Abstract
Hyaluronic acid (HA) is a biopolymer with wide biomedical and cosmetic applications, wherein the molecular weight of HA (MWHA) is an important quality parameter that determines its suitability for the targeted application. To produce HA with desired molecular weight, it is important to identify parameters that offer tunability and control of MWHA at a desired value during fermentation. In this work, two tunable parameters, viz. glucose concentration and combination of HA biosynthetic genes expressed, were used to produce HA of different molecular weights. Three recombinant strains of Lactococcus lactis were constructed, using a combination of the has-operon genes from Streptococcus zooepidemicus (hasA, hasB, hasE) and the α-phosphoglucomutase gene (pgmA) from L. lactis. Batch fermentations of these recombinant strains at different initial glucose concentrations enabled production of HA with different molecular weights. Co-expression of hasABE was observed to be particularly effective in improving the MWHA. It was observed during batch fermentations of all these recombinant L. lactis cultures that the MWHA decreases steadily during the later part of the fermentation and the final value is 19–43% lower than the peak MWHA produced. Analysis of the fermentation data showed that the decrease in MWHA correlated strongly with the decrease in specific productivity of the culture. To overcome this decrease in MWHA, a glucostat strategy was successfully devised which could maintain a high value of specific productivity throughout the glucostat phase and result in constant-MW HA production. Glucostat processes were designed with the three recombinant L. lactis strains at two different glucose concentrations to produce constant molecular weight HA ranging from 0.4 to 1.4 MDa. This is the first report of its kind in literature that demonstrates production of controlled MW HA over a wide range by using a combination of tunable parameters and suitable process control strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyaluronic acid (HA) is a biopolymer with wide applications in the fields of medicine and cosmetics. Molecular weight of HA (MWHA) is an important quality parameter that determines its suitability for various end applications (Table S1, Supplementary data). Therefore, strategies to produce HA with the desired molecular weight will be beneficial to industry (Marcellin et al. 2014). Developing such strategies requires identifying parameters that allow production of HA over a wide range of molecular weights and control of molecular weight at a desired value. There is very limited work on identifying tunable parameters which affect MWHA (Jing and DeAngelis 2004; Sheng et al. 2009) and on controlling MWHA at a desired value during fermentation (Jin et al. 2016).
Literature reports show many genetic and process parameters which affect MWHA. Genetic parameters include the combination of heterologous has genes (in the HA biosynthetic pathway) being overexpressed (Yu and Stephanopoulos 2008; Chauhan et al. 2014); whether the has genes were plasmid-encoded or chromosomally integrated (Hmar et al. 2014); gene-knockout in competing pathways which enhanced co-factor availability for HA-precursor synthesis (Kaur and Jayaraman 2016); downregulation of the glycolytic pathway and regulated production of hyaluronidase (Jin et al. 2016) (Table S2, Supplementary data). Process parameters such as pH, agitation rate, aeration rate, temperature, initial glucose concentration, C/N ratio (Kim et al. 1996; Armstrong and Johns 1997; Duan et al. 2008; Jagannath and Ramachandran 2010; Lai et al. 2011) have also been shown to affect MWHA (Table S3, Supplementary data). However, the main objective in most literature reports is to optimize the parameters in order to obtain the maximum possible MWHA. A few literature reports have demonstrated modulation of MWHA using tunable parameters. Sheng et al. (2009) varied the hasA/hasB mRNA ratio, which resulted in HA molecular weight variation only in a narrow range of 0.6–0.9 MDa. Jin et al. (2016) used a combination of genetic strategies to produce HA in the molecular weight range of 2.2 kDa to 1.42 MDa in engineered Bacillus subtilis strains. They initially produced HA in the MW range of 1.4–1.83 MDa by co-expression of multiple genes in the HA pathway and downregulation of the pfk gene in the glycolytic pathway. Then, they co-expressed regulated amounts of engineered leech hyaluronidase to produce HA over a wide molecular weight range. Jing and DeAngelis (2004) varied the precursor to acceptor oligosaccharide ratio during in vitro HA polymerization to obtain monodisperse HA with molecular weight in a wide range of 16 kDa to 2 MDa. However, this strategy offers poor scalability owing to its expensive nature.
Another drawback with most literature reports on HA production is that these studies have only reported the MWHA obtained at the end of a batch fermentation process. There are hardly any reports on variation of MWHA during the course of the batch fermentation. Only four reports on HA production using S. zooepidemicus provide HA molecular weight trend as a part of the fermentation profile. Armstrong and Johns (1997) and Im et al. (2009) observed an initial increase in MWHA followed by a constant molecular weight trend for the HA produced. Two other reports have shown that, after an initial increase, the MWHA decreased by 30–60% during the course of the fermentation (Pires and Santana 2010; Jagannath and Ramachandran 2010). A recent study using four recombinant B. subtilis strains showed a 11–31% decrease in MWHA during flask cultivation (Westbrook et al. 2018). None of the above studies investigated the reasons for the decrease in MWHA or provided any strategies to control MWHA during the fermentation.
Similar to the above studies, in this work, we have shown a decrease in MWHA produced by recombinant Lactococcus lactis strains during the course of batch fermentations. We have analyzed the process parameters and correlated them with variation in MWHA and devised a process strategy for controlling MWHA during the fermentation process. The molecular weight control strategy developed was employed in fermentations involving different sets of genetic and process parameters which affect molecular weight, thus allowing production of constant molecular weight HA over a wide range of values.
Materials and methods
Chemicals and kits
M17 media components, glucose, and cetyltrimethyl ammonium bromide (CTAB) were purchased from HiMedia Laboratories (India). Isopropyl alcohol, sulfuric acid, and sodium nitrate were purchased from Merck (USA). Hyaluronan standards with Mv-0.1, 0.6, and 1.59 MDa (measured using viscometry) were purchased from Lifecore Biomedical (USA).
The GenElute Bacterial Genomic Kit, chloramphenicol, and nisin were obtained from Sigma-Aldrich (USA). QIAprep Spin Miniprep kit for plasmid isolation, QIAquick™ Gel Extraction kit and the QIAquick™ PCR purification kit were purchased from Qiagen (Germany). The enzymes NcoI, KpnI, PmlI, Phusion™ DNA Polymerase, and T4 DNA ligase were bought from New England Biolabs (USA).
Construction of L. lactis clones overexpressing hasA, hasB, hasE, and pgmA genes
Bacterial strains and plasmids
Streptococcus equi subsp. zooepidemicus ATCC 35246 was obtained from American Type Culture Collection (USA). The plasmid pNZ8148 and Lactococcus lactis NZ9000 were obtained from NIZO (Netherlands). E. coli MC1061 was a kind gift from Dr. Devinder Sehgal, National Institute of Immunology, New Delhi.
Construction of recombinant Lactococcus lactis
The gene sequences of hasA, hasB, and hasE were obtained from the partial sequence of Streptococcus equi subsp. zooepidemicus has operon, with GenBank accession ID-AF347022.1. The base positions of has genes in this partial sequence were hasA-512 to 1784; hasB-2023 to 3242; hasE-590 to 7268. Similarly, pgmA gene sequence was obtained from the complete genome sequence of Lactococcus lactis subsp. cremoris NZ9000 with GenBank accession ID-CP002094.1. The base position of pgmA gene in this complete genome sequence was 540,061 to 540,819.
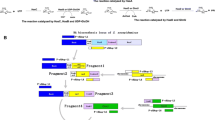
Nisin inducible plasmid, pNZ8148, was used to construct recombinant plasmids pGJP2 (containing hasA and hasB genes), pGJP5 (containing hasA, hasB and hasE genes) and pGJP6 (containing hasA, hasB and pgmA genes). The pGJP2 plasmid was constructed first, followed by cloning of hasE and pgmA genes into pGJP2 to obtain pGJP5 and pGJP6 constructs, respectively. The step-wise cloning strategy used for constructing the recombinant plasmids pGJP2, pGJP5 and pGJP6 is shown schematically in Fig. S1a and S1b, Supplementary data. The recombinant clones after transformation of pGJP2, pGJP5 and pGJP6 into L. lactis NZ9000 were assigned as L. lactis GJP2, GJP5 and GJP6, respectively.
The genomic DNA of S. equi subsp. zooepidemicus ATCC 35246 and L. lactis NZ9000 were isolated using GenElute™ Bacterial Genomic DNA Kit. Using the Phusion™ High-Fidelity DNA Polymerase, hasE gene and a single DNA fragment comprising hasA and hasB genes were amplified from S. zooepidemicus genomic DNA. The pgmA gene was amplified from L. Lactis NZ9000 genomic DNA. The primers used for amplification of different gene fragments and the associated restriction sites are shown in Table S4 (Supplementary data). The PCR conditions for amplification of the gene fragments were as follows: after an initial denaturation at 98 °C for 2 min, subsequent denaturation was carried out at 98 °C for 30 s. Annealing was carried out at 52 °C for hasAB gene fragment and at 47 °C for hasE and pgmA genes, each for 45 s. The extension step was carried out at 72 °C for varied time periods for different fragments as follows: hasAB for 90 s, hasE for 30 s and pgmA for 30 s. The total number of cycles (starting from second denaturation to extension) was 35 cycles for all amplifications. The final extension for all gene combinations was at 72 °C for 5 min. The amplicons were then purified using QIA quick™ PCR purification kit.
The vector pNZ8148 and the gene fragment were double digested with respective restriction enzymes (Fig. S1c, Supplementary data) for 3 h at 37 °C and purified using QIAquick™ Gel Extraction kit and ligated with T4 DNA ligase at 16 °C for 16 h. The ligated products were transformed into ultra-competent E. coli MC1061 and plated on Luria-Bertani agar plates containing chloramphenicol (10 μg/ml) and streptomycin (10 μg/ml). The positive recombinants screened using restriction digestion and PCR were confirmed by DNA sequencing. The positive recombinant plasmids were then transformed into electrocompetent L. lactis by electroporation.
Fermentation experiments
All bioreactor experiments were carried out in duplicate in an unaerated 2.4 L bioengineering (Switzerland) bioreactor at 30 °C, 200 rpm, and pH 7. Modified M17 media (1.2 L) with the following composition was autoclaved in situ: yeast extract-5 g/l, brain heart infusion-5 g/l, MgSO4·7H2O-0.5 g/l, ascorbic acid-0.5 g/l, KH2PO4-0.5 g/l, and K2HPO4-1.5 g/l. Autoclaved glucose added with chloramphenicol (10 μg/ml) was aseptically transferred into the reactor. Inoculum was prepared from glycerol stocks stored at − 80 °C by sub-culturing twice in flasks and was aseptically introduced into the fermenter to a final concentration of 8%.
For glucostat experiments, glucose concentration in the bioreactor was maintained constant by using pH feedback strategy (Papagianni et al. 2007). The glucose consumed for lactic acid production can be monitored from the amount of base added by the pH controller and a correlation can be obtained between the two. It was found that for every gram of glucose consumed, 0.578 g of KOH was pumped in (Fig. S2, Supplementary data). Glucose feed and KOH were both connected to the base pump of the fermenter. Glucose and KOH solutions were prepared such that, with every instance of addition of base, the corresponding amount of glucose consumed in the reactor will be replenished by the base pump. For all bioreactor experiments, samples were drawn every 2 h and stored at − 20 °C until they were analyzed for concentrations of glucose, biomass, HA, lactate, and HA molecular weight.
Analytical techniques
Determination of biomass concentration
Biomass concentration was determined from absorbance measured at 600 nm. Fermentation broth was treated with equal volume of 0.1% SDS for 10 min at room temperature and centrifuged at 9500×g for 10 min. The pellet was re-suspended in 0.9% NaCl and absorbance was measured at 600 nm using UV-visible spectrophotometer (Jasco, US).
Determination of glucose and lactate concentration
Glucose and lactate concentration in the broth were measured using ion exchange chromatography. Phenomenex Rezex R-OA column (320 mm × 4.5 mm) along with Phenomenex Rezex R-OA guard column fitted to Shimadzu HPLC-PDA-RID detector system was used for the analysis. Isocratic elution using 5 mM sulfuric acid with a flow rate of 0.6 ml/min was carried out. Lactate concentration was measured from absorbance at 210 nm and glucose concentration was measured using RID.
Determination of HA concentration
HA concentration was measured using CTAB method (Oueslati et al. 2014). Fermentation broth was treated with equal volume of 0.1% SDS for 10 min at room temperature and centrifuged at 9500×g for 10 min. The supernatant obtained was used for the CTAB assay.
Determination of HA molecular weight
MWHA was measured using size exclusion chromatography (SEC). Calibration of SEC and molecular weight estimation of HA in fermentation samples using SEC were carried out according to Shanmuga Doss et al. (2017).
Statistical analysis
The statistical analysis was performed using t test available in Minitab 16 with 5% level of significance. All fermentation experiments and analyses were performed at least in duplicate, and the results were expressed as a mean ± standard deviation. Statistical significance was represented by p values *p < 0.05, **p < 0.01, ***p < 0.001.
Results
The two objectives of this work were (i) to devise process strategies to control variation in MWHA during fermentation and (ii) to produce constant-MW HA over a wide range of molecular weights. Batch experiments were carried out using the three recombinant L. lactis strains to validate production of HA with different molecular weights. During batch fermentation with all three recombinant strains, the MWHA showed a decreasing trend after a small initial phase of increase. From the analysis of bioprocess parameters during the batch fermentation experiments, the decrease in MWHA was inferred to be due to the drastic reduction of glucose flux towards HA biosynthetic pathway. This observation correlated with the decreasing glucose concentration towards the later part of the batch fermentation. Therefore, a glucostat strategy was developed and tested for production of constant-MW HA during fermentation. The simultaneous manipulation of process parameter (glucose concentration) and genetic parameter (combination of HA biosynthetic genes overexpressed) in a glucostat enabled production of constant-MW HA over a 0.4–1.4 MDa range.
Construction of recombinant L. lactis strains
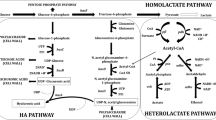
Three recombinant L. lactis clones, overexpressing different combinations of HA biosynthetic pathway genes, were constructed using the nisin inducible pNZ8148 expression system. The genes hasA, hasB and hasE (Fig. 1) were amplified from the has-operon in the genomic DNA of the natural HA producer S. zooepidemicus while the gene coding for α-phosphoglucomutase (pgmA) was obtained from L. lactis. The recombinant plasmids pGJP2 (containing hasAB genes), pGJP5 (containing hasABE genes) and pGJP6 (containing hasAB and pgmA genes) were transformed into L. lactis NZ9000. The construction of the recombinant strains is detailed in the “Materials and methods” section.
While L. lactis does not have the hyaluronan synthase (hasA) gene, it has the homolog (ugd) of the hasB gene, coding for UDP-glucose dehydrogenase. Since ugd is tightly transcriptionally regulated (Prasad et al. 2012), overexpression of both hasA and hasB is required for substantial production of HA by recombinant L. lactis cultures. Earlier studies with recombinant L. lactis strains have shown that overexpression of a third HA pathway gene (hasC or hasD), in addition to hasA and hasB, results in enhancement of HA production in recombinant L. lactis cultures (Prasad et al. 2010; Prasad et al. 2012; Chauhan et al. 2014). In this study, we chose hasE and pgmA as the third gene, in addition to hasA and hasB, for overexpression in the recombinant L. lactis strains GJP5 and GJP6, respectively. As shown in Fig. 1, hasE and pgmA express the nodal enzymes from glucose-6-phosphate in the HA biosynthetic pathway. Hence, by selective overexpression of hasE and pgmA, the carbon flux distribution could be varied in the two parallel pathways leading to the formation of the two precursors of HA, viz., UDP-glucuronic acid and UDP-N-acetyl glucosamine. As the intracellular ratio of the two precursors (and their pathway fluxes) has been shown to have a significant effect on MWHA (Badle et al. 2014), we expected the clones overexpressing hasE and pgmA genes along with hasAB to produce HA of different molecular weights. While overexpression of pgmA gene in S. zooepidemicus was shown earlier to not affect HA yield and MWHA (Chen et al. 2014), overexpression of hasE has been shown to enhance MWHA in S. zooepidemicus (Chen et al. 2009).
Effect of co-expression of hasE and pgmA genes on HA production and molecular weight
Batch fermentation experiments were carried out using the three recombinant L. lactis strains at 10 g/l and 30 g/l initial glucose concentration. For sake of clarity, Fig. 2 shows only the profiles of MWHA along with glucose and HA concentration. Complete batch fermentation profiles including biomass and lactate concentration are shown in Fig. S3 (Supplementary data). In accordance with previous literature reports (Jagannath and Ramachandran 2010; Chauhan et al. 2014), a higher initial glucose concentration gave rise to higher HA concentration and MWHA at the end of the batch fermentations for all the three recombinant strains (Fig. 3).
The effect of overexpression of a third gene (hasE/pgmA) in addition to hasAB, on HA concentration and MWHA, is illustrated in Fig. 3. Compared to the L. lactis GJP2 strain (expressing hasAB), overexpression of hasABE in L. lactis GJP5 resulted in 120% and 56% improvement in HA concentration for the batch fermentations using 10 g/l and 30 g/l initial glucose concentrations, respectively (Fig. 3a). Similarly, additional overexpression of pgmA resulted in 150% and 47% increase in HA concentration under the respective conditions. This implies that overexpression of the third gene improved the carbon flux towards the respective HA-precursor, thus improving HA concentration and yield for L. lactis GJP5 and GJP6 cultures.
Overexpression of hasE gene resulted in significantly higher MWHA towards the end of the batch fermentation (end-MWHA) in comparison to pgmA overexpression (Fig. 3b). Compared to L. lactis GJP2, L. lactis GJP5 cultures produced 84–95% higher end-MWHA, while L. lactis GJP6 produced only 21–51% higher end-MWHA during batch fermentations using 10 and 30 g/l initial glucose concentrations. Thus, co-expression of pgmA and hasE (along with hasAB) exhibited an enhancement in MWHA, with hasE co-expression showing a more pronounced effect. This increase in MWHA upon coexpression of a third gene along with hasAB is in contrast to the results reported by Prasad et al. (2010) and Chauhan et al. (2014), wherein they observed no enhancement of MWHA upon the co-expression of hasABC or hasABD in L. lactis strains.
Molecular weight variation of HA during batch fermentation
The HA molecular weight profile for all the six batch fermentations shows that, after a brief initial increase, the MWHA decreases steadily from its peak value until end of the fermentation (Fig. 2). Across the three recombinant strains, MWHA showed 26–35% decrease and 19–43% decrease during the 10 g/l and 30 g/l batch fermentations, respectively (Fig. 3b). Since MWHA measured is the average molecular weight of all HA chains present in the broth, a decrease in the average molecular weight would mean introduction of shorter chains into the broth with progress of fermentation. Shorter chains can be introduced into the broth either by the cell or by degradation of existing HA chains due to shear or hyaluronidase action inside the reactor. Since L. lactis is hyaluronidase negative, a control experiment was carried out to check shear degradation of HA inside the reactor. In a batch experiment with un-induced recombinant L. lactis culture, 1 g of HA was spiked into the fermenter a few hours after inoculation. Molecular weight profiling revealed that MWHA remained constant throughout the experiment (Fig. S4, Supplementary data), thus ruling out shear degradation during HA production. From Fig. 2, it can be observed that decrease in MWHA is accompanied by increase in HA accumulation during all six cultivations. Hence, the decreasing trend of MWHA might be due to production of shorter HA chains by the cell towards the later part of the batch fermentations.
However, further analysis was needed to explain the decreasing trend of MWHA. We focused on the fermentation parameters such as specific growth rate (μ), specific glucose uptake rate (qs), specific production rate of HA (qp) and the various yield coefficients based on glucose uptake, viz. biomass (YX/S), HA (YHA/S), and lactate (YLA/S) yield coefficients (Table 1). Furthermore, the batch fermentation process was divided into two phases, viz. phase of increasing MWHA (phase 1) and phase of decreasing MWHA (phase 2) and the averaged values of the bioprocess parameters were calculated independently for the two phases. One of the critical parameters is the specific production rate of HA (qp), which is determined by specific glucose uptake rate (qs) and the proportion of glucose fluxed into HA formation, measured by YHA/S (C-mol/C-mol). From Table 1, qs and YHA/S were observed to decrease with transition from phase 1 to phase 2 for the 10 g/l as well as 30 g/l glucose concentration experiments. Consequently, the qp values were much lower in phase 2 as compared to phase 1 (Fig. 4a, b) and might reflect lower precursor availability for HA formation. This reduction in qp and the probable decrease in precursor availability could be the reason for formation of shorter chains and decreasing MWHA during phase 2. The values of YX/S were consistently lower while that of YL/S were consistently higher for phase 2 in comparison to phase 1 (Table 1). Papagianni et al. (2007) observed in batch cultures of L. lactis, carried out at 10 g/l initial glucose concentration, the specific lactate production rate increased sharply when the glucose concentration dropped to ~ 5–6 g/l. We observed similar trends in some of the 10 g/l batch fermentations (Fig. S3, Supplementary data). The decrease in qs, owing to decreasing glucose concentration in the batch fermentation, leads to a redistribution of carbon flux in favor of a higher lactate yield at the expense of biomass and product yield. Therefore, to prevent decrease in MWHA, it is important to develop strategies to prevent drastic decreases in qs, YHA/S, and qp. To sustain high values of these parameters during fermentation, we attempted a glucostat strategy as explained in the next section.
Glucostat strategy for control of MWHA during fermentation
We designed a glucostat process as a possible strategy to prevent the decrease in MWHA which was observed in batch fermentations. Glucostat can be described as a fermentation process in which glucose concentration is maintained constant at a desired value by continuous feeding of glucose. Figure 5 shows the glucose, HA and MWHA profiles for the 10 g/l and 30 g/l glucostat experiments using the three recombinant L. lactis strains. Detailed profiles can be found in Fig. S5 (Supplementary data). Figure 5 shows that glucostat fermentation resulted in constant-MW HA production. By carrying out the glucostat fermentation at different glucose concentrations and using the three recombinant L. lactis clones overexpressing different combinations of HA biosynthetic genes, constant-MW HA production in a range of 0.4–1.4 MDa was achieved (Fig. S6, Supplementary data).
The continuous production of HA during the glucostat shows that, on the average, HA chains of similar length were produced during this period. This observation is in contrast to the decreasing trend of MWHA during batch fermentations and clearly shows the power of glucose concentration as a control parameter. A constant glucose concentration in the glucostat allows maintenance of a high value of specific productivity (qp) throughout the glucostat phase. The qp value during glucostat phase of 30 g/l glucostats (Fig. 6b) was comparable to qp value during phase 1 of 30 g/l batch experiments (Fig. 4b). Similarly, the MWHA in the 30 g/l glucostats is comparable to the peak MWHA obtained during 30 g/l batch fermentations.
Similar to the observations in the batch experiments, MWHA showed a decreasing trend during the post-glucostat phase. MWHA maintained during glucostat phase and at the end of post-glucostat phase is as shown in Table S5 (Supplementary data). The decrease in MWHA during post-glucostat phase also validates the batch fermentation data, illustrating the strong influence of glucose concentration on MWHA. The decrease in MWHA during post-glucostat phase was also correlated with a decrease in qp (Fig. 6a, b). The decrease in qp during transition from glucostat phase to post-glucostat phase can be explained by decreasing qs and YHA/S values along with an increase in YLA/S (Table 2), corroborating the similar trends observed in batch fermentation (Table 1).
Effect of glucose concentration on bioprocess parameters and MWHA in glucostats
We analyzed how different glucose concentrations affect qs and YHA/S, thereby influencing qp and MWHA in the glucostat experiments. Figure 7 shows the comparison of bioprocess parameters — qs, μ, YHA/S, and qp for the three recombinant L. lactis strains in the 10 and 30 g/l glucostat fermentations. The 10 g/l glucostat supported higher qs than the 30 g/l glucostat (Fig. 7a). This observation is in accordance with the one made by Papagianni et al. (2007) during glucostat studies with L. lactis cultures. They observed that the specific glucose uptake rate and the activities of key glycolytic enzymes are maximum at 10 g/l in glucostat cultures of L. lactis. We observed that the higher qs in 10 g/l glucostat fermentation supported higher growth rates (Fig. 7b) and biomass yields (Table 2) when compared to 30 g/l glucostat fermentation. High substrate flux into biomass formation resulted in lower YHA/S values in 10 g/l glucostat when compared to 30 g/l glucostat (Fig. 7c). This leads to lower qp values in 10 g/l glucostat fermentation as compared to 30 g/l glucostat fermentation (Fig. 7d) which explains the lower MWHA obtained in 10 g/l glucostat fermentation as opposed to 30 g/l glucostat fermentation (Fig. 5, Table S5). The correlation of MWHA with qs, μ, YHA/S, and qp during glucostat fermentation is shown in Fig. 8. The inverse correlation observed for MWHA with specific growth rate (Fig. 8a) is supported by chemostat studies with recombinant L. lactis cultures (Badle et al. 2014). Earlier studies in our lab with fed-batch and chemostat cultures of recombinant L. lactis have shown that MWHA correlates inversely with qs (unpublished results). We also observed inverse correlations between MWHA and qs (Fig. 8b). A strong positive correlation is observed for MWHA with YHA/S (Fig. 8c) and qp (Fig. 8d), which corroborates the inferences drawn for the decrease in MWHA during phase 2 of the batch fermentations and post-glucostat phase.
Discussion
In this study, we observed that specific HA production rate (qp) correlated strongly with MWHA. To produce HA with different MW or to control MWHA at a constant value during fermentation, qp could serve as a suitable control parameter. The parameter qp is influenced by the specific glucose uptake rate (qs) and the fraction of glucose fluxed into the HA biosynthetic pathway (YHA/S). From Tables 1 and 2, it can be understood that the percentage drop in YHA/S was higher when compared to percentage drop in qs with progress of fermentation into phase 2 in case of batch fermentation and into post-glucostat phase during glucostat fermentation. So, decrease in glucose concentration affects resource allocation into HA pathway more than specific glucose uptake rate, which results in decrease of qp and MWHA. Thus, the variation in qp and MWHA values is more sensitive to the variation in YHA/S than that in qs.
The YHA/S values are determined by carbon flux distribution to biomass formation (YX/S) and formation of by-products such as lactate (YLA/S). The latter two yield coefficients depend on process conditions, including the concentration of glucose. For example, in phase 2 of batch fermentations and in the post-glucostat phase, decreasing concentration of glucose resulted in higher values of YLA/S, thereby lowering the YHA/S values (Tables 1 and 2). With reducing glucose concentration and uptake rate, increasing the lactate yield would be a natural strategy for the cell to improve its energy availability through substrate-level phosphorylation. Redon et al. (2005) reported downregulation of genes involved in transcription, translation, nucleotide biosynthesis, cell envelope biosynthesis, and cell division in Lactococcus lactis cultures during progression to nutrient depleted conditions in the later hours of batch fermentation. Redon et al. (2005) also observed glycolytic enzymes to be downregulated in the study. However, the measurement of specific activities of glycolytic enzymes indicated otherwise. Ercan et al. (2015) did a transcriptional analysis of Lactococcus lactis adapting to starvation conditions. They found the involvement of a transcriptional regulator (codY) in the downregulation of genes involved in transcriptional and cell division machinery, purine metabolism, and natural transformation. Hence, the shift in the carbon allocation from biomass and HA formation to glycolysis might be a result of transcriptional and allosteric regulation mechanisms. We also observed that the μ and YX/S values were lower for the 30 g/l glucostats as compared to those of 10 g/l glucostats, resulting in higher values of YHA/S (Table 2) and thereby leading to higher MWHA in 30 g/l glucostats. For a given process condition, the YHA/S values can also be enhanced by the overexpression of additional HA pathway genes as seen from the glucostat data (Table 2).
As shown in Tables S2 and S3 (Supplementary data), a range of genetic and process parameters can also be varied to obtain HA of different molecular weights. Metabolic engineering strategies for decreasing the glycolytic flux (Jin et al. 2016) or lactate formation (Kaur and Jayaraman 2016) have shown to enhance HA yield and MWHA. Co-expression of HA pathway genes has also been a successful strategy to improve HA yield and MWHA (Prasad et al. 2010; Chauhan et al. 2014). MWHA can also be varied by manipulation of process parameters such as pH, agitation, aeration, initial glucose concentration, and temperature. However, the variation obtained in MWHA is rather small in such studies (Tables S2 and S3). In this work, we have shown that YHA/S values can be varied using simultaneous genetic (co-expression of HA biosynthetic genes) and process (glucose concentration) manipulations, thus enabling the production of HA over a 3-fold range of molecular weights.
While MWHA is determined by YHA/S (which strongly influences qp), the maintenance of a constant molecular weight during fermentation is dependent on maintaining a high value of qs and YHA/S. As can be discerned from Table 2, the qs values are similar for a specific glucose concentration and are therefore controlled only by that process parameter. Therefore, glucose concentration serves as an ideal control parameter to sustain higher values of qs and YHA/S as demonstrated by the constant-MW HA production during glucostat fermentations in this work. Thus, by combining glucostat strategy with genetic and process parameters that offer tunability of MWHA, we can modulate the production of constant-MW HA over a wide range of values.
Normally, glucose should be the only nutrient which influences values of qs and YHA/S during a glucostat strategy, for the production of constant-MW HA. But, when the reducing concentrations of other nutrients influence product formation rates, value of qs and YHA/S cannot be controlled by only controlling glucose concentration. Similar to glucostat strategy, nitrogen or phosphorus-controlled processes might also be able to maintain qs and YHA/S, especially if the C/N ratio is kept constant. However, this is difficult to implement from an operational standpoint (especially for complex nitrogen sources), since it is difficult to measure or control nitrogen or phosphorous concentration online. Moreover, there is no indirect mechanism (such as lactate formation in case of glucose) which can indicate consumption of nitrogen or phosphorus. Therefore, a simpler strategy for maintaining glucose concentration and thereby qs has been adopted in this study.
Molecular weight variation and its control during fermentation is a less explored area in literature on HA fermentation. This work has been an effort to understand MWHA variation during fermentation, to develop strategies to control MWHA during fermentation, and to employ the strategy developed to produce constant-MW HA over a range of molecular weights. The insights obtained from this study on molecular weight variation and the molecular weight control strategy developed in this work have significant implications for production of HA of desired molecular weights required for specific applications.
References
Armstrong DC, Johns MR (1997) Culture conditions affect the molecular weight properties of hyaluronic acid produced by Streptococcus zooepidemicus. Appl Environ Microbiol 63:2759–2764
Badle SS, Jayaraman G, Ramachandran KB (2014) Ratio of intracellular precursors concentration and their flux influences hyaluronic acid molecular weight in Streptococcus zooepidemicus and recombinant Lactococcus lactis. Bioresour Technol 163C:222–227. https://doi.org/10.1016/j.biortech.2014.04.027
Chauhan AS, Badle SS, Ramachandran KB, Jayaraman G (2014) The P170 expression system enhances hyaluronan molecular weight and production in metabolically-engineered Lactococcus lactis. Biochem Eng J 90:73–78. https://doi.org/10.1016/j.bej.2014.05.012
Chen WY, Marcellin E, Hung J, Nielsen LK (2009) Hyaluronan molecular weight is controlled by UDP-N-acetylglucosamine concentration in Streptococcus zooepidemicus. J Biol Chem 284:18007–18014. https://doi.org/10.1074/jbc.M109.011999
Chen WY, Marcellin E, Steen JA, Nielsen LK (2014) The role of hyaluronic acid precursor concentrations in molecular weight control in Streptococcus zooepidemicus. Mol Biotechnol 56:147–156. https://doi.org/10.1007/s12033-013-9690-4
Duan X-J, Yang L, Zhang X, Tan W-S (2008) Effect of oxygen and shear stress on molecular weight of hyaluronic acid. J Microbiol Biotechnol 18:718–724
Ercan O, Wels M, Smid EJ, Kleerebezem M (2015) Genome-Wide Transcriptional Responses to Carbon Starvation in Nongrowing Lactococcus lactis. Appl Environ Microbiol 81:2554–2561. https://doi.org/10.1128/AEM.03748-14
Hmar RV, Prasad SB, Jayaraman G, Ramachandran KB (2014) Chromosomal integration of hyaluronic acid synthesis (has) genes enhances the molecular weight of hyaluronan produced in Lactococcus lactis. Biotechnol J 9:1554–1564. https://doi.org/10.1002/biot.201400215
Im JH, Song JM, Kang JH, Kang DJ (2009) Optimization of medium components for high-molecular-weight hyaluronic acid production by Streptococcus sp. ID9102 via a statistical approach. J Ind Microbiol Biotechnol 36:1337–1344. https://doi.org/10.1007/s10295-009-0618-8
Jagannath S, Ramachandran KB (2010) Influence of competing metabolic processes on the molecular weight of hyaluronic acid synthesized by Streptococcus zooepidemicus. Biochem Eng J 48:148–158. https://doi.org/10.1016/j.bej.2009.09.003
Jin P, Kang Z, Yuan P, Du G, Chen J (2016) Production of specific-molecular-weight hyaluronan by metabolically engineered Bacillus subtilis 168. Metab Eng 35:21–30. https://doi.org/10.1016/j.ymben.2016.01.008
Jing W, DeAngelis PL (2004) Synchronized chemoenzymatic synthesis of monodisperse hyaluronan polymers. J Biol Chem 279:42345–42349. https://doi.org/10.1074/jbc.M402744200
Kaur M, Jayaraman G (2016) Hyaluronan production and molecular weight is enhanced in pathway-engineered strains of lactate dehydrogenase-deficient Lactococcus lactis. Metab Eng Commun 3:15–23. https://doi.org/10.1016/j.meteno.2016.01.003
Kim J-H, Yoo S-J, Oh D-K, Kweon Y-G, Park D-W, Lee C-H, Gil G-H (1996) Selection of a Streptococcus equi mutant and optimization of culture conditions for the production of high molecular weight hyaluronic acid. Enzym Microb Technol 19:440–445. https://doi.org/10.1016/S0141-0229(96)00019-1
Lai ZW, Rahim RA, Ariff AB, Mohamad R (2011) Medium formulation and impeller design on the biosynthesis of high molecular weight hyaluronic acid by Streptococcus zooepidemicus ATCC 39920. Afr J Microbiol Res 5:2114–2123. https://doi.org/10.5897/AJMR11.305
Marcellin E, Steen JA, Nielsen LK (2014) Insight into hyaluronic acid molecular weight control. Appl Microbiol Biotechnol 98:6947–6956. https://doi.org/10.1007/s00253-014-5853-x
Oueslati N, Leblanc P, Harscoat-schiavo C, Rondags E, Meunier S, Kapel R, Marc I (2014) CTAB turbidimetric method for assaying hyaluronic acid in complex environments and under cross-linked form. Carbohydr Polym 112:102–108. https://doi.org/10.1016/j.carbpol.2014.05.039
Papagianni M, Avramidis N, Filiousis G (2007) Glycolysis and the regulation of glucose transport in Lactococcus lactis spp. lactis in batch and fed-batch culture. Microb Cell Factories 6:16. https://doi.org/10.1186/1475-2859-6-16
Pires AMB, Santana MHA (2010) Metabolic effects of the initial glucose concentration on microbial production of hyaluronic acid. Appl Biochem Biotechnol 162:1751–1761. https://doi.org/10.1007/s12010-010-8956-6
Prasad SB, Jayaraman G, Ramachandran KB (2010) Hyaluronic acid production is enhanced by the additional co-expression of UDP-glucose pyrophosphorylase in Lactococcus lactis. Appl Microbiol Biotechnol 86:273–283. https://doi.org/10.1007/s00253-009-2293-0
Prasad SB, Ramachandran KB, Jayaraman G (2012) Transcription analysis of hyaluronan biosynthesis genes in Streptococcus zooepidemicus and metabolically engineered Lactococcus lactis. Appl Microbiol Biotechnol 94:1593–1607. https://doi.org/10.1007/s00253-012-3944-0
Redon E, Loubiere P, Cocaign-bousquet M (2005) Transcriptome Analysis of the Progressive Adaptation of Lactococcus lactis to Carbon Starvation. J Bacteriol 187:3589–3592. https://doi.org/10.1128/JB.187.10.3589
Shanmuga Doss S, Bhatt NP, Jayaraman G (2017) Improving the accuracy of hyaluronic acid molecular weight estimation by conventional size exclusion chromatography. J Chromatogr B Anal Technol Biomed Life Sci 1060:255–261. https://doi.org/10.1016/j.jchromb.2017.06.006
Sheng JZ, Ling PX, Zhu XQ, Guo XP, Zhang TM, He YL, Wang FS (2009) Use of induction promoters to regulate hyaluronan synthase and UDP-glucose-6-dehydrogenase of Streptococcus zooepidemicus expression in Lactococcus lactis: a case study of the regulation mechanism of hyaluronic acid polymer. J Appl Microbiol 107:136–144. https://doi.org/10.1111/j.1365-2672.2009.04185.x
Westbrook AW, Ren X, Perry MMC (2018) Engineering of cell membrane to enhance heterologous production of hyaluronic acid in Bacillus subtilis. Biotechnol Bioeng 115:216–231. https://doi.org/10.1002/bit.26459
Yu H, Stephanopoulos G (2008) Metabolic engineering of Escherichia coli for biosynthesis of hyaluronic acid. Metab Eng 10:24–32. https://doi.org/10.1016/j.ymben.2007.09.001
Acknowledgements
The authors would like to acknowledge Indian Institute of Technology Madras for providing infrastructure to carry out this work and Ministry of Human Resource Development, Government of India for providing Fellowships to Sreeja Shanmuga Doss and Pandeeswari Jeeva.
Funding
The Department of Biotechnology (Ministry of Science and Technology, Govt. of India) provided funds to execute this project through Project No. BT/IN/Finland/30/GJ/2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1188 kb)
Rights and permissions
About this article
Cite this article
Jeeva, P., Shanmuga Doss, S., Sundaram, V. et al. Production of controlled molecular weight hyaluronic acid by glucostat strategy using recombinant Lactococcus lactis cultures. Appl Microbiol Biotechnol 103, 4363–4375 (2019). https://doi.org/10.1007/s00253-019-09769-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09769-0