Abstract
Purpose
Continuous cropping of tomato (Solanum lycopersicum Mill.) causes soil degradation, accumulating Ralstonia solanacearum that induce Ralstonia wilt notably in plastic shed soils. Arbuscular mycorrhizal (AM) fungi play a crucial role in protecting hosts against such soil-borne pathogens, but comprehensive understanding of the soil–plant defense systems upon mycorrhization is not clear yet, especially at the later period of fruit production. The aim of this study was to investigate the underlining mechanisms in both soil and plant.
Materials and methods
A 10-week greenhouse pot experiment with four treatments, including control and inoculation with Funneliformis caledonium (Fc), R. solanacearum (Rs), and both strains (Rs + Fc), was carried out on a sterilized soil. Pots with two tomato plants each were randomly arranged with six replicates per treatment. The wilt severity; the tissue biomass and nutrient content; the root mycorrhizal colonization and total phenolic compounds; the leaf peroxidase (POD), polyphenol oxidase (PPO), and phenylalanine ammonia lyase (PAL) activities; and soil AM fungi and R. solanacearum abundances, soil pH, organic C and nutrient concentrations, and phosphatase activity were all tested. Both redundancy analysis (RDA) and structural equation modeling (SEM) were performed to illustrate plant overall performance among treatments and to elucidate the major influencing pathways of AM fungi.
Results and discussion
The additional inoculation with F. caledonium resulted in significant decreases of soil R. solanacearum abundance and Olsen-P concentration, as well as increases of soil pH, organic C concentration, and phosphatase activity, as compared to the soil only inoculated with R. solanacearum. Mycorrhizal inoculation also increased root total phenolic compound content, and leaf POD and PPO activities, but reduced shoot/root K ratio in plants under the attack of R. solanacearum, thereby alleviating Ralstonia wilt severity by 65.7% and yield loss by 46.5%. The RDA and SEM results revealed significant variation in plant overall performance among treatments, and the contribution of AM fungi in suppressing tomato Ralstonia wilt and yield damage particularly via ameliorating soil quality and alleviating plant metabolic pressure.
Conclusions
This study verified the bio-protection of AM fungi in both soil and plant systems against tomato Ralstonia wilt. Mycorrhization shifted the soil environment and suppressed soil R. solanacearum population, and also modulated plant nutrient translocation, increased phenolic compounds synthetization, and activated defense enzymes. Through establishing the integrated defense systems in both rhizosphere and plant, AM fungi alleviated the severity of Ralstonia disease and ameliorated yield damage in tomato.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil-borne pathogens, as signs of biological imbalances of soil ecosystems and also soil degradation, are usually associated with conventional agricultural managements (Keys 2004; Pankhurst and Lynch 2005; Liu et al. 2019b). Ralstonia wilt caused by Ralstonia solanacearum is the second most considerable bacterial pathogen in the world, which threatens many economically important crops, especially Solanaceae (Yabuuchi et al. 1995; Elphinstone et al. 2005; Mansfield et al. 2012). The bacterium can survive in soil for long periods in the absence of host, and once in the root, it rapidly enters and multiplies in the xylem, producing exopolysaccharide that blocks the vascular system and water flow, concomitant with shoot wilt and plant death (Kim et al. 2016). However, conventional chemical and rotation approaches seemed either ineffective or unpractical in preventing the disease (Aloyce et al. 2019). Other countermeasures, such as physical (e.g., solarization, fumigation, and grafting) (Zeist et al. 2019), agronomic practices (e.g., resistant cultivars and soil amendments) (Costa et al. 2019; Yang et al. 2019), biocontrol agents (e.g., antagonistic microbes) (Elazouni et al. 2019; Elsayed et al. 2020), and integrated strategies (Bhai et al. 2019), have also been implemented, among which biocontrol has become the most common and promising method with advantages such as self-sustaining and environmental friendly (Yuliar and Toyota 2015).
Arbuscular mycorrhizal (AM) fungi colonize the roots of most terrestrial plant species, forming mutually beneficial symbioses that are important in nutrient acquisition by plants, particularly phosphorus (P) (Smith and Read 2008). Besides nutritional benefits, AM fungi can also improve host resistance to abiotic (e.g., drought, salinity and surface pollutants) and biotic (e.g. soil-borne pathogenic fungi, bacteria, nematodes, and pests) stresses (Smith et al. 2010; Cui et al. 2013). Different mechanisms regarding the protection by AM fungi on plant health have been proposed, such as the compensation for nutritional losses, the direct competition with invading pathogens for carbon (C) sources and niches, and the indirect protection via changing root morphology and/or exudation (Lopez-Raez et al. 2010b; Tahat et al. 2012; Vos et al. 2014). In addition, the establishment of AM symbiosis is accompanied by hormonal, transcriptional, and metabolic changes in hosts, leading to primed plant systemic defense in response to future attackers, known as mycorrhiza-induced resistance (MIR) (Pozo and Azcon-Aguilar 2007; Lopez-Raez et al. 2010b; Jung et al. 2012). For example, peroxidase (POD) and polyphenol oxidase (PPO) have important functions for cell-wall lignification and antibiotic and cytotoxic activities to pathogens (Peter 1989; War et al. 2012; Taheri and Kakooee 2017). In addition, phenylalanine ammonia lyase (PAL) is a key enzyme in phenylpropanoid metabolism, associated with the biosynthesis of phenolics and phytoalexins (Mariutto et al. 2011). The relationships between the MIR-induced systemic defense and the activities of these defense-related enzymes have been demonstrated by studies (Ren et al. 2010; Eke et al. 2016; Jaiti et al. 2017; Wang et al. 2018).
Tomato (Solanum lycopersicum Mill.) is one of the most widely cultivated economical vegetable crops, and with high mycorrhizal growth dependency (Baum et al. 2015). Former studies have shown that AM fungi are effective biocontrol agents to manage various soil-borne diseases of tomato, including root disease caused by Phytophthora parasitica Dastur (Pozo et al. 2002), Verticillium wilt caused by Verticillium dahlia Kleb. (Karagiannidis et al. 2002), Fusarium wilt caused by F. oxysporum f. sp. lycopersici (Saccardo) Snyder et Hansen (Nair et al. 2015), and early blight disease caused by Alternaria solani Sorauer (Song et al. 2015). The protection by AM fungi against Ralstonia wilt has also been widely studied. Yuan et al. (2016) reported that the application of Funneliformis mosseae decreased the abundance of R. solanacearum by 80% at the end of a pot experiment. Tahat et al. (2012) observed anatomical changes in tomato root architecture with the presence of F. mosseae, which protected the plant from the invasion of R. solanacearum. Zhu and Yao (2004) found that Glomus versiformae induced phenolic production in tomato roots, enhancing plant resistance to R. solanacearum infection both locally and systemically. Chave et al. (2017) showed that Rhizophagus irregularis delayed the development of tomato Ralstonia wilt under in vitro culture condition, resulting in a final reduction in wilt incidence.
However, comprehensive understanding regarding the AM fungi-involved defense systems in the rhizosphere soil and plant tissues is still lacking. In addition, previous studies mostly reported a transitory effect of AM fungi on disease suppression, whereas the subsequent influence in plant development and production has been less investigated. Taiwo et al. (2007) found that only 33 and 50% of control and F. mosseae-treated plants survived when tomato fruit harvesting if R. solanacearum was inoculated 1 week after seedling transplanting. Consequently, it has been demonstrated that the bio-protection by AM fungi against soil-borne pathogens only in pre-mycorrhizal (usually at least two or three weeks) plants (Cordier et al. 1996; Chave et al. 2017). Fortunately, the large gathering of R. solanacearum in the soil commonly occurs 4 weeks after planting of tomatoes (Wei et al. 2019). Here, a pot-culture experiment was conducted to evaluate the effectiveness of pre-mycorrhization (four weeks) on tomato production under the attack of Ralstonia wilt. It was hypothesized that mycorrhizal symbiosis could help tomato plants deal with the disease through establishing defense systems in both soil and plant. This study was expected to enhance the understanding of AM fungi-involved biocontrol processes against soil-borne diseases.
2 Materials and methods
2.1 Soil, seedling, fungal, and bacterial materials
The experimental soil (sandy clay loam Orthic Anthrosol) was collected from a plastic shed with continuous tomato growing history at the Science Park (31°43′17″N, 118°46′20″E) of Nanjing Institute of Vegetable and Flower Sciences, Jiangsu province, China. Soil was air-dried, homogenized using a 5-mm sieve, and sterilized (121 °C for 1 h, twice), with pH of 5.9, organic C of 1.3 g kg−1, total nitrogen (N) of 0.61 g kg−1, available P (i.e., Olsen-P) of 48 mg kg−1, and available potassium (K) of 90 mg kg−1. Tomato seeds Hezuo 903 (Changzhong Tomato Seeds, Shanghai, China) were disinfected with 0.05% NaClO for 5 min, rinsed with distilled water, and germinated in the dark on moist filter paper at 25 °C for 48 h. Afterwards, seeds were sown in seedling trays with sterilized (121 °C for 1 h, twice) peat moss (Hengaoda Fertilizer Technology, Lianyungang, China), and transplanted after 4 weeks (when the seedlings had at least 6 leaves).
The AM fungus F. caledonium (Nicol. & Gerd.) Trappe & Gerdemann 90036 was selected based on its great antagonistic potential against soil-borne pathogens (Hu et al. 2010, 2020). It was originally isolated from a fluvo-aquic soil in Henan province, China (Liao et al. 2003), deposited at Institute of Soil Science, Chinese Academy of Sciences, Nanjing, China, and propagated by a cycle of white clover (Trifolium repens L.) and a cycle of sudangrass (Sorghum sudanense (Piper) Stapf.) in a sterilized (121 °C for 1 h, twice) substrate composed of sand, vermiculite, zeolite, and soil (4 months per cycle). The final inoculum was a mixture of rhizosphere soil containing mycorrhizal root fragments, hyphae, and spores and was air dried and passed through a 2-mm sieve.
The tested R. solanacearum phylotype 1 strain QL-Rs1115 (Wei et al. 2011) was kindly provided by the Department of Plant Nutrition and Fertilizer, Nanjing Agricultural University. The strain with pathogenicity was identified as pink colonies on triphenyl tetrazolium chloride (TTC) medium (Kelman et al. 1954). A single colony of the isolate was grown in Luria–Bertani (LB) medium and incubated at 28 °C for 48 h on a rotary shaker at 150 rpm to reach an optical density of 1.46 at 600 nm (2.1 × 108 cfu mL−1). The pathogen inoculant was prepared by centrifuging in a 50-mL tube at 8000 rpm to remove supernatant and resuspended with the same amount of sterilized water.
2.2 Pot experiment
There were four treatments: non-inoculation (control), inoculation with F. caledonium (Fc), inoculation with R. solanacearum (Rs), and inoculation with both R. solanacearum and F. caledonium (Rs + Fc), with six replicates for each treatment. Planting took place on April 9, 2018. A base fertilizer of urea (0.105 g kg−1), superphosphate (0.567 g kg−1) and potassium sulfate (0.071 g kg−1), based on the fertilization rates of local tomato greenhouses, was thoroughly mixed with the soil (4.5 kg) of each pot. The bottom layer was filled with 3.375 kg soil, followed by a thin layer of 225 g AM inoculum (an equal amount of sterilized inoculum for the non-mycorrhizal treatments). Tomato seedlings were selected for uniformity prior to transplanting, 2 seedlings for each pot. Afterwards, another 1.125 kg of soil was added as the top layer. The experiment was carried out in a sunlit greenhouse with 35/25 °C day/night temperature and 40–60% relative humidity. Pots were randomly arranged, and weighed and watered regularly to maintain soil at 70% water-holding capacity. On May 7, inoculation with R. solanacearum was conducted by evenly injecting 15 mL of bacterial suspension into 4 points of each pot soil. In early flowering and fruit forming stages, 500 mL 50% Hoagland nutrient solution (without any phosphate) was added to each pot.
2.3 Analyses of wilt severity and harvesting
On June 8, the Ralstonia wilt severity was evaluated at the late fruiting stage of tomato based on a 0–4 scale according to Kempe and Sequeira (1983), where 0 = no wilting, 1 = ≤ 25% wilting, 2 = 26–50% wilting, 3 = 51–75% wilting, and 4 = > 75% wilting or dead, and calculated as
Harvesting took place on June 17 when most fruits were ripe. Fresh fruits were weighed immediately, then chopped and oven-dried (60 °C). Leaves (0.5 g) were collected from the same position of plants for each pot, frozen in liquid N, and stored at −70 °C, until use for enzymatic analysis. Shoots were cut at the soil surface. Roots were picked up and thoroughly rinsed with tap water. A subsample of fresh roots was immediately used to assess mycorrhizal colonization. Another subsample of fresh roots (0.25 g) was frozen in liquid N and stored at −70 °C for measurement of total phenolic compounds. Shoots and the rest of root samples were dried at 60 °C, and shoot and root biomasses were recorded, respectively. The dry plant samples were then ground for following nutrient measurements. The composite soil samples were subsequently collected. A subsample of soil was kept in −70 °C for later DNA extraction. The rest of the soil samples were air-dried and homogenized by a 2-mm sieve, to determine soil chemical characteristics.
2.4 Soil analyses
Soil pH was determined using a soil-to-water ratio of 1: 2.5 (m/m). Soil organic C was determined by the method of Mebius (1960). Soil mineral N was extracted with potassium chloride and determined with a Continuous Flow Analytical System (Skalar San++) (Meisinger et al. 1992). Soil available P was extracted with sodium bicarbonate and examined following the molybdenum-blue method (Olsen et al. 1954). Soil phosphatase activity was assessed based on the method of Tabatabai (1994), and was represented as units of mg p-nitrophenol produced per gram of soil 24 h−1. All these results were calibrated based on an oven-dried soil weight (105 °C, 24 h).
Genomic DNA was extracted from 0.5 g soil per sample using a FastDNA® SPIN Kit for Soil (MP Biomedicals, OH, USA). DNA quality was assessed by a NanoDrop 1000 Spectrophotometer (Thermo Scientific, USA). Real-time PCR was performed in a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, Shanghai, China) using primer pairs NS31/AML2 (Liu et al. 2011) and Rsol_fliC (Schonfeld et al. 2003) for soil AM fungi (with Fc and Rs + Fc) and R. solanacearum (with Rs and Rs + Fc) enumeration, respectively. Both reactions were conducted in a 20-μL system, containing 10 μL of 2 TB\(\times\) Green Premix EX Taq™ II supermix (Takara Bio Inc.), 0.4 μL of each primer, and 2 μL of template (tenfold diluted). The thermal profile for AM fungi was 2 min at 94 °C, 35 cycles of 30 s at 94 °C, 1 min at 60 °C; that for R. solanacearum was 5 min at 95 °C; 35 cycles of 30 s at 95 °C, 30 s at 59 °C.
2.5 Mycorrhizal colonization and plant analyses
After clear washing in 10% potassium hydroxide (w/v) and staining with 0.05% (v/v) trypan blue in lactophenol, root (around 1.5 g fresh weight per sample) mycorrhization was measured by the visual method according to Giovannetti and Mosse (1980). For each sample, around 300 root segments (0.5 cm) were checked based on the presence of AM fungal colonization under a compound microscope (× 40). The colonization rate was calculated as the number of colonized roots divided by total number of roots examined. Nutrient concentrations in the dried and pulverized root, shoot, and fruit samples were measured by digesting in concentrated sulfuric acid and hydrogen peroxide mixture, followed by Kjeldahl digestion, molybdenum ascorbic acid colorimetry, and flame photometry for N, P, and K concentrations, respectively (Lu 2000). The shoot/root ratios were calculated as the ratios of N, P, and K concentrations in shoot to those in root for each pot.
Fresh roots (0.25 g) were ground in liquid N. Ethanol and 10% trichloroacetic acid were added in the homogenized roots, followed by centrifuging at 5000 rpm. The supernatant was used to determine the phenolic content with Folin-Ciocalteu Reagent (Lowry et al. 1951) on an Epoch Microplate Spectrophotometer (BioTek® Instruments, Inc., Winooski, VT, USA) at 765 nm. Fresh leaf samples (0.2 g) were ground using liquid N and homogenized in 10 mL of 50 mM L−1 sodium phosphate buffer (pH 7.0). The homogenate was centrifuged at 10,000 rpm for 30 min. Activities of POD, PPO, and PAL in the supernatant were measured using ELISA Kit following the manufacturer’s instruction (Shenlian Biotech, Shanghai, China).
2.6 Statistical analysis
Statistical analysis was carried out by SPSS 13.0 software. All data were analyzed using a one-way analysis of variance (ANOVA) with significant differences among means identified by Duncan’s multiple-range method (P < 0.05), except for soil AM fungi and R. solanacearum population, mycorrhizal colonization, and wilt severity, which were analyzed through Student’s T-test (P < 0.05). Normality and homogeneity of variances were checked prior to ANOVA, and data for soil mineral N, shoot and root P concentration, shoot/root K ratio, leaf POD activity were firstly Box-Cox transformed using the ‘car’ package (Fox and Weisberg 2019) in R v. 4.0.2 (R Development Core Team 2018).
Redundancy analysis (RDA) was performed to illustrate plant overall performance (shoot and root biomass, fruit yield, mycorrhizal colonization and Ralstonia wilt severity, response variables) among treatments, and their relationships with soil microbial population, soil chemical and biochemical properties, and plant nutritional and defensive features (explanatory variables), using the R package, “vegan” (Oksanen et al. 2018). Data were normalized using a MIN–MAX normalization method. Based on the variance inflation factor (VIF), soil available K was removed to reduce the possibility of collinearity among variables. The significance of RDA result was tested based on 999 permutations. The Pearson correlation coefficients between variables were calculated.
Structural equation modeling (SEM) in IBM® SPSS® AMOS™ 24.0 (IBM Corp, Armonk, NY, USA) was further employed to explore the major influencing pathways of AM fungi on tomato Ralstonia wilt severity and fruit yield. The SEM was developed from a fully conceptual model based on maximum likelihood estimation. The variances of soil quality (e.g., pH, organic C and phosphatase activity) and nutrient (e.g., mineral N and available P) properties, shoot/root N and P, and leaf enzymatic activities (e.g., PAL, POD, and PPO) were represented using the first axes in principal component analyses (PCA). Other data were standardized using Z-scores. The SEM met the following criteria: a nonsignificant chi-square test (P > 0.05); the root mean square error of approximation (RMSEA) < 0.05; the comparative fit index (CFI) > 0.90; and the root mean square residual (RMR) < 0.08 (Kline 2015).
3 Results
3.1 Soil chemical, biochemical, and biological properties
Compared with control, Rs significantly decreased (P < 0.05) soil organic C concentration and phosphatase activity, but significantly increased (P < 0.05) the soil available P and mineral N concentrations (Table 1). Compared with Rs, Rs + Fc significantly increased (P < 0.05) the soil pH, organic C concentration, and phosphatase activity but decreased (P < 0.05) the soil available P concentration. The abundance of soil AM fungi was significantly reduced (P < 0.05) by 56.4% with R. solanacearum inoculation, and a significant decrease (P < 0.05) of 76.0% was also shown in the R. solanacearum population upon mycorrhization.
3.2 Mycorrhizal colonization, Ralstonia wilt severity, plant biomass, and fruit yield
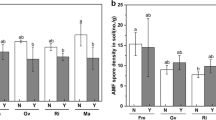
Mycorrhization was observed following inoculation of F. caledonium. The average colonization rates in Fc and Rs + Fc were 63.5% and 65.1%, respectively (Fig. 1a). Wilt symptoms were only observed in the two R. solanacearum-inoculated treatments. The severity reached 72.9% in Rs, and significantly reduced (P < 0.05) to 25.0% in Rs + Fc (Fig. 1b). Infection with R. solanacearum seriously decreased the shoot and root biomass and yield production as compared to control (Fig. 1c, d). Inoculation with F. caledonium only had positive influence (P < 0.05) in the fruit production of pathogenic plants, alleviating the yield loss by 46.5%.
Mycorrhizal colonization (a), Ralstonia wilt severity (b), plant biomass (c), and fruit yield (d) of tomato. Control, non-inoculation; Fc, inoculation with Funneliformis caledonium; Rs, inoculation with Ralstonia solanacearum; Rs + Fc, inoculation with both R. solanacearum and F. caledonium. Vertical T bars indicate standard deviations. Bars topped with asterisk or not topped by the same letter indicate a significant difference in values (P < 0.05)
3.3 Tissue concentrations and shoot/root ratios of N, P, and K
Compared with control, Rs significantly increased (P < 0.05) the shoot K concentration (Fig. 2a), decreased (P < 0.05) the root K concentration (Fig. 2b), and therefore elevated (P < 0.05) the ratio of shoot/root K (Fig. 2c). In contrast, there were significantly higher (P < 0.05) root N and P concentrations and lower ratios of shoot/root N and P in Rs relative to control. Compared with Rs, Rs + Fc showed significant decreases (P < 0.05) of P and K concentrations in shoots, but increases (P < 0.05) in P and K concentrations in roots, and decreases (P < 0.05) in shoot/root ratios of P and K. Nutrient concentrations in fruits were not affected by treatments (Fig. 2d).
The concentrations of N, P, and K in tomato shoot (a), root (b), shoot/root ratios (c), and fruit (d). Control, non-inoculation; Fc, inoculation with Funneliformis caledonium; Rs, inoculation with Ralstonia solanacearum; Rs + Fc, inoculation with both F. caledonium and R. solanacearum. Vertical T bars indicate standard deviations. Bars not topped by the same letter indicates a significant difference in values (P < 0.05)
3.4 Root total phenolic compound content, and leaf POD, PPO, and PAL activities
Without R. solanacearum inoculation, the selected plant defense features were not influenced by AM fungi. Compared with control, Rs significantly reduced (P < 0.05) the total phenolic compound content in roots and POD, PPO, and PAL activities in leaves (Fig. 3). Compared with Rs, significantly higher (P < 0.05) root total phenolic compound content and leaf POD and PPO activities were shown in Rs + Fc, whereas no difference was detected with leaf PAL activity.
Root total phenolic compounds (TPC) content (a), and leaf peroxidase (POD) (b), polyphenol oxidase (PPO) (c), and phenylalanine ammonia lyase (PAL) (d) activities of tomato. Control, non-inoculation; Fc, inoculation with Funneliformis caledonium; Rs, inoculation with Ralstonia solanacearum; Rs + Fc, inoculation with both F. caledonium and R. solanacearum. Vertical T bars indicate standard deviations. Bars not topped by the same letter indicates a significant difference in values (P < 0.05)
3.5 RDA and SEM results
The RDA plot revealed significant variation in plant overall performance among treatments (P < 0.001), 79.3% of which could be explained by the detected soil microbial abundances and other properties and plant nutrient and defense features (Fig. 4a). The fruit yield and shoot and root biomasses all showed negative correlations (P < 0.01) with Ralstonia wilt severity (Table 2), which exerted positive correlations (P < 0.001) with soil R. solanacearum abundance and mineral N and available P concentrations, and shoot/root K ratio, and negative correlations (P < 0.05) with soil AM fungal abundance, pH, organic C and root total phenolic compound content, and leaf PPO and PAL activities. Furthermore, soil R. solanacearum abundance and root mycorrhizal colonization had negative and positive correlations (P < 0.05) with AM fungal abundance, respectively.
Redundancy analysis (RDA) plot, exploring the relationships (*, **, and *** denote significances at P < 0.05, P < 0.01, and P < 0.001, respectively) between plant overall performance, soil microbial population and other properties, and plant nutrient translocation and defense features (a), and structural equation modeling (SEM), disentangling the major influencing pathways of arbuscular mycorrhizal (AM) fungi on tomato Ralstonia wilt severity and fruit yield (b). Control, non-inoculation; Fc, inoculation with Funneliformis caledonium; Rs, inoculation with Ralstonia solanacearum; Rs + Fc, inoculation with both F. caledonium and R. solanacearum; POD, peroxidase; PPO, polyphenol oxidase, PAL, phenylalanine ammonia lyase. The standardized path coefficients are reflected in the width of arrows, while solid and dashed lines indicate positive and negative path coefficients (*P < 0.05; **P < 0.01; ***P < 0.001), respectively. R2 values indicate the proportion of variance explained for each endogenous variable. Model fit parameters: chi-square = 20.897, df = 29, P = 0.863; RMSEA = 0.000; CFI = 1.000; RMR = 0.055
The SEM explained 88.6% and 66.1% of the total variances in Ralstonia wilt severity and fruit yield, respectively (Fig. 4b). Soil AM fungal abundance showed a direct positive effect (λ = 0.468, P < 0.05) on soil quality parameters (e.g., soil pH, organic C, and phosphatase activity), which had negative relationships with soil R. solanacearum abundance (λ = −0.422, P < 0.05) and Ralstonia wilt severity (λ = −0.324, P < 0.001). Root mycorrhizal colonization showed a negative effect (λ = −0.341, P < 0.05) on shoot/root K, which had a negative and a positive relationship with fruit yield (λ = −0.418, P < 0.01) and Ralstonia wilt severity (λ = 0.518, P < 0.001), respectively. However, root mycorrhizal colonization also showed a negative impact on shoot/root N and P (λ = −0.520, P < 0.01), which was positively associated with fruit yield (λ = 0.451, P < 0.001).
4 Discussion
This study aimed at revealing the protective effects and underlying mechanism of AM fungi against tomato Ralstonia wilt. As a soil-borne disease, the incidence of Ralstonia wilt can be related with the pathogen population in soil (Wei et al. 2011). In the current study, the strong relationship between Ralstonia wilt severity and soil R. solanacearum population was shown (Table 2). And there may be direct competition between the AM fungal and pathogenic strains, as suggested by the negative effects of either AM fungus or R. solanacearum treatment on their opponent’s population in soil (Table 1). In addition, soil acidity and deterioration are commonly accompanied with the development of soil-borne pathogens (Yuliar and Toyota 2015), while there is also great potential for AM fungi in preventing such processes, through ameliorating soil structure, C deposition, nutrient levels, and activities of key soil enzymes (Gianinazzi et al. 2010; Chen et al. 2018; Qin et al. 2020). This study reported increases of soil pH and organic C concentration in the pathogenic soil in response to mycorrhizal colonization (Table 1). The increase in soil phosphatase activity with mycorrhizal inoculation suggested more available P released for better nourished plants to resist the disease, while the decrease in soil available P concentration upon mycorrhization under pathogen attack was possibly due to the enhanced P uptake (Figs. 1 and 2).
Ameliorated nutrient status or fitness of mycorrhizal plant can benefit plant resistance to pathogens (Azcon-Aguilar and Barea 1996). While increasing P levels in the host plant has been reported as a common benefit of mycorrhization (Smith and Smith 2015), there is also support on plant K uptake (Liu et al. 2019a). In this study, tomato plants exhibited an extremely high shoot/root K ratio in response to pathogen attack (Fig. 2), which had a positive correlation with Ralstonia wilt severity (Fig. 4b). It is noteworthy that tomato fruit yield and root biomass and mycorrhizal colonization all showed negative correlations with the shoot/root K ratio. Potassium is crucial to tomato yield and quality (Hartz et al. 1999), and might be associated with plant pathogen resistance (Hou et al. 2020). It was possible that mycorrhization relieved the K threaten due to Ralstonia wilt, thus allowing normal K translocation rate in tomato plant. Nevertheless, the relationships among AM fungi, plant K translocation, and tomato Ralstonia wilt deserve further investigation.
Both localized and induced systemic resistance is involved in pathogen control by AM fungi (Cordier et al. 1998). On the one hand, the present study showed increased root total phenolic compounds in the mycorrhizal plants under the attack of R. solanacearum (Fig. 3a). Phenolic compounds may cause contiguous lignification in plant cell walls to prevent pathogen infection or act as signaling molecules modulating plant–microbe interactions (Matern et al. 1995; Lopez-Raez et al. 2010a). Our result was in agreement with Zhu and Yao (2004), who reported that AM fungal inoculation increased the root phenolic compounds both locally and systemically (to a less extent), hence suppressing the R. solanacearum population in both soil and roots. On the other hand, the enhanced plant systemic defense via MIR was indicated by the activation of leaf POD and PPO in the plants under pathogen attack (Fig. 3b, c). Similar to our findings, Wang et al. (2018) reported that pre-inoculation of F. mosseae could enhance tomato plant resistance to mould disease (Cladosporium fulvum) via increasing leaf POD activity. Ren et al. (2010) found that tomato leaf PPO activity at 10 weeks after transplant was decreased with inoculation of F. oxysporum but maintained a high level with the dual inoculation of F. oxysporum and G. etunicatum. The reduction with F. oxysporum and maintenance with AM fungal inoculant were also shown in root PPO, but the changes occurred earlier and to a less extent than leaf PPO. Although no mycorrhization-induced changes were shown here as findings in other studies (Eke et al. 2016), PAL activity was still an important defensive indicator that strongly correlated with plant performance (Fig. 4d). However, such leaf enzyme activities may only provide an apparent understanding of systemic defense induced by AM fungi; further exploration of the defense enzymes in roots and harmonic and transcriptomic profiles is still needed (Ghareeb et al. 2011; De Coninck et al. 2015; Rivero et al. 2015).
The biocontrol of R. solanacearum by AM fungi was thought to exert at the level of the tissues (Chave et al. 2017), since biofilms of the pathogenic bacterium required for its virulence are produced on the surface of tomato cells adjacent to intercellular spaces (Mori et al. 2016), whereas a recent study suggested that plant health can be predetermined by initial soil microbiome composition and functioning (Wei et al. 2019). In the present study, plant overall performance was largely explained by the soil microbial population and other soil properties, as well as the selected plant nutritional and defensive features, and AM fungi played key roles in the integrated defense mechanisms in both soil and plant to counter Ralstonia wilt (Fig. 4), while the SEM result also showed the negative influence of AM fungi on plant N and P translocation, indicating a possible trade-off between mycorrhiza-induced plant fitness to cope with the disease and down-regulated photosynthesis and growth-related processes (Bernsdorff et al. 2016). The causations between AM fungi, R. solanacearum, and changes in soil and plant systems, as well as the potential trade-offs involved, should be further explored. And for an improved protective efficiency, understanding of the variant biocontrol abilities of mycorrhizal species (Rivero et al. 2018) and potential synergistic effects in the mycorrhizosphere (Singh et al. 2013; Perez-de-Luque et al. 2017) is also needed.
5 Conclusions
This study verified the bio-protection of AM fungi against tomato Ralstonia wilt in an integrated soil–plant system. Firstly, inoculation with F. caledonium ameliorated soil quality and suppressed the soil R. solanacearum population. Meanwhile, mycorrhization modulated plant nutrient translocation, increased root phenolic compounds, and activated leaf defense enzymes, indicating strengthened plant immunity to the pathogen. Through establishing the integrated defense system, AM fungi alleviated the severity of Ralstonia disease and ameliorated yield damage in tomato.
References
Aloyce A, Ndakidemi PA, Mbega ER (2019) Survey and conventional management methods of bacterial wilt disease in open fields and greenhouses in Tanzania. J Plant Pathol 101:1107–1114
Azcon-Aguilar C, Barea JM (1996) Arbuscular mycorrhizas and biological control of soil-borne plant pathogens-an overview of the mechanisms involved. Mycorrhiza 6:457–464
Bernsdorff F, Doring AC, Gruner K, Schuck S, Brautigam A, Zeier J (2016) Pipecolic acid orchestrates plant systemic acquired resistance and defense priming via salicylic acid-dependent and -independent pathways. Plant Cell 28:102–129
Baum C, El-Tohamy W, Gruda N (2015) Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review. Sci Hortic 187:131–141
Bhai RS, Prameela TP, Vincy K, Biju CN, Srinivasan V, Babu KN (2019) Soil solarization and amelioration with calcium chloride or Bacillus licheniformis-an effective integrated strategy for the management of bacterial wilt of ginger incited by Ralstonia pseudosolanacearum. Eur J Plant Pathol 154:903–917
Chave M, Crozilhac P, Deberdt P, Plouznikoff K, Declerck S (2017) Rhizophagus irregularis MUCL 41833 transitorily reduces tomato bacterial wilt incidence caused by Ralstonia solanacearum under in vitro conditions. Mycorrhiza 27:719–723
Chen M, Arato M, Borghi L, Nouri E, Reinhardt D (2018) Beneficial services of arbuscular mycorrhizal fungi - From ecology to application. Front Plant Sci 9:1270
Cordier C, Gianinazzi S, Gianinazzi-Pearson V (1996) Colonisation patterns of root tissues by Phytophthora nicotianae var. parasitica related to reduced disease in mycorrhizal tomato. Plant Soil 185:223–232
Cordier C, Pozo MJ, Barea JM, Gianinazzi S, Gianinazzi-Pearson V (1998) Cell defense responses associated with localized and systemic resistance to Phytophthora parasitica induced in tomato by an arbuscular mycorrhizal fungus. Mol Plant Microbe Interact 11:1017–1028
Cui XC, Hu JL, Lin XG, Wang FY, Chen RR, Wang JH, Zhu JG (2013) Arbuscular mycorrhizal fungi alleviate ozone stress on nitrogen nutrition of field wheat. J Agric Sci Technol 15:1043–1052
Costa KDD, dos Santos PR, dos Santos AMM, Silva AMF, Chagas JTB, de Carvalho JLS, Pereira JWD, Silva MD, da Silva JR, Menezes D (2019) Genetic control of tomato resistance to Ralstonia solanacearum. Euphytica 215:136
De Coninck B, Timmermans P, Vos C, Cammue BPA, Kazan K (2015) What lies beneath: belowground defense strategies in plants. Trends Plant Sci 20:91–101
Eke P, Chatue GC, Wakam LN, Kouipou RMT, Fokou PVT, Boyom FF (2016) Mycorrhiza consortia suppress the Fusarium root rot (Fusarium solani f. sp Phaseoli) in common bean (Phaseolus vulgaris L.). Biol Control 103:240–250
Elazouni I, Abdel-Aziz S, Rabea A (2019) Microbial efficacy as biological agents for potato enrichment as well as bio-controls against wilt disease caused by Ralstonia solanacearum. World J Microbiol Biotechnol 35:30
Elphinstone J, Allen C, Prior P, Hayward A (2005) The current bacterial wilt situation: a global overview. In: Allen C, Prior P, Hayward AC (eds) Bacterial wilt disease and the Ralstonia solanacearum species complex. American Phytophatological Society Press, St. Paul, Minnesota, pp 9–28
Elsayed TR, Jacquiod S, Nour EH, Sorensen SJ, Smalla K (2020) Biocontrol of bacterial wilt disease through complex interaction between tomato plant, antagonists, the indigenous rhizosphere microbiota, and Ralstonia solanacearum. Front Microbiol 10:15
Fox J, Weisberg S (2019). An R companion to Applied Regression (3rd ed). Sage Publications
Ghareeb H, Bozso Z, Ott PG, Repenning C, Stahl F, Wydra K (2011) Transcriptome of silicon-induced resistance against Ralstonia solanacearum in the silicon non-accumulator tomato implicates priming effect. Physiol Mol Plant Pathol 75:83–89
Gianinazzi S, Gollotte A, Binet MN, van Tuinen D, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20:519–530
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Hartz TK, Miyao G, Mullen RJ, Cahn MD, Brittan KL (1999) Potassium requirements for maximum yield and fruit quality of processing tomato. J Am Soc Hortic Sci 124:199–204
Hou S, Zhang Y, Li M, Liu H, Wu F, Hu J, Lin X (2020) Concomitant biocontrol of pepper Phytophthora blight by soil indigenous arbuscular mycorrhizal fungi via upfront film-mulching with reductive fertilizer and tobacco waste. J Soils Sediments 20:452–460
Hu J, Lin X, Wang J, Shen W, Wu S, Peng S, Mao T (2010) Arbuscular mycorrhizal fungal inoculation enhances suppression of cucumber Fusarium wilt in greenhouse soils. Pedosphere 20:586–593
Hu J, Hou S, Li M, Wang J, Wu F, Lin X (2020) The better suppression of pepper Phytophthora blight by arbuscular mycorrhizal (AM) fungus than Purpureocillium lilacinum alone or combined with AM fungus. J Soils Sediments 20:792–800
Jaiti F, Meddich A, Hadrami IE (2017) Effectiveness of arbuscular mycorrhizal fungi in the protection of date palm (Phoenix dactylifera L.) against bayoud disease. Physiol Mol Plant Pathol 71:166–173
Jung SC, Martinez-Medina A, Lopez-Raez JA, Pozo MJ (2012) Mycorrhiza-induced resistance and priming of plant defenses. J Chem Ecol 38:651–664
Karagiannidis N, Bletsos F, Stavropoulos N (2002) Effect of Verticillium wilt (Verticillium dahliae Kleb.) and mycorrhiza (Glomus mosseae) on root colonization, growth and nutrient uptake in tomato and eggplant seedlings. Sci Hortic 94:145–156
Kelman Z, Farkas FI, Lovredkovich I (1954) Hypersensitive reaction induced by phytophathogenic bacteria in the tobacoo leaf. Phytopathology 54:474–477
Kempe J, Sequeira L (1983) Biological-control of bacterial wilt of potatoes – attempts to induce resistance by treating tubers with bacteria. Plant Dis 67:499–503
Keys E (2004) Commercial agriculture as creative destruction or destructive creation: a case study of chili cultivation and plant-pest disease in the southern Yucatan region. Land Degrad Dev 15:397–409
Kim BS, French E, Caldwell D, Harrington EJ, Iyer-Pascuzzi AS (2016) Bacterial wilt disease: host resistance and pathogen virulence mechanisms. Physiol Mol Plant Pathol 95:37–43
Kline RB (2015) Principles and practice of structural equation modeling. Guilford Publications.
Liao JP, Lin XG, Cao ZH, Shi YQ, Wong MH (2003) Interactions between arbuscular mycorrhizae and heavy metals under sand culture experiment. Chemosphere 50:847–853
Liu JJ, Liu JL, Liu JH, Cui MM, Huang YJ, Tian Y, Chen AQ, Xu GH (2019a) The potassium transporter SIHAK10 is involved in mycorrhizal potassium uptake. Plant Physiol 180:465–479
Liu J, Yao Q, Li Y, Zhang W, Mi G, Chen X, Yu Z, Wang G (2019b) Continuous cropping of soybean alters the bulk and rhizospheric soil fungal communities in a Mollisol of Northeast PR China. Land Degrad Dev 30:1725–1738
Liu YJ, He JX, Shi GX, An LZ, Feng OM, HY, (2011) Diverse communities of arbuscular mycorrhizal fungi inhabit sites with very high altitude in Tibet Plateau. FEMS Microbiol Ecol 78:355–365
Lopez-Raez JA, Flors V, Garcia JM, Pozo MJ (2010a) AM symbiosis alters phenolic acid content in tomato roots. Plant Signal Behav 5:1138–1140
Lopez-Raez JA, Verhage A, Fernandez I, Garcia JM, Azcon-Aguilar C, Flors V, Pozo MJ (2010b) Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. J Exp Bot 61:2589–2601
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lu R (2000) Analyticle methods for soil and agro-chemistry. China Agicultural Science and Technology Press, Beijing
Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA, Toth I, Salmond G, Foster GD (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13:614–629
Mariutto M, Duby F, Adam A, Bureau C, Fauconnier ML, Ongena M, Thonart P, Dommes J (2011) The elicitation of a systemic resistance by Pseudomonas putida BTP1 in tomato involves the stimulation of two lipoxygenase isoforms. BMC Plant Biol 11:29
Matern U, Grimmig B, Kneusel RE (1995) Plant cell wall reinforcement in the disease-resistance response: molecular composition and regulation. Can J Bot 73:511–517
Mebius LJ (1960) A rapid method for the determination of organic carbon in soil. Anal Chim Acta 22:120–124
Meisinger JJ, Bandel VA, Angle JS, O’Keefe BE, Reynolds CM (1992) Presidedress soil nitrate test evaluation in Maryland. Soil Sci Soc Am J 56:575–578
Mori Y, Inoue K, Ikeda K, Nakayashiki H, Higashimoto C, Ohnishi K, Kiba A, Hikichi Y (2016) The vascular plant-pathogenic bacterium Ralstonia solanacearum produces biofilms required for its virulence on the surfaces of tomato cells adjacent to intercellular spaces. Mol Plant Pathol 17:890–902
Nair A, Kolet SP, Thulasiram HV, Bhargava S (2015) Role of methyl jasmonate in the expression of mycorrhizal induced resistance against Fusarium oxysporum in tomato plants. Physiol Mol Plant Pathol 92:139–145
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens H, Szocs E, Wagner H (2018) vegan: Community Ecology Package. Ordination methods, diversity analysis and other functions for community and vegetation ecologists. Ver 2.5–1
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Department of Agriculture, Washington
Pankhurst CE, Lynch JM (2005) Biocontrol of soil-borne plant diseases. In: Hillel D (ed) Encyclopedia of soils in the environment. Elsevier, Oxford, pp 129–136
Perez-de-Luque A, Tille S, Johnson I, Pascual-Pardo D, Ton J, Cameron DD (2017) The interactive effects of arbuscular mycorrhiza and plant growth-promoting rhizobacteria synergistically enhance host plant defences against pathogens. Sci Rep 7:16409
Peter MG (1989) Chemical modifications of bio-polymers by quinones and quinone methides. Angew Chem Int Ed 28:555–570
Pozo MJ, Azcon-Aguilar C (2007) Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol 10:393–398
Pozo MJ, Cordier C, Dumas-Gaudot E, Gianinazzi S, Barea JM, Azcon-Aguilar C (2002) Localized versus systemic effect of arbuscular mycorrhizal fungi on defense responses to Phytophthora infection in tomato plants. J Exp Bot 53:525–534
Qin M, Zhang Q, Pan J, Jiang S, Liu Y, Bahadur A, Peng Z, Yang Y, Feng H (2020) Effect of arbuscular mycorrhizal fungi on soil enzyme activity is coupled with increased plant biomass. Eur J Soil Sci 71:84–92
R Development Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ren LX, Lou YS, Sakamoto K, Inubushi K, Amemiya Y, Shen QR, Xu GH (2010) Effects of arbuscular mycorrhizal colonization on microbial community in rhizosphere soil and Fusarium wilt disease in tomato. Commun Soil Sci Plant Anal 41:1399–1410
Rivero J, Alvarez D, Flors V, Azcon-Aguilar C, Pozo MJ (2018) Root metabolic plasticity underlies functional diversity in mycorrhiza-enhanced stress tolerance in tomato. New Phytol 220:1322–1336
Rivero J, Gamir J, Aroca R, Pozo MJ, Flors V (2015) Metabolic transition in mycorrhizal tomato roots. Front Microbiol 6:598
Schonfeld J, Heuer H, van Elsas JD, Smalla K (2003) Specific and sensitive detection of Ralstonia solanacearum in soil on the basis of PCR amplification of fliC fragments. Appl Environ Microbiol 69:7248–7256
Singh R, Soni SK, Kalra A (2013) Synergy between Glomus fasciculatum and a beneficial Pseudomonas in reducing root diseases and improving yield and forskolin content in Coleus forskohlii Briq. under organic field conditions. Mycorrhiza 23:35–44
Smith FA, Smith SE (2015) How harmonious are arbuscular mycorrhizal symbioses? Inconsistent concepts reflect different mindsets as well as results. New Phytol 205:1381–1384
Smith SE, Facelli E, Pope S, Smith FA (2010) Plant performance in stressful environments: interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 326:3–20
Smith SE, Read D (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press
Song YY, Chen DM, Lu K, Sun ZX, Zeng RS (2015) Enhanced tomato disease resistance primed by arbuscular mycorrhizal fungus. Front Plant Sci 6:13
Tabatabai MA (1994) Soil enzymes. In: Weaver RW, Angle GS, Bottomley PS, Bezdicek D, Smith S, Tabatabai MA, Wollum A (eds) Methods of soil analysis. Soil Science Society of America, Madison, Wisconsin, pp 775–833
Tahat MM, Sijam K, Othman R (2012) Ultrastructural changes of tomatoes (Lycopersicon esculentum) root colonizaed by Glomus mosseae and Ralstonia solanacearum. Afr J Biotechnol 11:6681–6686
Taheri P, Kakooee T (2017) Reactive oxygen species accumulation and homeostasis are involved in plant immunity to an opportunistic fungal pathogen. J Plant Physiol 216:152–163
Taiwo L, Adebayo DT, Adebayo OS, Adediran JA (2007) Compost and Glomus mosseae for management of bacterial and Fusarium wilts of tomato. Int J Veg Sci 13:49–61
Vos CM, Yang Y, De Coninck B, Cammue BPA (2014) Fungal (-like) biocontrol organisms in tomato disease control. Biol Control 74:65–81
Wang YY, Yin QS, Qu Y, Li GZ, Hao L (2018) Arbuscular mycorrhiza-mediated resistance in tomato against Cladosporium fulvum-induced mould disease. J Phytopathol 166:67–74
War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7:1306–1320
Wei Z, Gu Y, Friman VP, Kowalchuk GA, Xu Y, Shen Q, Jousset A (2019) Initial soil microbiome composition and functioning predetermine future plant health. Sci Adv 5:eaaw0759
Wei Z, Yang XM, Yin SX, Shen QR, Ran W, Xu YC (2011) Efficacy of Bacillus-fortified organic fertiliser in controlling bacterial wilt of tomato in the field. Appl Soil Ecol 48:152–159
Yang CL, Dong Y, Friman VP, Jousset A, Wei Z, Xu YC, Shen QR (2019) Carbon resource richness shapes bacterial competitive interactions by alleviating growth-antibiosis trade-off. Funct Ecol 33:868–875
Yabuuchi E, Kosako Y, Yano I, Hotta H, Nishiuchi Y (1995) Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen. Nov. and Ralstonia eutropha (Davis 1969) comb Nov. Microbiol Immunol 39:897–905
Yuan S, Li M, Fang Z, Liu Y, Shi W, Pan B, Wu K, Shi J, Shen B, Shen Q (2016) Biological control of tobacco bacterial wilt using Trichoderma harzianum amended bioorganic fertilizer and the arbuscular mycorrhizal fungi Glomus mosseae. Biol Control 92:164–171
Yuliar NYA, Toyota K (2015) Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ 30:1–11
Zeist AR, de Resende JTV, Pozzebon BC, Gabriel A, da Silva AA, Zeist RA (2019) Combination of solarization, biofumigation and grafting techniques for the management of bacterial wilt in tomato. Hortic Bras 37:260–265
Zhu HH, Yao Q (2004) Localized and systemic increase of phenols in tomato roots induced by Glomus versiforme inhibits Ralstonia solanacearum. J Phytopathol 152:537–542
Acknowledgements
We are grateful to Dr. Zhong Wei for supplying the strain of Ralstonia solanacearum QL-Rs1115, and to Dr. Jiangang Li, Dr. Feifei Sun, and Ms. Qi Zhao for their assistance in field soil sampling. We also acknowledge two anonymous reviewers and the editor for their valuable suggestions on the manuscript revision.
Funding
This work was supported by the National Key R&D Program of China (2017YFD0200603), the National Natural Science Foundation of China (No. 41671265), and the Knowledge Innovation Program of Chinese Academy of Sciences (CAS) (ISSASIP1634). J. Hu is supported by the Youth Innovation Promotion Association, CAS (No. 2016285).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human participant and/or animal rights and informed consent
None.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Dulce Flores-Rentería
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, M., Hou, S., Wang, J. et al. Arbuscular mycorrhizal fungus suppresses tomato (Solanum lycopersicum Mill.) Ralstonia wilt via establishing a soil–plant integrated defense system. J Soils Sediments 21, 3607–3619 (2021). https://doi.org/10.1007/s11368-021-03016-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-021-03016-8