Abstract
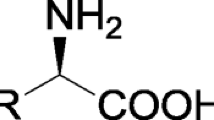
l-Tyrosine is an aromatic, polar, non-essential amino acid that contains a highly reactive α-amino, α-carboxyl, and phenolic hydroxyl group. Derivatization of these functional groups can produce chemicals, such as l-3,4-dihydroxyphenylalanine, tyramine, 4-hydroxyphenylpyruvic acid, and benzylisoquinoline alkaloids, which are widely employed in the pharmaceutical, food, and cosmetics industries. In this review, we summarize typical l-tyrosine derivatizations catalyzed by enzymatic biocatalysts, as well as the strategies and challenges associated with their production processes. Finally, we discuss future perspectives pertaining to the enzymatic production of l-tyrosine derivatives.
Key points
• Summary of recent advances in enzyme-catalyzed l-tyrosine derivatization.
• Highlights of relevant strategies involved in l-tyrosine derivatives biosynthesis.
• Future perspectives on industrial applications of l-tyrosine derivatization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
l-Tyrosine is a valuable compound widely used in the food, health care, and cosmetics industries (Dennig et al. 2015; Li et al. 2020). As an aromatic polar amino acid, l-tyrosine contains a highly reactive α-amino group, α-carboxyl group, and phenolic hydroxyl group Their derivatization generates a variety of high-value chemicals, such as unsaturated aromatic compounds, α-hydroxy acids, and aromatic alcohols, which are commonly applied in the feed, pharmaceutical, and fine chemical industries (Chen et al. 2019; Lukito et al. 2019; Rodriguez et al. 2017).
Two main strategies have been developed for l-tyrosine derivatization: chemical synthesis and biosynthesis (Luetke et al. 2007; Sarlaslani 2007). Heterocyclic compounds can be obtained directly from l-tyrosine via oxidation and hydrolysis reactions using chemical catalysts (Cox et al. 2019; Glachet et al. 2019); however, these processes commonly require extreme reaction conditions such as high temperatures, multi-step procedures, and high-cost precursors, resulting in various toxic intermediates and limited economic benefit (Mujumdar et al. 2019; Wen et al. 2016). Biosynthetic strategies, including microbial fermentation and enzymatic catalysis, offer an alternative approach. Although microbial fermentation usually exhibits good enantioselectivity, the separation and purification processes are complex owing to the inclusion of other substrates and proteins in the culture broth (Haslinger and Prather 2020; Noda et al. 2015). Enzyme catalysis provides a promising and efficient approach for synthesizing chemicals from l-tyrosine due to its elevated specificity, diversity, and atom economy. Enzymatic derivatization relies on the ability of specific enzymes to recognize a particular functional group on l-tyrosine and synthesize certain derivatives. An example is given by alkylation of the phenolic hydroxyl group (Kim et al. 2018) to O-alkylated l-tyrosine derivatives, or decarboxylation of the α-carboxyl group to generate tyramine (Zhang et al. 2016). Moreover, wild-type or mutant enzymes can be selected to convert l-tyrosine to derivatives under specific conditions, such as high substrate loads or non-aqueous media (Almhjell et al. 2018; Li et al. 2019b; Sheldon and Pereira 2017). Furthermore, the elevated catalytic efficiency of enzymes, such as tyrosine aminomutase (TAM; EC 5.4.3.6) or phenylalanine aminomutase (PAM; EC 5.4.3.11), enables high atom economy and a shift of the α-amino group between α- and β-positions to generate (R)-β-tyrosine (Wang et al. 2020).
The present review aims to provide a summary of typical enzyme-catalyzed l-tyrosine derivatization reactions (Fig. 1). Based on the reaction characteristics, we can divide the reactions into four categories: side chain, α-carboxyl group, α-amino group, and multi-group derivatization. Additionally, we summarize the strategies associated with these enzymatic processes and discuss methods for producing l-tyrosine derivatives as well as the associated challenges.
Derivatization of the l-tyrosine side chain
As shown in Fig. 2, the phenol group comprises a benzene ring (–C6H5) and a hydroxyl moiety (–OH). The benzene ring can be hydroxylated and prenylated to generate l-3,4-dihydroxyphenylalanine (l-DOPA) and 3-dimethylallyl-l-tyrosine, respectively. Additionally, the hydroxyl can be easily substituted through direct O-alkylation to produce O-alkylated l-tyrosine derivatives.
Derivatization of the l-tyrosine side chain. a Derivatization of the benzene ring on the phenol group. b Derivatization of the hydroxyl group on the phenol group. TYR, tyrosinase; FgaPT2, 4-dimethylallyltryptophan synthase; TyrPT, 4-O-dimethylallyl-l-tyrosine synthase; SirD, 4-O-dimethylallyl-l-tyrosine synthase; DMAPP, dimethylallyl diphosphate; MAPP, methylallyl diphosphate; 2-pen-pp, 2-pentenyl diphosphate
Derivatization of the benzene ring on the phenol group
Owing to the presence of the hydroxyl group, the C3 position of the l-tyrosine benzene ring becomes more reactive and can undergo either hydroxylation to produce l-DOPA or prenylation to yield 3-dimethylallyl-l-tyrosine (Fig. 2a). Two main enzymatic approaches have been developed for the hydroxylation of the benzene ring: simple enzymatic synthesis and the electro-enzymatic system (Min et al. 2019; Min et al. 2015). Synthesis of l-DOPA by tyrosinase (EC 1.14.18.1) can occur either via reactor engineering or immobilization engineering. Reactor engineering achieves sustainable and efficient operation by allowing full mixing of tyrosinase and l-tyrosine (Rinaldi et al. 2020; Sheldon and Woodley 2018). The most representative example involves a packed-bed reactor, in which tyrosinase is trapped in a copper-alginate gel, resulting in 7.7 mg/L l-DOPA and productivity of 110 mg/L/h (Ates et al. 2007). Immobilization engineering can further increase the l-DOPA titer by preventing enzyme denaturation and promoting enzyme recycling (Sheldon and Woodley 2018). Recently, a new nano-biocatalyst was prepared by immobilizing tyrosinase from Verrucomicrobium spinosum on a polyhydroxyalkanoate nanoparticle, resulting in an l-DOPA titer of 446.20 mg/L, with 90.62% conversion and productivity of 148.70 mg/L/h (Tan et al. 2019). Even though a variety of reactor types and materials for immobilization of the recycled biocatalysts have been explored, the productivity of l-DOPA has remained below 500 mg/L/h, which does not meet the requirements of industrial production. To overcome this limitation, an electro-enzymatic system designed to utilize electrical power to directly reduce the undesirable byproduct dopaquinine back to l-DOPA has been developed (Wu and Zhu 2018). The system achieved 76.6 g/L of l-DOPA at an optimal productivity of 15,300 mg/L/h and 77.7% conversion after a 5-h reaction (Min et al. 2013) (Fig. 2a). The prominent conversion rate and productivity of the electro-enzymatic system are attractive for industrial-scale applications.
C3-prenylation of the benzene ring on the phenol group can be catalyzed by 4-dimethylallyltryptophan synthase (FgaPT2; EC 2.5.1.34) from Aspergillus fumigatus. Scheme 1 illustrates a hypothetical mechanism for FgaPT2 reaction with l-tyrosine. Accordingly, protonation of dimethylallyl diphosphate (DMAPP) yields the dimethylallyl carbocation, which is then attacked by C3 of l-tyrosine to generate intermediate I; finally, the latter undergoes proton elimination to form 3-dimethylallyl-l-tyrosine (Fan et al. 2015a). FgaPT2, which traditionally catalyzes C4-prenylation of the indole ring of l-tryptophan, was mutated to an l-tyrosine C3-prenylating enzyme (FgaPT2K174F) by protein engineering. This strategy led to the synthesis of 0.5 mM 3-dimethylallyl-l-tyrosine at a 50% conversion (Fan et al. 2015b) (Fig. 2a). Although this value is far from the requirements of industrial production, it demonstrates the potential benefit of changing the substrate specificity of other prenyltransferases and the industrial application of l-tyrosine derivatization.
Derivatization of the hydroxyl on the phenol group
Due to easy dissociation of the phenolic –OH group, l-tyrosine can undergo direct O-alkylation to generate O-alkylated l-tyrosine derivatives characterized by numerous pharmacological and biological activities (Chen et al. 2020; Li 2016; Morita et al. 2018). As a member of the dimethylallyl tryptophan synthase superfamily (Burkhardt et al. 2019), 4-O-dimethylallyl-l-tyrosine synthase (TyrPT; EC 2.5.1.122) from Aspergillus niger and 4-O-dimethylallyl-l-tyrosine synthase (SirD; EC 2.5.1.122) from Leptosphaeria maculans can specifically transfer alkyl moieties from three different alkyl donors to an l-tyrosine acceptor (Fig. 2b) (Fan et al. 2015a; Yu et al. 2015). Using DMAPP as the alkyl donor, TyrPT and SirD catalyzed the O-prenylation of l-tyrosine to 4-O-dimethylallyl-l-tyrosine, with 89.1% and 99.3% conversion, respectively. Transfer of methylallyl from methylallyl diphosphate (MAPP) to l-tyrosine also yielded 4-O-methylallyl-l-tyrosine, albeit at a conversion rate of only 21.2% (TyrPT) and 28.0% (SirD). The presence of 2-pentenyldiphosphate (2-pen-PP) achieved 56.6% (TyrPT) and 44.3% (SirD) conversion of l-tyrosine to 4-O-(2-pentenyl)-l-tyrosine (Fig. 2b) (Bandari et al. 2017). These results enriched the libraries of alkyl donors for l-tyrosine O-alkylation, but the turnover of TyrPT and SirD with MAPP and 2-pen-PP remained 0.1–1.0 per minute. Hence, site-directed mutagenesis should be used in the future to improve the catalytic efficiency of TyrPT and SirD on MAPP and 2-pen-PP as alkyl donors.
Derivatization of the l-tyrosine α-carboxyl group
As a stable functional group on a conjugated system, the carboxyl group of l-tyrosine can undergo two different derivatizations: direct decarboxylation by tyrosine decarboxylase (TDC; EC 4.1.1.25) to form tyramine or facile reduction by l-tyrosine reductases (EC 1.2.1.101) to generate l-tyrosine aldehyde (Fig. 3).
Decarboxylation of the α-carboxyl group by tyrosine decarboxylase
The α-carboxyl group of l-tyrosine can undergo decarboxylation by TDC to generate tyramine (Ni et al. 2019; Toy et al. 2015), which is a precursor of textile materials and drugs (Jiang et al. 2019; Zhang and Ni 2014). In the previous decade, membrane engineering, immobilization engineering, and expression optimization became the focus of l-tyrosine decarboxylation. Substrate uptake and product release are mediated by the cell membrane (Britton et al. 2018); therefore, increasing cell membrane permeability could potentially augment the tyramine titer (Westbrook et al. 2019; Westbrook et al. 2018). Use of 1% hexane-treated Origami (DE3) expressing TDC from Lactobacillus brevis (LbTDC) as the biocatalyst successfully converted 18 g/L l-tyrosine to 18 g/L tyramine (> 99%) after 4.5 h (Zhao et al. 2017). To prevent tyramine from being toxic to cells (Zhang and Ni 2014), an encapsulating biocatalyst with alginate (Zhao et al. 2011) achieved 377 mM tyramine, corresponding to a 91.2% yield (Zhang et al. 2016). Improving the expression of soluble TDC in a heterologous host can further increase the tyramine titer. Accordingly, the addition of 1% glucose under acidic conditions, increased the specific activity of LbTDC in Escherichia coli BL21 (DE3) to 46.3 U/mg (Zhang and Ni 2014), allowing 400 mM tyrosine to be completely converted into 400 mM tyramine (> 99.9%) within 24 h (Fig. 3a) (Jiang et al. 2019). Further development of this whole-cell decarboxylation process could lead to highly productive and selective industrial manufacturing of tyramine.
Reduction of the α-carboxyl group by l-tyrosine reductases
The mechanism of the l-tyrosine α-carboxyl group to an aldehyde group by l-tyrosine reductases comprises four steps (Qu et al. 2018). In the first step, the carboxylic acid substrate is activated to form an acyl-AMP intermediate in the adenylation domain. In the second step, the acyl group is transferred to phosphopantetheine to form an enzyme-bound thioester. Third, the peptidyl carrier protein domain delivers the thioester to the reductase domain. Finally, the thioester is reduced by NADPH to yield the aldehyde product (Scheme 2). An enzyme-screening strategy (Derrington et al. 2019; Winkler 2018) led to the identification of l-tyrosine reductases LnaA and LnbA from Aspergillus flavus, which reduced the stable α-carboxyl group using NADPH as a cofactor. LnaA and LnbA could convert almost 1 mM l-tyrosine to l-tyrosine aldehyde after a 2-h reaction (Fig. 3b) (Kalb et al. 2014). As this reaction was conducted in a total volume of only 5 mL, with 1 mM l-tyrosine and 250 μM purified enzyme, further work (e.g., discovery and performance improvement of novel enzymes) is required to test its applicability at an industrial scale.
Mechanism of l-tyrosine reductases biocatalysts. (I) Activation of the acid in the adenylation domain (A-domain); (II) transesterification of AMP-ester; (III) movement of the phosphopantetheine arm to the reductase domain (R-domain); (IV) thioester reduction to produce the aldehyde. A-domain, adenylation domain; PCP-domain, peptidyl carrier protein domain; R-domain, reductase domain
Derivatization of the l-tyrosine α-amino group
The chiral α-amino group of l-tyrosine can undergo five different derivatizations: (1) deamination via l-amino acid deaminases (l-AADs; EC 1.4.3.2); (2) elimination catalyzed by tyrosine ammonia lyase (TAL; EC 4.3.1.5); (3) α-amino shifting by TAM or PAM; (4) catalysis by a three-enzyme cascade composed of l-AAD, meso-diaminopimelate dehydrogenase (DAPDH; EC 1.4.1.16), and formate dehydrogenase (FDH) for the stereo-inversion of the α-amino group; and (5) type conversion of the α-amino group by a three-enzyme cascade including l-AAD, 2-hydroxyisocaproate dehydrogenase (HicDH), and FDH (Fig. 4).
Derivatization of the l-tyrosine α-amino group. a Deamination to produce 4-hydroxyphenylpyruvic acid. b Elimination to produce p-coumaric acid. c Shifting to produce (R)-β-tyrosine. d Stereo-inversion to produce d-tyrosine. e Type conversion to produce (R)- or (S)-4-hydroxyphenyl lactic acid. l-AAD, l-amino acid deaminases; PAM, phenylalanine aminomutase; TAL, tyrosine ammonia lyase; DAPDH, meso-diaminopimelate dehydrogenase; FDH, formate dehydrogenase; d- or l-HicDH, 2-hydroxyisocaproate dehydrogenase
Deamination of the α-amino group via l-amino acid deaminase
Conversion of the l-tyrosine α-amino group (–NH2) to a carbonyl group (–CO–) is catalyzed by four different kinds of enzymes: l-amino acid dehydrogenase (EC1.4.1.5), l-tyrosine aminotransferases (EC 2.6.1.5), l-amino acid oxidase (EC 1.4.3.2), and l-AADs. Membrane-bound l-AAD is an ideal enzyme to prepare 4-hydroxyphenylpyruvic acid (4-HPPA) as no amino acceptor is needed and no H2O2 is produced during the reaction (Fig. 4a) (Ju et al. 2016; Wang et al. 2020). A widely accepted hypothetical mechanism envisions that, during the first half of the reaction, l-AAD employs FAD to catalyze the deamination of l-amino acid, yielding the corresponding α-imino acid, while the FADH2 cofactor is recycled by transferring electrons to molecular oxygen through a membrane electron transfer chain, yielding H2O instead of H2O2. In the second half of the reaction, the corresponding α-keto acid and ammonia are obtained via spontaneous hydrolysis of the α-imino acid (Molla et al. 2017) (Scheme 3). Because l-AADs are anchored to the outside of the cell membrane through an N-terminal transmembrane helix (Ju et al. 2016; Motta et al. 2016), optimization of the protein-secretion system could improve the release of l-AADs to the periplasm. Replacing the integrated pelB-Tat signal peptide with a twin-arginine signal peptide increased secretion efficiency of whole-cell E. coli BL21 (DE3) expressing l-AAD from Proteus vulgaris to 18.9%, resulting in the transformation of 100 mM l-tyrosine into 72.72 mM 4-HPPA with 72.7% yield in 10 h (Ding et al. 2018). This bioprocess, which is more eco-friendly and economical than the use of transaminase or oxidoreductase, has strong potential for industrial application.
Elimination of the α-amino group by tyrosine ammonia lyase
Elimination of the l-tyrosine α-amino group can be achieved by 3,5-dihydro-5-methylidene-4H-imidazol-4-one (MIO)-dependent TAL (EC 4.3.1.5) (Parmeggiani et al. 2018; Wu and Li 2018). The mechanism of MIO-dependent ammonia lyases has been discussed extensively over the past 50 years. The generally accepted hypothesis is that, first, the MIO cofactor is spontaneously generated as a potent electrophile from the three-amino acid motif Ala-Ser-Gly (Scheme 4a). In the second step, the amino group becomes attached to the MIO cofactor, which enhances the leaving ability of the amino group. In the third step, deprotonation of the β-carbon results in the elimination of the MIO-bound amine and product formation. Finally, the MIO-bound amine becomes protonated and released to the broth as free ammonia, regenerating the functional MIO-prosthetic group (Attanayake et al. 2018) (Scheme 4b, path a). To date, three strategies, including protein engineering combined with codon optimization, immobilization engineering, and enzyme screening, have been used to convert l-tyrosine to p-coumaric acid. As a promising tool, protein engineering has been used extensively to improve the catalytic efficiency of codon-optimized TAL and its affinity for l-tyrosine. The most representative example is a triple mutant of TAL from Rhodotorula glutinis (RgTALS9N/A11T/E518V) (Zhou et al. 2016), whose synthesis of 280 mg/L of p-coumaric acid was 65.9% higher than that of wild-type RgTAL. To obtain an even higher p-coumaric acid titer, a calcium alginate matrix cross-linked with glutaraldehyde and polyethyleneimine (GA/PEI) was employed to provide the mechanical stability necessary for repeated use in consecutive batch reactions (Trotman et al. 2007). As a result, 50 g/L l-tyrosine was converted to ~ 39 g/L p-coumaric acid with an average yield of 88% in a 1-L batch reaction run for 41 cycles. The enzymatic screening revealed a thermostable TAL from the wood-rotting fungus Phanerochaete chrysosporium (PcTAL) (Xue et al. 2007). This discovery allowed the reaction temperature to be raised from 30 to 45 °C, resulting in 42.2 g/L p-coumaric acid being produced from 50 g/L l-tyrosine, with 1.11 g/g dcw/L and only 2 g/L PcTAL (Fig. 4b). Recently, another thermostable TAL identified in Streptomyces sp. NRRL F-4489 was characterized as having much higher efficiency, corresponding to a kcat/Km of 78.31/μM/min (Cui et al. 2020). Therefore, the above process could replace traditional chemical synthesis for the industrial production of p-coumaric acid.
Shifting of the α-amino group by tyrosine or phenylalanine aminomutase
The α-amino group of l-tyrosine can move from the α-position to the β-position to generate (R)-β-tyrosine by two different MIO-dependent aminomutases: TAM or PAM (Parmeggiani et al. 2018; Turner 2011). MIO-dependent aminomutases likely share the same catalytic mechanism for the amine removal step with ammonia lyases, but rather than releasing the MIO-bound amine into the surrounding environment, they catalyze a Michael addition that appends the amine to the α,β-unsaturated carboxylic acid intermediate, creating a β-amino acid as the final product (Attanayake et al. 2018) (Scheme 4b, path b). A eukaryotic (R)-selective TAM from rice (Oryza sativa) was found to generate enantiopure (R)-β-tyrosine with 52% conversion and 96.5% ee after 24 h of reaction (Walter et al. 2016; Yan et al. 2015). To further improve conversion, an engineered PAM from Taxus wallichiana (TwPAMC107S) was obtained by sequence alignment and rational mutagenesis, resulting in (R)-β-tyrosine with > 99% ee and without any trace of the undesirable (S)-enantiomer or p-coumarate byproducts (Fig. 4c) (Wu et al. 2010). However, the lower titer, productivity, and yield of (R)-β-tyrosine limit its industrial application. Therefore, continued enzyme discovery and engineering efforts are required to transform aminomutases into effective commercial biocatalysts for l-tyrosine derivatization.
Stereo-inversion of the α-amino group by a three-enzyme cascade
The α-amino group of l-tyrosine can be stereo-inverted by cascading deamination and reductive amination (Song et al. 2018). In one study, a biocatalytic reaction route was designed based on the modification of the α-amino group (Fig. 4d) (Zhang et al. 2019): l-tyrosine was first deaminated to 4-HPPA by l-AAD from Proteus mirabilis, followed by stereoselective reductive amination with a recombinant DAPDH from Symbiobacterium thermophilum (StDAPDHH227V). By incorporating an NADPH-recycling system based on FDH from Burkholderia stabilis, d-tyrosine was obtained with 45.3% conversion and > 99% ee. Recently, the identification and functional characterization of two novel DAPDHs from Numidum massiliense (NmDAPDH) and Thermosyntropha lipolytica (TlDAPDH) were reported. NmDAPDH was found to use both NADP+ and NAD+ as coenzymes and exhibited ~ 2 times higher kcat/Km toward meso-2,6-diaminopimelate than StDAPDH (Akita et al. 2020b). Moreover, the activity of TlDAPDH remained above 80% after incubation for 30 min at 50 °C–65 °C and pH 4.5–pH 9.5 (Akita et al. 2020a). While these studies are still at the laboratory stage, they offer some guidance on the industrial production of d-tyrosine.
Type conversion of the α-amino group by a three-enzyme cascade
Direct conversion of the α-amino group to a hydroxyl group to produce the corresponding chiral α-hydroxy acid cannot be achieved by natural enzymes (Song et al. 2019). Thus, designing an artificial biocatalytic cascade and balancing the expression levels of the cascade enzymes represent two strategies for preparing stereocomplementary (R)- or (S)-4-hydroxyphenyl lactic acid (4-HPLA) from l-tyrosine (Diethelm et al. 2010; Gourinchas et al. 2015). As shown in Fig. 4e, l-tyrosine was first converted to 4-HPPA by l-AAD from Proteus myxofaciens and then asymmetrically reduced by HicDH from Lactobacillus paracasei DSM 20008 (L-HicDH) or from Lactobacillus confuses DSM 20196 (D-HicDH). By incorporating FDH from Candida boidinii to recycle NADH, 100 mg of l-tyrosine was successfully transformed into 78.4 mg (R)-4-HPLA and 80 mg of (S)-4-HPLA with 78–80% isolated yield and 95–99% ee (Busto et al. 2014). To implement this cascade in a designed cell catalyst, the expression levels of the cascade enzymes were balanced by two different promoters (the stronger T7/lacO promoter and the weaker pBAD promoter) (Gourinchas et al. 2015), which resulted in 18.1 g/L (R)-4-HPLA and 36.1 g/L (S)-4-HPLA, with > 99% conversion and > 98% ee (Fig. 4e). Such artificial biocatalytic cascade with both prominent conversion rate and balanced protein expression level might improve the feasibility of 4-HPLA production at an industrial scale.
Derivatization of multiple groups on l-tyrosine
The different groups on l-tyrosine can undergo three types of derivatizations: (1) combinations of convergent and divergent reactions that produce non-natural benzylisoquinoline alkaloids (BIAs); (2) coupling of transamination and deoxygenation reactions to produce (S)-4-hydroxymandelic acid [(S)-HMA]; and (3) a five-enzyme linear cascade that generates 4-hydroxybenzyl alcohol (Fig. 5).
Derivatization of multiple groups on l-tyrosine. a Combinations of convergent and divergent reactions to produce non-natural benzylisoquinoline alkaloids. b Coupling transamination and deoxygenation to produce (S)-4-hydroxymandelic acid. c Five-enzyme linear cascade to generate 4-hydroxybenzyl alcohol TDC, tyrosine decarboxylase; TYR, tyrosinase; NCS, norcoclaurine synthase; TAm, transaminase; TyrB, beta-methylphenylalanine transaminase; HMS, 4-hydroxymandelate synthase; SMDH, (S)-mandelate dehydrogenase; BFD, benzoylformate decarboxylase; PAR, phenylacetaldehyde reductase
Combinations of convergent and divergent reactions to produce BIAs
Combining convergent and divergent reactions affecting the three functional groups on l-tyrosine leads to the synthesis of three non-natural BIAs (Wang et al. 2019). A convergent reaction with phenylacetaldehyde as substrate plus tyrosinase from Candidatus Nitrosopumilus salaria BD31Q, TDCs from Enterococcus faecalis DC32, and norcoclaurine synthase (EC 4.2.1.78) from Thalictrum flavum as catalysts enabled the synthesis of 1-benzyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline at 66% isolated yield and > 97% ee (Fig. 5a). Introduction of transaminase from Chromobacterium violaceum and l-tyrosine as the sole starting material led to production of (S)-norlaudanosoline with 53% isolated yield and > 97% ee (Fig. 5a). If the order of those four enzymes was changed, (S)-norcoclaurine was produced with an isolated yield of 62% and > 97% ee (Fig. 5a). However, although bio-based production of BIAs can be achieved with excellent enantioselectivity, the low BIAs titer is far from the industrial scale. In the future, directed evolution or expression optimization should be used to improve the activity and stability of key enzymes in the pathway and make the transformation more effective.
Coupling of transamination and deoxygenation reactions to produce (S)-HMA
A sequential cascade of transamination and deoxygenation can be created by coupling β-methylphenylalanine transaminase TyrB (EC 2.6.1.107) with 4-hydroxymandelate synthase (HMS; EC 1.13.11.46) from Amycolatopsis orientalis. As shown in Fig. 5b, l-tyrosine was first transaminated to 4-HPPA using α-ketoglutarate as the amino acceptor and then directly converted to (S)-HMA in the presence of oxygen (Youn et al. 2020). Using recombinant E. coli as whole-cell biocatalysts over a period of 6 h yielded ~ 4.5 mM (S)-HMA with 84% conversion and > 97% ee. Nevertheless, the application of this route on an industrial scale has been hampered by low productivity and the additional cost of the co-substrate α-ketoglutarate. Use of l-AAD instead of β-methylphenylalanine transaminase TyrB and a reactor engineering strategy to improve catalytic efficiency could bring this process closer to industrial application.
A five-enzyme linear cascade to produce 4-hydroxybenzyl alcohol
Cascading a deamination reaction with four other reactions allowed the conversion of l-tyrosine α-amino and α-carboxyl groups into a hydroxyl group (Fig. 5c) (Liu et al. 2020). Specifically, 4-HPPA was first obtained through direct deamination of l-tyrosine catalyzed by l-AAD, and subsequent transformation of 4-HPPA to 4-hydroxybenzyl alcohol was achieved through four heterologous enzymatic steps involving HMS, (S)-mandelate dehydrogenase (EC 1.1.99.31), benzoylformate decarboxylase (EC 4.1.1.7), and phenylacetaldehyde reductase from Solanum lycopersicum. Using this method, 580.98 mg/L of 4-hydroxybenzyl alcohol was synthesized from l-tyrosine with 93.6% conversion. Even though an NADPH-recycling system can be incorporated to reduce the cost for cofactor addition, NADPH is still too expensive to be applied at an industrial scale. Recently, a novel short-chain carbonyl reductase from Bacillus aryabhattai (BaSDR1; EC 1.1.1.184) was cloned and expressed in E. coli. Notably, BaSDR1 displayed excellent catalytic performance toward various acetophenone analogs with NADH as a cofactor and yielded chiral phenethyl alcohol with 99% ee for all the tested substrates (Li et al. 2019a). Replacing phenylacetaldehyde reductase with NADH-dependent carbonyl reductase or changing cofactor preference of phenylacetaldehyde reductase will likely enable the industrial production of 4-hydroxybenzyl alcohol.
Conclusion and future prospects
As an aromatic polar amino acid, l-tyrosine contains a highly reactive α-amino, α-carboxyl, and phenolic group. These functional groups allow diverse derivatizations of l-tyrosine, such as hydroxylation catalyzed by tyrosinase, decarboxylation of the α-carboxyl group by TDC, and deamination of the α-amino group via l-AAD. Single- or multi-group derivatizations guided by various enzymes enable highly enantioselective conversion of l-tyrosine to value-added chemical scaffolds, including non-proteinogenic amino acids, α-hydroxy acids, ketoacids, unsaturated aromatic compounds, and non-natural alkaloids. However, unsatisfactory yields of l-tyrosine derivatives, as well as the restrictive category of l-tyrosine, continue to limit the industrial application of the derivatization process. Therefore, future efforts should focus on the following three aspects: (1) discovery and performance improvement of novel enzymes; (2) design and extension of derivatization reactions; and (3) optimization and development of the catalytic process. With the explosive growth in enzyme discovery (e.g., metagenome screening and gene mining), the prospect of using new functional enzymes for l-tyrosine derivatization increases the chances of overcoming multiple limitations. Additionally, protein engineering technologies promote enzyme modification to address defects associated with enzyme activity and stability, resulting in increased tolerance for high substrate loads required in industrial applications (Sun et al. 2019). l-Tyrosine derivatization has clearly advanced from single-step transformations to multi-step cascade reactions using either isolated biocatalysts or whole-cell systems. Therefore, a deeper understanding of the catalytic performance of the associated enzymes will foster the continued development of derivatization strategies by extending linear enzyme cascades or designing different cascade types (e.g., orthogonal or cyclic cascades) via biocatalytic retrosynthetic analysis (Schrittwieser et al. 2018). Furthermore, to meet the demands of industrial settings, novel techniques aimed at optimizing the catalytic process, such as media, immobilization, and reactor engineering, should be implemented to improve the cost-effectiveness of l-tyrosine derivatives production. As a result of more effective biocatalysts, novel l-tyrosine derivatives can be soon expected on a large scale.
References
Akita H, Nakamichi Y, Morita T, Matsushika A (2020a) Characterization of an NAD(P)+-dependent meso-diaminopimelate dehydrogenase from Thermosyntropha lipolytica. Biochim Biophys Acta, Proteins Proteomics 1868(10):9. https://doi.org/10.1016/j.bbapap.2020.140476
Akita H, Nakamichi Y, Morita T, Matsushika A (2020b) Identification and functional characterization of NAD(P)+-dependent meso-diaminopimelate dehydrogenase from Numidum massiliense. MicrobiologyOpen 9(8):10. https://doi.org/10.1002/mbo3.1059
Almhjell PJ, Boville CE, Arnold FH (2018) Engineering enzymes for noncanonical amino acid synthesis. Chem Soc Rev 47(24):8980–8997. https://doi.org/10.1039/c8cs00665b
Ates S, Cortenlioglu E, Bayraktar E, Mehmetoglu U (2007) Production of L-DOPA using cu-alginate gel immobilized tyrosinase in a batch and packed bed reactor. Enzym Microb Technol 40(4):683–687. https://doi.org/10.1016/j.enzmictec.2006.05.031
Attanayake G, Walter T, Walker KD (2018) Understanding which residues of the active site and loop structure of a tyrosine aminomutase define its mutase and lyase activities. Biochemistry 57(25):3503–3514. https://doi.org/10.1021/acs.biochem.8b00269
Bandari C, Scull EM, Masterson JM, Tran RHQ, Foster SB, Nicholas KM, Singh S (2017) Determination of alkyl-donor promiscuity of tyrosine-O-prenyltransferase SirD from Leptosphaeria maculans. ChemBioChem 18(23):2323–2327. https://doi.org/10.1002/cbic.201700469
Britton J, Majumdar S, Weiss GA (2018) Continuous flow biocatalysis. Chem Soc Rev 47(15):5891–5918. https://doi.org/10.1039/c7cs00906b
Burkhardt I, Ye ZF, Janevska S, Tudzynski B, Dickschat JS (2019) Biochemical and mechanistic characterization of the fungal reverse N-1-dimethylallyltryptophan synthase DMATS1Ff. ACS Chem Biol 14(12):2922–2931. https://doi.org/10.1021/acschembio.9b00828
Busto E, Richter N, Grischek B, Kroutil W (2014) Formal inversion or retention of L-alpha-amino acids to enantiopure (R)- or (S)-hydroxyacids. Chem Eur J 20(35):11225–11228. https://doi.org/10.1002/chem.201403195
Chen W, Yao J, Meng J, Han W, Tao Y, Chen Y, Guo Y, Shi G, He Y, Jin J-M, Tang S-Y (2019) Promiscuous enzymatic activity-aided multiple-pathway network design for metabolic flux rearrangement in hydroxytyrosol biosynthesis. Nat Commun 10:960. https://doi.org/10.1038/s41467-019-08781-2
Chen Y-R, Naresh A, Liang S-Y, Lin C-H, Chein R-J, Lin H-C (2020) Discovery of a dual function cytochrome P450 that catalyzes enyne formation in cyclohexanoid terpenoid biosynthesis. Angew Chem Int Ed 59(32):13537–13541. https://doi.org/10.1002/anie.202004435
Cox JB, Kimishima A, Wood JL (2019) Total synthesis of herquline B and C. J Am Chem Soc 141(1):25–28. https://doi.org/10.1021/jacs.8b10212
Cui P, Zhong W, Qin Y, Tao F, Wang W, Zhan J (2020) Characterization of two new aromatic amino acid lyases from actinomycetes for highly efficient production of p-coumaric acid. 43(7):1287–1298. https://doi.org/10.1007/s00449-020-02325-5
Dennig A, Busto E, Kroutil W, Faber K (2015) Biocatalytic one-pot synthesis of L-tyrosine derivatives from monosubstituted benzenes, pyruvate, and ammonia. ACS Catal 5(12):7503–7506. https://doi.org/10.1021/acscatal.5b02129
Derrington SR, Turner NJ, France SP (2019) Carboxylic acid reductases (CARs):an industrial perspective. J Biotechnol 304:78–88. https://doi.org/10.1016/j.jbiotec.2019.08.010
Diethelm S, Schindler CS, Carreira EM (2010) Synthesis of Microcin SF608 through nucleophilic opening of an oxabicyclo [2.2.1] heptane. Org Lett 12(17):3950–3953. https://doi.org/10.1021/ol1017189
Ding H, Zhao W, Lu C, Huang J, Hu S, Yao S, Mei L, Wang J, Mei J (2018) Biosynthesis of 4-hydroxyphenylpyruvic acid from L-tyrosine using recombinant Escherichia coli cells expressing membrane bound L-amino acid deaminase. Chin J Chem Eng 26(2):380–385. https://doi.org/10.1016/j.cjche.2017.08.009
Fan A, Xie X, Li S (2015a) Tryptophan prenyltransferases showing higher catalytic activities for Friedel-crafts alkylation of o- and m-tyrosines than tyrosine prenyltransferases. Org Biomol Chem 13(27):7551–7557. https://doi.org/10.1039/c5ob01040c
Fan A, Zocher G, Stec E, Stehle T, Li SM (2015b) Site-directed mutagenesis switching a dimethylallyl tryptophan synthase to a specific tyrosine C-3-prenylating enzyme. J Biol Chem 290(3):1364–1373. https://doi.org/10.1074/jbc.M114.623413
Glachet T, Marzag H, Rosa NS, Colell JFP, Zhang G, Warren WS, Franck X, Theis T, Reboul V (2019) Iodonitrene in action: direct transformation of amino acids into terminal diazirines and 15N2-diazirines and their application as hyperpolarized markers. J Am Chem Soc 141(34):13689–13696. https://doi.org/10.1021/jacs.9b07035
Gourinchas G, Busto E, Killinger M, Richter N, Wiltschi B, Kroutil W (2015) A synthetic biology approach for the transformation of L-α-amino acids to the corresponding enantiopure (R)- or (S)-α-hydroxy acids. Chem Commun 51(14):2828–2831. https://doi.org/10.1039/c4cc08286a
Haslinger K, Prather KLJ (2020) Heterologous caffeic acid biosynthesis in Escherichia coli is affected by choice of tyrosine ammonia lyase and redox partners for bacterial cytochrome P450. Microb Cell Factories 19(1):26. https://doi.org/10.1186/s12934-020-01300-9
Jiang MY, Xu GC, Ni J, Zhang K, Dong JJ, Han RZ, Ni Y (2019) Improving soluble expression of tyrosine decarboxylase from lactobacillus brevis for tyramine synthesis with high total turnover number. Appl Biochem Biotechnol 188(2):436–449. https://doi.org/10.1007/s12010-018-2925-x
Ju YC, Tong SL, Gao YX, Zhao W, Liu Q, Gu Q, Xu J, Niu LW, Teng MK, Zhou HH (2016) Crystal structure of a membrane-bound L-amino acid deaminase from Proteus vulgaris. J Struct Biol 195(3):306–315. https://doi.org/10.1016/j.jsb.2016.07.008
Kalb D, Lackner G, Hoffmeister D (2014) Functional and phylogenetic divergence of fungal adenylate-forming reductases. Appl Environ Microbiol 80(19):6175–6183. https://doi.org/10.1128/aem.01767-14
Kim S, Sung BH, Kim SC, Lee HS (2018) Genetic incorporation of L-dihydroxyphenylalanine (DOPA) biosynthesized by a tyrosine phenol-lyase. Chem Commun 54(24):3002–3005. https://doi.org/10.1039/c8cc00281a
Li A, Yuchi Q, Li X, Pang W, Li B, Xue F, Zhang L (2019a) Discovery of a novel ortho-haloacetophenones-specific carbonyl reductase from Bacillus aryabhattai and insight into the molecular basis for its catalytic performance. Int J Biol Macromol 138:781–790. https://doi.org/10.1016/j.ijbiomac.2019.07.153
Li G, Lian J, Xue H, Jiang Y, Ju S, Wu M, Lin J, Yang L (2020) Biocascade synthesis of L-tyrosine derivatives by coupling a thermophilic tyrosine phenol-lyase and L-lactate oxidase. Eur J Org Chem 2020(8):1050–1054. https://doi.org/10.1002/ejoc.202000061
Li W (2016) Bringing bioactive compounds into membranes: the UbiA superfamily of intramembrane aromatic prenyltransferases. Trends Biochem Sci 41(4):356–370. https://doi.org/10.1016/j.tibs.2016.01.007
Li X, Ma M, Xin X, Tang Y, Zhao G, Xiao X (2019b) Efficient acylation of gastrodin by Aspergillus oryzae whole-cells in non-aqueous media. RSC Adv 9(29):16701–16712. https://doi.org/10.1039/c9ra01605h
Liu L, Zhu Y, Chen Y, Chen H, Fan C, Mo Q, Yuan J (2020) One-pot cascade biotransformation for efficient synthesis of benzyl alcohol and its analogs. Chem Asian J 15(7):1018–1021. https://doi.org/10.1002/asia.201901680
Luetke ET, Santos CNS, Stephanopoulos G (2007) Perspectives of biotechnological production of L-tyrosine and its applications. Appl Microbiol Biotechnol 77(4):751–762. https://doi.org/10.1007/s00253-007-1243-y
Lukito BR, Sekar BS, Wu SK, Li Z (2019) Whole cell-based cascade biotransformation for the production of (S)-mandelic acid from styrene, L-phenylalanine, glucose, or glycerol. Adv Synth Catal 361(15):3560–3568. https://doi.org/10.1002/adsc.201900373
Min K, Kathavarayan T, Park K, Yoo YJ (2013) Novel strategy for enhancing productivity in L-DOPA synthesis: the electroenzymatic approach using well-dispersed L-tyrosine. J Mol Catal B Enzym 90:87–90. https://doi.org/10.1016/j.molcatb.2013.01.027
Min K, Park GW, Yoo YJ, Lee JS (2019) A perspective on the biotechnological applications of the versatile tyrosinase. Bioresour Technol 289:121730. https://doi.org/10.1016/j.biortech.2019.121730
Min K, Park K, Park DH, Yoo YJ (2015) Overview on the biotechnological production of L-DOPA. Appl Microbiol Biotechnol 99(2):575–584. https://doi.org/10.1007/s00253-014-6215-4
Molla G, Melis R, Pollegioni L (2017) Breaking the mirror: L-amino acid deaminase, a novel stereoselective biocatalyst. Biotechnol Adv 35(6):657–668. https://doi.org/10.1016/j.biotechadv.2017.07.011
Morita M, Hao Y, Jokela JK, Sardar D, Lin ZJ, Sivonen K, Nair SK, Schmidt EW (2018) Post-translational tyrosine geranylation in cyanobactin biosynthesis. J Am Chem Soc 140(19):6044–6048. https://doi.org/10.1021/jacs.8b03137
Motta P, Molla G, Pollegioni L, Nardini M (2016) Structure-function relationships in L-amino acid deaminase, a flavoprotein belonging to a novel class of biotechnologically relevant enzymes. J Biol Chem 291(20):10457–10475. https://doi.org/10.1074/jbc.M115.703819
Mujumdar P, Kopecka J, Bua S, Supuran CT, Riganti C, Poulsen S-A (2019) Carbonic anhydrase XII inhibitors overcome temozolomide resistance in glioblastoma. J Med Chem 62(8):4174–4192. https://doi.org/10.1021/acs.jmedchem.9b00282
Ni J, Xu GC, Dai W, Zhao YL, Ni Y (2019) Hyperconjugation promoted by hydrogen bonding between His98/His241 and a carboxyl group contributes to tyrosine decarboxylase catalysis. Catal Sci Technol 9(22):6222–6226. https://doi.org/10.1039/c9cy01290g
Noda S, Kawai Y, Tanaka T, Kondo A (2015) 4-vinylphenol biosynthesis from cellulose as the sole carbon source using phenolic acid decarboxylase- and tyrosine ammonia lyase-expressing Streptomyces lividans. Bioresour Technol 180:59–65. https://doi.org/10.1016/j.biortech.2014.12.064
Parmeggiani F, Weise NJ, Ahmed ST, Turner NJ (2018) Synthetic and therapeutic applications of ammonia-lyases and aminomutases. Chem Rev 118(1):73–118. https://doi.org/10.1021/acs.chemrev.6b00824
Qu G, Guo JG, Yang DM, Sun ZT (2018) Biocatalysis of carboxylic acid reductases: phylogenesis, catalytic mechanism and potential applications. Green Chem 20(4):777–792. https://doi.org/10.1039/c7gc03046k
Rinaldi F, Fernandez-Lucas J, de la Fuente D, Zheng C, Bavaro T, Peters B, Massolini G, Annunziata F, Conti P, de la Mata I, Terreni M, Calleri E (2020) Immobilized enzyme reactors based on nucleoside phosphorylases and 2- deoxyribosyltransferase for the in -flow synthesis of pharmaceutically relevant nucleoside analogues. Bioresour Technol 307. https://doi.org/10.1016/j.biortech.2020.123258
Rodriguez A, Chen Y, Khoomrung S, Ozdemir E, Borodina I, Nielsen J (2017) Comparison of the metabolic response to over-production of p-coumaric acid in two yeast strains. Metab Eng 44:265–272. https://doi.org/10.1016/j.ymben.2017.10.013
Sarlaslani FS (2007) Development of a combined biological and chemical process for production of industrial aromatics from renewable resources. Annu Rev Microbiol 61:51–69. https://doi.org/10.1146/annrev.micro.61.080706.093248
Schrittwieser JH, Velikogne S, Hall M, Kroutil W (2018) Artificial biocatalytic linear cascades for preparation of organic molecules. Chem Rev 118(1):270–348. https://doi.org/10.1021/acs.chemrev.7b00033
Sheldon RA, Pereira PC (2017) Biocatalysis engineering: the big picture. Chem Soc Rev 46(10):2678–2691. https://doi.org/10.1039/c6cs00854b
Sheldon RA, Woodley JM (2018) Role of biocatalysis in sustainable chemistry. Chem Rev 118(2):801–838. https://doi.org/10.1021/acs.chemrev.7b00203
Song W, Chen X, Wu J, Xu J, Zhang W, Liu J, Chen J, Liu L (2019) Biocatalytic derivatization of proteinogenic amino acids for fine chemicals. Biotechnol Adv 40:107496. https://doi.org/10.1016/j.biotechadv.2019.107496
Song W, Wang J-H, Wu J, Liu J, Chen X-L, Liu L-M (2018) Asymmetric assembly of high-value alpha-functionalized organic acids using a biocatalytic chiral-group-resetting process. Nat Commun 9:3818. https://doi.org/10.1038/s41467-018-06241-x
Sun Z, Liu Q, Qu G, Feng Y, Reetz MT (2019) Utility of B-factors in protein science: interpreting rigidity, flexibility, and internal motion and engineering thermostability. Chem Rev 119(3):1626–1665. https://doi.org/10.1021/acs.chemrev.8b00290
Tan D, Zhao JP, Ran GQ, Zhu XL, Ding Y, Lu XY (2019) Highly efficient biocatalytic synthesis of L-DOPA using in situ immobilized Verrucomicrobium spinosum tyrosinase on polyhydroxyalkanoate nano-granules. Appl Microbiol Biotechnol 103(14):5663–5678. https://doi.org/10.1007/s00253-019-09851-7
Toy N, Ozogul F, Ozogul Y (2015) The influence of the cell free solution of lactic acid bacteria on tyramine production by food borne-pathogens in tyrosine decarboxylase broth. Food Chem 173:45–53. https://doi.org/10.1016/j.foodchem.2014.10.001
Trotman RJ, Camp CE, Ben BA, DiCosimo R, Huang LX, Crum GA, Sariaslani FS, Haynie SL (2007) Calcium alginate bead immobilization of cells containing tyrosine ammonia lyase activity for use in the production of p-hydroxycinnamic acid. Biotechnol Prog 23(3):638–644. https://doi.org/10.1021/bp060379e
Turner NJ (2011) Ammonia lyases and aminomutases as biocatalysts for the synthesis of α-amino and β-amino acids. Curr Opin Chem Biol 15(2):234–240. https://doi.org/10.1016/j.cbpa.2010.11.009
Walter T, King Z, Walker KD (2016) A tyrosine aminomutase from rice (Oryza sativa) lsomerizes (S)-α- to (R)-β-tyrosine with unique high enantioselectivity and retention of configuration. Biochemistry 55(1):1–4. https://doi.org/10.1021/acs.biochem.5b01331
Wang J, Song W, Wu J, Liu J, Chen X, Liu L (2020) Efficient production of phenylpropionic acids by an amino-group-transformation biocatalytic cascade. Biotechnol Bioeng 117(3):614–625. https://doi.org/10.1002/bit.27241
Wang Y, Tappertzhofen N, Mendez-Sanchez D, Bawn M, Lyu B, Ward JM, Hailes HC (2019) Design and use of de novo cascades for the biosynthesis of new benzylisoquinoline alkaloids. Angew Chem Int Ed 58(30):10120–10125. https://doi.org/10.1002/anie.201902761
Wen J, Bao Y, Niu Q, Liu J, Yang J, Wang W, Jiang T, Fan Y, Li K, Wang J, Zhao L, Liu D (2016) Synthesis, biological evaluation and molecular modeling studies of psammaplin A and its analogs as potent histone deacetylases inhibitors and cytotoxic agents. Bioorg Med Chem Lett 26(17):232–236. https://doi.org/10.1016/j.bmcl.2015.12.094
Westbrook AW, Miscevic D, Kilpatrick S, Bruder MR, Moo-Young M, Chou CP (2019) Strain engineering for microbial production of value-added chemicals and fuels from glycerol. Biotechnol Adv 37(4):538–568. https://doi.org/10.1016/j.biotechadv.2018.10.006
Westbrook AW, Ren X, Moo-Young M, Chou CP (2018) Engineering of cell membrane to enhance heterologous production of hyaluronic acid in Bacillus subtilis. Biotechnol Bioeng 115(1):216–231. https://doi.org/10.1002/bit.26459
Winkler M (2018) Carboxylic acid reductase enzymes (CARs). Curr Opin Chem Biol 43:23–29. https://doi.org/10.1016/j.cbpa.2017.10.006
Wu B, Szymanski W, Wijma HJ, Crismaru CG, de Wildeman S, Poelarends GJ, Feringa BL, Janssen DB (2010) Engineering of an enantioselective tyrosine aminomutase by mutation of a single active site residue in phenylalanine aminomutase. Chem Commun 46(43):8157–8159. https://doi.org/10.1039/c0cc02768e
Wu R, Zhu Z (2018) Self-powered enzymatic electrosynthesis of L-3,4-dihydroxyphenylalanine in a hybrid bioelectrochemical system. ACS Sustain Chem Eng 6(10):12593–12597. https://doi.org/10.1021/acssuschemeng.8b03862
Wu SK, Li Z (2018) Whole-cell cascade biotransformations for one-pot multistep organic synthesis. ChemCatChem 10(10):2164–2178. https://doi.org/10.1002/cctc.201701669
Xue Z, McCluskey M, Cantera K, Ben-Bassat A, Sariaslani RS, Huang L (2007) Improved production of p-hydroxycinnamic acid from tyrosine using a novel thermostable phenylalanine/tyrosine ammonia lyase enzyme. Enzym Microb Technol 42(1):58–64. https://doi.org/10.1016/j.enzmictec.2007.07.025
Yan J, Aboshi T, Teraishi M, Strickler SR, Spindel JE, Tung C-W, Takata R, Matsumoto F, Maesaka Y, McCouch SR, Okumoto Y, Mori N, Jander G (2015) The tyrosine aminomutase TAM1 is required for β-tyrosine biosynthesis in rice. Plant Cell 27(4):1265–1278. https://doi.org/10.1105/tpc.15.00058
Youn JW, Albermann C, Sprenger GA (2020) In vivo cascade catalysis of aromatic amino acids to the respective mandelic acids using recombinant E. coli cells expressing hydroxymandelate synthase (HMS) from Amycolatopsis mediterranei. Mol Catal 483:110713. https://doi.org/10.1016/j.mcat.2019.110713
Yu H, Liebhold M, Xie X, Li S (2015) Tyrosine O-prenyltransferases TyrPT and SirD displaying similar behavior toward unnatural alkyl or benzyl diphosphate as their natural prenyl donor dimethylallyl diphosphate. Appl Microbiol Biotechnol 99(17):7115–7124. https://doi.org/10.1007/s00253-015-6452-1
Zhang DP, Jing XR, Zhang WL, Nie Y, Xu Y (2019) Highly selective synthesis of D-amino acids from readily available L-amino acids by a one-pot biocatalytic stereoinversion cascade. RSC Adv 9(51):29927–29935. https://doi.org/10.1039/c9ra06301c
Zhang H, Wei Y, Lu Y, Wu S, Liu Q, Liu J, Jiao Q (2016) Three-step biocatalytic reaction using whole cells for efficient production of tyramine from keratin acid hydrolysis wastewater. Appl Microbiol Biotechnol 100(4):1691–1700. https://doi.org/10.1007/s00253-015-7054-7
Zhang K, Ni Y (2014) Tyrosine decarboxylase from Lactobacillus brevis: soluble expression and characterization. Protein Expr Purif 94:33–39. https://doi.org/10.1016/j.pep.2013.10.018
Zhao GH, Liu JZ, Dong K, Zhang F, Zhang HJ, Liu QA, Jiao QC (2011) Enzymatic synthesis of L-tryptophan from hair acid hydrolysis industries wastewater with tryptophan synthase. Bioresour Technol 102(3):3554–3557. https://doi.org/10.1016/j.biortech.2010.09.019
Zhao W, Hu S, Yu Y, Xu H, Huang J, Yao S, Mei L, Jin Z (2017) Biosynthesis of tyramine with permeabilized recombinant Escherichia Coli cells expressing tyrosine decarboxylase. J Chem Eng Chin Uni 31(6):1364–1371
Zhou SH, Liu PR, Chen J, Du GC, Li HZ, Zhou JW (2016) Characterization of mutants of a tyrosine ammonia-lyase from Rhodotorula glutinis. Appl Microbiol Biotechnol 100(24):10443–10452. https://doi.org/10.1007/s00253-016-7672-8
Funding
This work was financially supported by the national first-class discipline program of Light Industry Technology and Engineering (LITE2018-20), the National Natural Science Foundation of China (21878126), and the Key Technologies R & D Program of Jiangsu Province (BE2018623).
Author information
Authors and Affiliations
Contributions
Tan X. searched and organized published data. Tan X. wrote the manuscript with help from Song W. and Chen X. Liu L. and Wu J. conceived and designed the mini-review. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies on human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tan, X., Song, W., Chen, X. et al. Recent advances in biocatalytic derivatization of l-tyrosine. Appl Microbiol Biotechnol 104, 9907–9920 (2020). https://doi.org/10.1007/s00253-020-10949-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10949-6