 min at 3000 rpm, and the supernatant was transferred to a graduated flask. The external calibration method was used as an analytical procedure. The chemical characteristics of the substrate used in the experiments are shown in Supplemental Table S1.
Plant growth response tests
Selection of inoculum components—preliminary experiments
To select candidates for a multimicroorganismal inoculum, one representative strain of 9 identified species (O. maius, Oidiodendron griseum, P. fortinii, Hymenoscyphus sp., Meliniomyces variabilis, Helotiales sp., Cadophora finlandica, Cadophora sp. and Leptodontium orchidicola) was used for V. corymbosum inoculation in single inoculation experiment. Cylindrocarpon pauciseptatum, Sporothrix inflata, Sporothrix variecibatus and T. harzianum were not used due to difficulties in maintaining fungal cultures. Micropropagated rooted plants were transferred into pots with sterile substrate composed of peat and perlite (5:1; v:v) and inoculated with 4 mL of liquid inoculum. Twenty-four plants per treatment were prepared. Plants were cultivated in Sunbags (Sigma Aldrich, St. Louis, MO, USA) in greenhouse (16-h photoperiod, under 90 mmol·m−2·s−1 of light intensity, 21/17 °C day/night temperature and 70% humidity) and irrigated twice a week (once with 2 mL sterile water and once with 2 mL Long Ashton (Hewitt 1966)). Three-month-old plants were harvested for analysis.
We selected the three ericoid mycorrhizal fungal species that enhanced plant growth to prepare inoculum for V. corymbosum: O. maius, Hymenoscyphus sp. and P. fortinii. In this experiment, Xylaria sp. as a representative of endophytic fungi was used to coinoculate V. corymbosum. Xylaria sp. has been previously shown to enhance mycorrhiza development in Verbascum plants (Wężowicz et al. 2017). Plants were inoculated with 2 mL of solid inoculum of ericoid fungi and 3 mL of liquid inoculum of Xylaria sp., cultivated in a greenhouse (16-h photoperiod, 21/17 °C day/night temperature) and irrigated twice a week with sterile water or Long Ashton solution. Three-month-old plants were harvested for analysis.
Semi-industrial scale blueberry production in greenhouses
To verify the ability of the inoculum to affect the growth of plants at different stages of ontogenesis, we used 4-week-old plants while transferring them from in vitro to the peat substrate, and the 2-year-old plants that were cultivated in peat substrate in pots. For clarity, plants inoculated during transfer from in vitro will be referred to as young, and plants inoculated during transfer to 1-L pots will be referred to as older. Inoculums composed of selected fungal species were investigated in greenhouse blueberry production scale. Plants transferred from in vitro to peat substrate were inoculated by adding 2 mL of solid inoculum of ericoid fungi and 3 mL of liquid inoculum of Xylaria sp. to the planting hole just before planting them out. In the case of the older plants, inoculum was mixed with peat in a concrete mixer (5 mL of solid ericoid inoculum and 1.5 mL of liquid Xylaria sp. inoculum per 1 L of peat), which was used as a new substrate in 1-L pots, into which the plants from small pots were transferred. Plants were cultivated in a greenhouse under natural day/night conditions and were watered with tap water every 2 days. Plants were inoculated in May (250 seedlings per treatment) and harvested after the growing season in September.
Inoculum preparation
For the first experiment, the inoculum was prepared in a liquid medium. Fungi were cultured on 2% malt extract at 26 °C, in an orbital shaker at 130 rpm·min−1 for 7 days. Fungal liquid cultures were centrifuged at 7000 × g for 5 min and washed twice in sterile deionized water. Mycelium was homogenized with a blade and suspended in 50 mL sterile, deionized water. The plants were inoculated just before planting by adding 3 mL of inoculum to the substrate.
Selected for the semi-industrial scale experiment ericoid fungi were cultured on a carrier composed of wheat bran and diatomaceous earth. Carrier components were mixed (4:1, v:v), placed in jars (50 mL carrier/200-mL jar) and sterilized (121 °C, 20 min). Subsequently, 3 plugs of the mycelium (grown on PDA medium in the dark at 26 °C for 7 days) were placed on the carrier. The inoculum was incubated in the dark at 26 °C for 30 days, separately for each fungal species. To inoculate the plant, inocula of different species were mixed in equal volumes and 2 mL of inoculum was placed in each planting hole just before planting.
Photosynthetic efficiency
Chlorophyll fluorescence measurements were performed with a Handy Pea fluorimeter (Hansatech Instruments, King’s Lynn, UK). One mature leaf from each plant (10 replicates) was dark-adapted for 20 min in a special clips before the measurement. Data were processed with BIOLYZER software (Laboratory of Bioenergetics, Geneva, Switzerland). Each fluorescent transient was calculated according to the OJIP test (Tsimilli-Michael and Strasser 2008). The method of OJIP fluorescence relies on the rapid rise of in vivo chlorophyll fluorescence quantum yield from PSII after illumination with light. In dark adapted leaves, exposition to light induces a fluorescent transient that rises from a minimum level (O level) to a maximum (P level) via two intermediate stages termed J and I. The kinetics of this transient can be used to assess the plants performance (Stirbet and Govindjee 2011). The following multiparametric indices were used to assess plant vitality (Strasser et al. 2000):
-
PIABS is the performance of the photosynthesis apparatus expressed in relation to absorption:
$${PI}_{ABS}=\frac{RC}{ABS}\bullet \frac{{\varphi }_{P0}}{1-{\varphi }_{P0}}\bullet \frac{{\psi }_{0}}{{1-\psi }_{0}}$$
where RC/ABS is a measure of the fraction of reaction centre chlorophyll (ChlRC) per chlorophyll of the antennae (ChlAntenna). φP0/(1 − φP0) indicates the contribution of the light reactions to primary photochemistry according to the JIP-test. Electron transport beyond Qa (primary quinone acceptor) is quantified as ψ0/(1 − ψ0).
-
Performance index (PItotal):where RE/ABS indicates the contribution of the reduction of end equivalents.
$${PI}_{total}={PI}_{ABS}\bullet \frac{RE}{ABS}$$
Phenolic compounds analysis
The total phenolic, tartaric ester and flavonol concentrations were estimated using the spectrophotometric method described by Fukumoto and Mazza (2000). Four replicates of 100 mg of frozen leaf tissue were homogenized in 5 mL of 80% methanol and centrifuged at 4800 × g at 4 °C for 15 min. Leaf extract (0.25 mL) was mixed with 0.25 mL 0.1% HCl in 96% ethanol and 4.50 mL 2% HCl in H2O and incubated at dark at room temperature for 15 min. Absorbance was measured at wavelengths of 280, 320, 360 and 520 nm. Calibration curves for calculating total phenolics, tartaric esters and flavonols were made using the following standards: chlorogenic acid, caffeic acid and quercetin, respectively. Calculation of phenolic compounds was expressed as mg of standard equivalents per 1 g of leaf dry weight.
Statistical analysis
Statistical analysis was performed using Statistica 13 (Tibco, Palo Alto, USA) and was considered significant at p ≤ 0.05. Data normal distribution and variance homogeneity were assessed with Shapiro–Wilk’s and Levene’s tests, respectively. Differences were tested using t-test or analysis of variance (ANOVA) followed by Tuckey’s or Dunnett post hoc tests.
Results
Selection of inoculum components
Fungi isolated from blueberry hairy roots
Thirty-nine pure cultures of fungi were isolated from 273 V. corymbosum hairy root fragments. According to the sequences of the ITS rRNA region, a total of 13 endophytic fungal taxa inhabited plant hairy roots (Supplemental Table S2, Supplemental Fig. S1). There were as follows: C. finlandica (C.J.K. Wang & H.E. Wilcox) T.C. Harr. & McNew, Cadophora sp. Lagerb. & Melin, C. pauciseptatum Schroers & Crous, Helotiales sp. Nannf., Hymenoscyphus sp. Gray, L. orchidicola Sigler et Currah, M. variabilis Hambl. & Sigler, O. griseum Robak, O. maius G.L. Barron, P. fortinii C.J.K. Wang & H.E. Wilcox, S. inflata de Hoog, S. variecibatus Roets, Z.W. de Beer & Crous and T. harzianum Rifai. All the fungi that we isolated belonged to the phylum Ascomycota. The most frequently isolated taxa were Hymenoscyphus sp. (11 isolates) and O. maius (5 isolates).
Screening for plant growth promoting fungi and inoculum testing
O. maius had a positive effect on most of the investigated parameters in comparison to control plants: plant height was improved by 40% (from 12.1 to 16.9 cm), plant fresh biomass by 47% (from 0.54 to 0.80 g) and dry biomass by 41% (from 0.12 to 0.17 g), leaf area by 45% (from 1.02 to 1.48 cm2) and root length by 59% (from 188 to 298 cm) (Fig. 2). P. fortinii increased only plant dry weight by 21% (from 0.12 to 0.15 g; Fig. 2). L. orchidicola negatively affected plant fresh weight; plants yielded 40% less dry weight than those not inoculated (from 0.12 to 0.07 g; Fig. 2). All of the other fungi showed a neutral effect on plant biometric parameters; plant height ranged from 9.0 (L. orchidicola) to 13.9 cm (Helotiales sp.), plant fresh weight—from 0.27 (L. orchidicola) to 0.58 g (O. griseum), plant dry weight—from 0.07 (L. orchidicola) to 0.13 g (O. griseum) (Fig. 2). Hymenoscyphus sp. accelerated the process of plant rooting 10 days after inoculation (Fig. 3).
Parameters of V. corymbosum inoculated with fungi isolated from blueberry hairy roots: plant height (A); plant fresh weight (B); plant dry weight (C); leaf number (D); leaf area (E); root length (F). For each treatment, 24 seedlings were inoculated and grown in peat and perlite (5:1, v:v). Plants were harvested for analysis 90 days after inoculation. Significant statistical differences between inoculated and control plants are indicated by asterisks (Dunnett test, p ≤ 0.05, n = 3–80)
O. maius, P. fortinii and Hymenoscyphus sp. were selected for the final composition of fungi used for induction of blueberry growth. Additionally, to test whether the endophytic fungus Xylaria sp. can enhance the plant growth-promoting effect, combinations of different inocula supplemented with Xylaria sp. were tested. We tested O. maius, P. fortinii and Hymenoscyphus sp. in different combinations to find the optimal composition of inoculum used for blueberry growth promotion. Inoculation with a single strain of O. maius, with a combination of O. maius and Hymenoscyphus sp. and with O. maius, Hymenoscyphus sp. and P. fortinii increased plant height 7 weeks after inoculation up to 32%, 17% and 34%, respectively (Fig. 4A). Twelve weeks after inoculation, we observed a positive effect of the combination of three microorganisms (O. maius, Hymenoscyphus sp. and P. fortinii) on plant height (Fig. 4B). Xylaria sp. improved the growth of plants inoculated with a single strain of O. maius, with the combination of O. maius and Hymenoscyphus sp. and with the combination of O. maius, Hymenoscyphus sp. and P. fortinii 7 weeks after inoculation (Fig. 4C). However, 12 weeks after inoculation, Xylaria sp. improved the growth of plants inoculated only with a combination of three ericoid fungi (O. maius, Hymenoscyphus sp. and P. fortinii) (Fig. 4D).
The height of plants inoculated with single or multiple inocula composed of the ericoid fungi Oidiodendron maius, Hymenoscyphus sp. and Phialocephala fortinii seven (A) and 12 (B) weeks after inoculation and the ericoid fungi and the endophytic fungus Xylaria sp. seven (C) and 12 (D) weeks after inoculation. For each treatment, 36 unrooted V. corymbosum seedlings were inoculated and grown in peat and perlite (5:1, v:v). Bars indicate ± SD; the statistical significance was tested using one-way analysis of variance (ANOVA) followed by the Dunnett (A, B) test and a t-test (C, D) at p ≤ 0.05 (N = 20)
Semi-industrial scale of blueberry biotization in greenhouse
Growth parameters
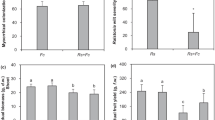
At the end of the season, the height of young plants reached 11.6 cm and was significantly better than that of control plants (35%; Fig. 5A). Additionally, inoculation of the young plants resulted in significantly increased leaf area (61%, from 3.8 to 6.1 cm2; Fig. 5B) and shoot fresh weight (33%, from 0.6 to 0.8 g; Fig. 5C). Plant dry weight was not affected by the inoculation of young plants (Fig. 5D). Root diameter and root volume were positively affected by inoculation (by 14% and 59%, respectively; Table 1).
Plant height (A, E); leaf area (B, F); fresh (C, G) and dry biomass (D, H) of V. corymbosum inoculated with inoculum composed of the ericoid fungi Oidiodendron maius, Hymenoscyphus sp. and Phialocephala fortinii and the endophytic fungus Xylaria sp. at the moment of transferring plants from in vitro to peat (A–D) and at the moment of transferring from small (0.4 L) to larger (1 L) pots (E–H). For each treatment, 250 seedlings were inoculated and grown in peat and perlite (5:1, v:v). Plants were harvested for analysis at the end of the growing season. Bars indicate ± SD; statistical significance was tested using t-test at p ≤ 0.05 (N = 20)
In the case of older plants, we did not observe any differences in plant height upon inoculation (Fig. 5E). However, inoculated plants had larger leaf area (100%; Fig. 5F) and fresh (Fig. 5G) and dry (Fig. 5H) root weight; root fresh and dry mass were improved by 57% (from 2.1 to 3.3 g) and 72% (from 0.4 to 0.7 g), respectively. Plant shoots yielded 62% (from 2.7 to 4.2 g) more dry and 55% (from 7.3 to 11.8 g) more fresh biomass. In total, plant biomass production was improved by 60% in relation to dry mass production and 57%, for fresh mass.
Plant vitality
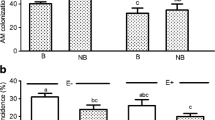
The contribution of light reactions to primary photochemistry (φP0/(1 − φP0)) was significantly higher for young, inoculated plants than for control plants (Fig. 6A). In older plants, this parameter did not change upon inoculation. However, other tested parameters (absorbance performance index, fraction of reaction centre chlorophyll per chlorophyll of the antennae, electron transport beyond primary quinone acceptor and contribution of the reduction of end equivalents) were significantly higher in inoculated plants than in control plants (Fig. 6B).
PSII efficiency of V. corymbosum inoculated with inoculum composed of the ericoid fungi Oidiodendron maius, Hymenoscyphus sp. and Phialocephala fortinii and the endophytic fungus Xylaria sp. at the moment of transferring plants from in vitro to peat (A) and at the moment of transferring from small (0.4 L) to larger (1 L) pots (B) compared to noninoculated plants (control). For each treatment, 250 seedlings were inoculated. Plants were grown in peat and perlite (5:1, v:v), irrigated with water. Plants were harvested for analysis at the end of the growing season. JIP-test parameters: PIabs—absorbance performance index, PItotal—total performance index, φP0/(1 − φP0)—contribution of light reactions for primary photochemistry, RC/ABS—fraction of reaction centre chlorophyll per chlorophyll of the antennae, ψ0/(1 − ψ0)—electron transport beyond primary quinone acceptor and RE/ABS—contribution of the reduction of end equivalents are presented relative to entirely noninoculated plants; statistically significant differences between particular treatments and those entirely noninoculated plants using t-test, p ≤ 0.05 (N = 10) are indicated by asterisk
Secondary metabolites in leaves
The concentrations of tartaric esters and flavonols in the leaves of young, inoculated plants reached 168 and 60 mg of standard equivalents and were significantly lower than those in control plants (Fig. 7A). The total phenolic concentration did not differ between the experimental treatments in young plants. In the case of older inoculated plants, the total phenolic, tartaric ester and flavonol concentrations reached 443, 214 and 65 mg of standard equivalents and did not differ significantly from the control, noninoculated plants (Fig. 7B).
Total phenolic, tartaric ester and flavonol concentrations in leaves of V. corymbosum inoculated with inoculum composed of the ericoid fungi Oidiodendron maius, Hymenoscyphus sp. and Phialocephala fortinii and endophytic fungus Xylaria sp. at the moment of transferring plants from in vitro to peat (A) and at the moment of transferring from small (0.4 L) to larger (1 L) pots (B). For each treatment, 250 seedlings were inoculated. Plants were grown in peat and perlite (5:1, v:v) irrigated with water. Plants were harvested for analysis at the end of the growing season. Bars indicate ± SD; statistical significance was tested using t-test at p ≤ 0.05 (N = 5)
Discussion
Thirteen endophytic fungal taxa were isolated from the hairy roots of V. corymbosum. Taxonomically, all the fungal strains belonged to the Ascomycota. Some of these taxa such as O. griseum, O. maius, Hymenoscyphus sp., C. finlandica and Cadophora sp. were previously shown to be mycorrhizal symbionts of ericoid plants (Dalpé 1986; Douglas et al. 1989; Cairney and Burke 1998; Vrålstad et al. 2002; Villarreal-Ruiz et al. 2004; Bizabani and Dames 2015). M. variabilis is known as an anamorph of root-associated fungi belonging to H. ericae (Hambleton and Sigler 2005). Others, L. orchidicola, P. fortinii, S. inflata, S. variecibatus and T. harzianum, are known as nonmycorrhizal, symbiotic fungi (Pearson and Read 1973; Hambleton and Currah 1997; Halmschlager and Kowalski 2003; Vohník et al. 2003; Fan et al. 2020). Recently, Meliniomyces spp. and P. fortinii have been shown to be colonizers not only of roots but also of aboveground compartments of V. myrtillus (Perotto et al. under review).
In the experiments, in which plants were inoculated with a single strain of ericoid fungi, the most beneficial for plant growth were O. maius and P. fortinii. Previously, the application of O. griseum, Pezizella ericae and H. ericae were shown to promote V. corymbosum growth (Scagel 2005; Scagel et al. 2005). Inoculation increased nutrient uptake, nutrient use efficiency and leaf biomass of Bluecrop, Earliblue, Georgia Gem and Patrion highbush blueberry cultivars (Scagel 2005). In another study, Bizabani and Dames (2015) showed that fungi from the Cadophora and Lachnum genera improved highbush blueberry growth. Thus, not only mycorrhizal fungi promoted highbush blueberry growth. Inoculation of blueberry with the endophytic fungus Anteaglonium improved root growth and shoot branching, which may be largely attributed to phytohormone signalling and biosynthesis in inoculated plants (Wu et al. 2021). Contrary to our results, Vohník et al. (2012) did not observe a biomass increase in V. corymbosum inoculated with the root symbiotic fungi: O. maius, H. ericae and P. fortinii. However, the addition of Agrocybe praecox, a saprotrophic basidiomycete, positively affected plant growth and biomass production. This effect may be associated with improved nutrient release from plant residues in the substrate by the lignin-degrading fungus (Vohník et al. 2012).
For the final composition of fungi used for the induction of blueberry growth, we added Xylaria sp. to the selected O. maius, P. fortinii and Hymenoscyphus sp.. Xylaria sp. is a representative of endophytic fungi shown to enhance mycorrhizal development in another plant species (Wężowicz et al. 2017). In our experiment, the addition of Xylaria sp. to inoculum composed of O. maius, Hymenoscyphus sp. and P. fortinii increased plant height. A previous study carried out on Lactuca serriola showed that the coinoculation with mycorrhizal and endophytic fungi was more beneficial for the plant than inoculation with a single fungus (Ważny et al. 2018). Blueberry biotization with ericoid and endophytic fungi on a semi-industrial scale improved plant growth parameters. The most pronounced increase was an increase in plant biomass production and a significant increase in plant leaf area. Significantly higher yields were obtained independently of the culture regime.
Accelerated growth increases plant demand for energy. Activation of photosynthesis and carbon assimilation in symbiotic plants has been shown previously (Sheng et al. 2008; Rozpądek et al. 2014, 2019; Ważny et al. 2018). This feature of plants treated with plant growth promoting microorganisms has been described on numerous occasions, however, never completely. Here, biotization of plants improved many photosynthesis indices (absorbance performance index, fraction of reaction centre chlorophyll per chlorophyll of the antennae, electron transport beyond the primary quinone acceptor and contribution to the reduction of end equivalents). This aspect of plant-microorganism interactions may play a significantly more important role than only improving plant growth. We cannot exclude the possibility that it also plays an important role in plant stress protection and disease resistance. This, however, requires further investigation.
Antioxidant compounds in plant tissues include mostly phenolic compounds (phenolic acid, flavonoids, tannins, stilbenes), carotenoids and vitamins (Larson 1988; Cao et al. 1997; Pietta 2000). Genetic factors, ontogenesis, intrafruit variation, field conditions, postharvest management and fruit processing are the main factors that affect the antioxidant capacity of crops (Manganaris et al. 2014; Rozpądek et al. 2015). Field management systems can play a significant role in antioxidant levels. In previous years, this system has focused on intraspecies crosses (Diamanti et al. 2012) and chemical growth regulators (Percival and MacKenzie 2007). The utilization of biotization methods is much less popular in agronomic practice. Our study shows that plant biotization with mycorrhizal and endophytic fungi did not increase secondary metabolites (tartaric esters, flavonols and total phenolics) in plant leaves. Most importantly, it did not have a negative effect on metabolite content. The total phenolics content in the leaves of blackberry, red raspberry and strawberry investigated by Wang and Lin (2000) varied with cultivar and developmental stage; young leaves had a higher total phenolic content than old leaves. These authors showed a positive correlation between phenolic content and oxygen radical absorbance capacity (ORAC); however, the correlation of ORAC values in fruits and leaves from some cultivars appears to be unclear.
In conclusion, the results presented here indicate that biotization of V. corymbosum with ericoid mycorrhizal and endophytic fungi significantly improved the biomass and vitality of plants cultivated on a semi-industrial scale. The biotization technology of highbush blueberry shown in this paper has been patented and may be utilized in horticulture (Turnau et al. 2021). This technology can be a way to modify conventional field management systems in blueberry fruit production in the direction of environmentally friendly practices and for organic blueberry production. Large-scale production of blueberries is based on in vitro plant material, cultivated in pots for one or a few seasons before planting out. These plants are mainly colonized by fungi that are already present in the soil, mutualists and pathogens. Inoculation of the soil with selected microorganisms can accelerate plant colonization by symbiotic microorganisms and, as a result, improve plant resistance to diseases. To improve plant growth and productivity (fruiting) with biotization, symbiotic microorganisms colonizing all plant tissues should also be taken into account.
Data availability
Data generated during this study (nucleotide sequences) have been deposited in the GenBank database with the primary accession numbers OM729672–OM729684.
References
Bizabani C, Dames J (2015) Effects of inoculating Lachnum and Cadophora isolates on the growth of Vaccinium corymbosum. Microbiol Res 181:68–74. https://doi.org/10.1016/j.micres.2015.08.005
Cairney JWG, Burke RM (1998) Extracellular enzyme activities of the ericoid mycorrhizal endophyte Hymenoscyphus ericae (Read) Korf and Kernan: their likely roles in decomposition of dead plant tissue in soil. Plant Soil 205:181–192. https://doi.org/10.1023/A:1004376731209
Cao G, Sofic E, Prior RL (1997) Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med 22:749–760. https://doi.org/10.1016/S0891-5849(96)00351-6
Colwell JD (1963) The estimation of the phosphorus fertilizer requirements of wheat in southern New South Wales by soil analysis. Aust J Exp Agric 3:190–197
Dalpé Y (1986) Axenic synthesis of ericoid mycorrhiza in Vaccinium angustifolium Ait. by Oidiodendron species. New Phytol 103:391–396
De Silva A, Patterson K, Rothrock C, Moore J (2000) Growth promotion of highbush blueberry by fungal and bacterial inoculants. HortScience 35:1228–1230. https://doi.org/10.21273/hortsci.35.7.1228
Diamanti J, Capocasa F, Balducci F, Battino M, Hancock J, Mezzetti B (2012) Increasing strawberry fruit sensorial and nutritional quality using wild and cultivated germplasm. PLoS ONE 7(10):e46470. https://doi.org/10.1371/journal.pone.0046470
Douglas GC, Heslin MC, Reid C (1989) Isolation of Oidiodendron maius from Rhododendron and ultrastructural characterization of synthesized mycorrhizas. Can J Bot 67:2206–2212. https://doi.org/10.1139/b89-280
Fan M, Chen X, Luo X, Zhang H, Liu Y, Zhang Y, Wu J, Zhao C, Zhao P (2020) Diversity of endophytic fungi from the leaves of Vaccinium dunalianum. Lett Appl Microbiol 71:479–489. https://doi.org/10.1111/lam.13345
Fehrer J, Réblová M, Bambasová V, Vohník M (2019) The root-symbiotic Rhizoscyphus ericae aggregate and Hyaloscypha (Leotiomycetes) are congeneric: phylogenetic and experimental evidence. Stud Mycol 92:195–225. https://doi.org/10.1016/j.simyco.2018.10.004
Fukumoto LR, Mazza G (2000) Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem 48:3597–3604. https://doi.org/10.1021/jf000220w
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gaskins MH, Albrecht SL, Hubbell DH (1985) Rhizosphere bacteria and their use to increase plant productivity: a review. Agric Ecosyst Environ 12:99–116. https://doi.org/10.1016/0167-8809(85)90071-4
Gianinazzi S, Schüepp H, Barea JM, Haselwandter K (2002) Mycorrhizal technology in agriculture. From genes to bioproducts. Birkhäuser Verlag, Basel, Boston, Berlin, pp 1–296
Gianinazzi S, Oubaha L, Chahbandar M, Blal B, Lemoine M-C (2003) Biotization of microplants for improved performance. Acta Hortic 625:165–172. https://doi.org/10.17660/ActaHortic.2003.625.17
Gollotte A, Secco B, Mercy L, Lemoine MC, Durand P, Prost M, Gianinazzi S (2009) Raspberry breeding and biotisation for increasing plant stress tolerance and antioxidant activity. Acta Hortic 838:145–150. https://doi.org/10.17660/ActaHortic.2009.838.24
Halmschlager E, Kowalski T (2003) Sporothrix inflata, a root-inhabiting fungus of Quercus robur and Q. petraea. Mycol Prog 2:259–266
Hambleton S, Currah RS (1997) Fungal endophytes from the roots of alpine and boreal Ericaceae. Can J Bot 75:1570–1581. https://doi.org/10.1139/b97-869
Hambleton S, Sigler L (2005) Meliniomyces, a new anamorph genus for root-associated fungi with phylogenetic affinities to Rhizoscyphus ericae (=Hymenoscyphus ericae), Leotiomycetes. Stud Mycol 53:1–27
Hamim A, Miché L, Douaik A, Mrabet R, Ouhammou A, Duponnois R, Hafidi M (2017) Diversity of fungal assemblages in roots of Ericaceae in two Mediterranean contrasting ecosystems. Comptes Rendus - Biol 340:226–237. https://doi.org/10.1016/j.crvi.2017.02.003
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition, 2nd edn. Farnham Royal, Bucks, England: The Commonwealth Agricultural Bureaux 22:1–241. https://doi.org/10.2136/sssaj1953.03615995001700030033x
Hu J, Wei Z, Friman VP, Gu SH, Wang XF, Eisenhauer N, Yang TJ, Ma J, Shen QR, Xu YC, Jousset A (2016) Probiotic diversity enhances rhizosphere microbiome function and plant disease suppression. Mbio 7(6):e01790-e1816. https://doi.org/10.1128/mBio.01790-16
Jansa J, Vosátka M (2000) In vitro and post vitro inoculation of micropropagated Rhododendrons with ericoid mycorrhizal fungi. Appl Soil Ecol 15:125–136. https://doi.org/10.1016/S0929-1393(00)00088-3
Jeffries P, Gianinazzi S, Perotto S, Turnau K, Barea JM (2003) The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol Fertil Soils 37:1–16. https://doi.org/10.1007/s00374-002-0546-5
Larson RA (1988) The antioxidants of higher plants. Phytochemistry 27:969–978. https://doi.org/10.1016/0031-9422(88)80254-1
Liu R, Dai M, Wu X, Li M, Liu X (2012) Suppression of the root-knot nematode [Meloidogyne incognita (Kofoid & White) Chitwood] on tomato by dual inoculation with arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria. Mycorrhiza 22:289–296. https://doi.org/10.1007/s00572-011-0397-8
Manganaris GA, Goulas V, Vicente AR, Terry LA (2014) Berry antioxidants: small fruits providing large benefits. J Sci Food Agric 94:825–833. https://doi.org/10.1002/jsfa.6432
Nguyen MP, Lehosmaa K, Martz F, Koskimäki JJ, Pirttilä AM, Häggman H (2021) Host species shape the community structure of culturable endophytes in fruits of wild berry species (Vaccinium myrtillus L., Empetrum nigrum L. and Vaccinium vitis-idaea L.). FEMS Microbiol Ecol 97:1–13. https://doi.org/10.1093/femsec/fiab097
Ortiz-Galeana MA, Hernández-Salmerón JE, Valenzuela-Aragón B, Delossantos-Villalobos S, del Carmen Rocha-Granados M, Santoyo G (2018) Diversidad de bacterias endófitas cultivables asociadas a plantas de arándano (Vaccinium corymbosum L.) cv. Biloxi con actividades promotoras del crecimiento vegetal. Chil J Agric Anim Sci 34(2):140–151. https://doi.org/10.4067/s0719-38902018005000403
Pearson V, Read DJ (1973) The biology of mycorrhiza in the Ericaceae, I. The isolation of the endophyte and synthesis of mycorrhizas in aseptic culture. New Phytol 72:371–379. https://doi.org/10.1111/j.1469-8137.1973.tb02044.x
Percival D, MacKenzie JL (2007) Use of plant growth regulators to increase polyphenolic compounds in the wild blueberry. Can J Plant Sci 87:333–336. https://doi.org/10.4141/P06-120
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042. https://doi.org/10.1021/np9904509
Prior RL, Cao G, Martin A, Sofic E, McEwen J, O’Brien C, Lischner N, Ehlenfeldt M, Kalt W, Krewer G, Mike C (1998) Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J Agric Food Chem 46:2686–2693
Remans R, Ramaekers L, Schelkens S, Hernandez G, Garcia A, Reyes JL, Mendez N, Toscano V, Mulling M, Galvez L, Vanderleyden J (2008) Effect of Rhizobium-Azospirillum coinoculation on nitrogen fixation and yield of two contrasting Phaseolus vulgaris L. genotypes cultivated across different environments in Cuba. Plant Soil 312:25–37. https://doi.org/10.1007/s11104-008-9606-4
Rozpądek P, Wężowicz K, Stojakowska A, Malarz J, Surówka E, Sobczyk T, Anielska T, Ważny R, Miszalski Z, Turnau K (2014) Mycorrhizal fungi modulate phytochemical production and antioxidant activity of Cichorium intybus L. (Asteraceae) under metal toxicity. Chemosphere 112:217–224. https://doi.org/10.1016/j.chemosphere.2014.04.023
Rozpądek P, Nosek M, Ślesak I, Kunicki E, Dziurka M, Miszalski Z (2015) Ozone fumigation increases the abundance of nutrients in Brassica vegetables: broccoli (Brassica oleracea var. italica) and Chinese cabbage (Brassica pekinensis). Eur Food Res Technol 240:459–462. https://doi.org/10.1007/s00217-014-2372-z
Rozpądek P, Nosek M, Domka A, Ważny R, Jędrzejczyk R, Tokarz K, Pilarska M, Niewiadomska E, Turnau K (2019) Acclimation of the photosynthetic apparatus and alterations in sugar metabolism in response to inoculation with endophytic fungi. Plant Cell Environ 42:1408–1423. https://doi.org/10.1111/pce.13485
Scagel CF (2005) Inoculation with ericoid mycorrhizal fungi alters fertilizer use of highbush blueberry cultivars. HortScience 40:786–794
Scagel CF, Wagner A, Winiarski P (2005) Inoculation with ericoid mycorrhizal fungi alters root colonization and growth in nursery production of blueberry plants from tissue culture and cuttings. Small Fruits Rev 4:113–135. https://doi.org/10.1300/J301v04n04_11
Sheng M, Tang M, Chen H, Yang B, Zhang F, Huang Y (2008) Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 18:287–296. https://doi.org/10.1007/s00572-008-0180-7
Stirbet A, Govindjee (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: basics and applications of the OJIP fluorescence transient. J Photochem Photobiol b: Biol 104(1–2):236–257. https://doi.org/10.1016/j.jphotobiol.2010.12.010
Strasser R, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanisms, regulation & adaptation. Taylor and Francis, London, pp 445–483
Strik BC, Yarborough D (2005) Blueberry production trends in North America, 1992 to 2003, and predictions for growth. Horttechnology 15:391–398. https://doi.org/10.21273/horttech.15.2.0391
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355. https://doi.org/10.4324/9781315839707
Tsimilli-Michael M, Strasser RJ (2008) In vivo assessment of stress impact on plant’s vitality: applications in detecting and evaluating the beneficial role of mycorrhization on host plants. In: Varma A (ed) Mycorrhiza, genetics and molecular biology, eco-function, biotechnology, eco-physiology, structure and systematics. Springer-Verlag, Berlin Heidelberg, 679–703. https://doi.org/10.1007/978-3-540-78826-3
Turnau K, Henriques FS, Anielska T, Renker C, Buscot F (2007) Metal uptake and detoxification mechanisms in Erica andevalensis growing in a pyrite mine tailing. Environ Exp Bot 61:117–123. https://doi.org/10.1016/j.envexpbot.2007.05.001
Turnau K, Ważny R, Rozpądek P (2021) Inoculum, a method of preparing inoculum and a method of biotization of highbush blueberry (in Polish). Patent Office of the Republic of Poland, Patent no, p 238335
Villarreal-Ruiz L, Anderson IC, Alexander IJ (2004) Interaction between an isolate from the Hymenoscyphus ericae aggregate and roots of Pinus and Vaccinium. New Phytol 164:183–192
Vohník M, Lukančič S, Bahor E, Regvar M, Vosátka M, Vodnik D (2003) Inoculation of Rhododendron cv. Belle-Heller with two strains of Phialocephala fortinii in two different substrates. Folia Geobot 38:191–200. https://doi.org/10.1007/BF02803151
Vohník M, Sadowsky JJ, Lukešová T, Albrechtová J, Vosátka M (2012) Inoculation with a ligninolytic basidiomycete, but not root symbiotic ascomycetes, positively affects growth of highbush blueberry (Ericaceae) grown in a pine litter substrate. Ligninolytic basidiomycete enhances growth of blueberry. Plant Soil 355:341–352. https://doi.org/10.1007/s11104-011-1106-2
Vrålstad T, Myhre E, Schumacher T (2002) Molecular diversity and phylogenetic affinities of symbiotic root-associated ascomycetes of the Helotiales in burnt and metal polluted habitats. New Phytol 155:131–148
Wang SY, Lin HS (2000) Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J Agric Food Chem 48:140–146. https://doi.org/10.1021/jf9908345
Wang MY, Bin HuL, Wang WH, Liu ST, Li M, Liu RJ (2009) Influence of long-term fixed fertilization on diversity of arbuscular mycorrhizal fungi. Pedosphere 19:663–672. https://doi.org/10.1016/S1002-0160(09)60161-2
Ważny R, Rozpądek P, Jędrzejczyk RJ, Śliwa M, Stojakowska A, Anielska T, Turnau K (2018) Does co-inoculation of Lactuca serriola with endophytic and arbuscular mycorrhizal fungi improve plant growth in a polluted environment? Mycorrhiza 28:235–246. https://doi.org/10.1007/s00572-018-0819-y
Wężowicz K, Rozpądek P, Turnau K (2017) Interactions of arbuscular mycorrhizal and endophytic fungi improve seedling survival and growth in post-mining waste. Mycorrhiza 27:1–13. https://doi.org/10.1007/s00572-017-0768-x
White T, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, White T (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Wilke B-M (2005) Determination of chemical and physical soil properties. In: Morgesin R, Schinner F (eds) Manual of soil analysis. Springer-Verlag, Berlin, Heidelberg, Monitoring and assessing soil bioremediation, pp 47–95
Wu F-L, Li Y, Tian W, Sun Y, Chen F, Zhang Y, Zhai Y, Zhang J, Su H, Wang L (2021) A novel dark septate fungal endophyte positively affected blueberry growth and changed the expression of plant genes involved in phytohormone and flavonoid biosynthesis. Tree Physiol 40:1080–1094. https://doi.org/10.1093/TREEPHYS/TPAA047
Yu YY, Da XuJ, Huang TX, Zhong J, Yu H, Qiu JP, Guo JH (2020) Combination of beneficial bacteria improves blueberry production and soil quality. Food Sci Nutr 8:5776–5784
Acknowledgements
The authors would like to acknowledge Teresa Anielska, Martyna Janicka and Weronika Janas (Jagiellonian University, Poland) for technical support and Niwa Hodowla Roślin Jagodowych (PL company) for cooperation in the project.
Funding
This work was supported by The National Science Centre and The National Centre for Research and Development, Poland, project TANGO (contract No. TANGO1/269101/NCBR/2015).
Author information
Authors and Affiliations
Contributions
RW, KT and PR conceived and designed the research. RW, RJJ and AD performed the research. RW analysed the data and wrote the original draft. RJJ, AD, PR and KT edited the manuscript. KT acquired funding. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ważny, R., Jędrzejczyk, R.J., Rozpądek, P. et al. Biotization of highbush blueberry with ericoid mycorrhizal and endophytic fungi improves plant growth and vitality. Appl Microbiol Biotechnol 106, 4775–4786 (2022). https://doi.org/10.1007/s00253-022-12019-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-12019-5