Abstract
Tyramine has been paid more attention in recent years as a significant metabolite of tyrosine and catecholamine drug and an intermediate of medicinal material and some drugs. In this study, an effective, green, and three-step biocatalytic synthesis method for production of tyramine starting from serine in keratin acid hydrolysis wastewater was developed and investigated. Serine deaminase from Escherichia coli was first combined with tyrosine phenol-lyase from Citrobacter koseri, to convert l-serine to l-tyrosine. l-Tyrosine can then be decarboxylated to tyramine by tyrosine decarboxylase from Lactobacillus brevis. All these enzymes originated from recombinant whole cells. Serine deaminase and tyrosine phenol-lyase could efficiently convert l-serine in wastewater to l-tyrosine at pH 8.0, 37 °C, and Triton X-100 of 0.04 % when tyrosine phenol-lyase and its corresponding substrates were sequentially added. Tyrosine conversion rate reached 98 % by l-tyrosine decarboxylase. In scale-up study, the conversion yield of l-serine in wastewater to tyrosine was up to 89 %. l-Tyrosine was decarboxylated to tyramine with a high yield 94 %. Tyramine hydrochloride was obtained with a total yield 84 %. This study has provided an efficient way of recycling keratin acid hydrolysis wastewater to produce tyramine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well known that amino acids play a significant role in productions of flavor materials, food additives, feed additives, medicine, health care products, and cosmetics. In China, the annual production of amino acids reached approximately 5,000,000 t in recent years (Wang et al. 2013). Extraction of amino acids from protein acid hydrolytes is one of the most prevailing methods due to the broad resource of keratin (e.g., hair and feather), so that multiple kinds of amino acids could be simultaneously collected such as cystine, arginine, tyrosine, phenylalanine, leucine, and isoleucine. However, the acid hydrolysis preparation method produces undesirable quantity of wastewater in that there are more than 180,000 t of keratin acid hydrolysis wastewater (KHW) per year generated in China, accounting for 60 % of KHW of the world (Xu et al. 2013; Zhao et al. 2011). In fact, the drained wastewater is still a rich resource of amino acid, containing valuable species such as serine, aspartic acid, threonine, glutamic acid, proline, and so on. If the wastewater was recycled to manufacture high-value-added products (e.g., tyramine), the environmental problem will be alleviated and the costs of the industrial production will be lowered. And, it will become greener to recycle the wastewater by biological method. There are cases in which the industrial wastewater cannot be avoided and resulted in many wasting and environmental problems. Accordingly, there is a growing need for more effective utilization of the industrial wastewater (Sheldon 2014). Many research groups have paid much attention to recycle some high-value products from industrial wastewater (Cheng et al. 2015; Coppens et al. 2014; Vu et al. 2009). It has been demonstrated that hair acid hydrolysis wastewater could be transformed into l-tryptophan and S-phenyl-l-cysteine by tryptophan synthase (Xu et al. 2013; Zhao et al. 2011). In the present study, the potential use of KHW as a raw material to synthesize tyramine, an important biogenic amine, was investigated.
Tyramine [2-(p-hydroxyphenyl) ethylamine], a biogenic amine, has gained tremendous interest in recent years as a catecholamine drug (Khwanchuea et al. 2008; Li et al. 2009), an intermediate of textile material (Krysin et al. 2001), and some drugs (e.g., bezafibrate, dopamine) (Li et al. 2009; Magnus et al. 2009; Panufnik and Kañska 2007). Tyramine is mainly prepared through chemical synthesis (Buck 1933; Wang and Xie 1994; Krysin et al. 2001; Li et al. 2009; Shimizu et al. 2012). Compared with chemical synthesis, enzymatic synthesis has its advantages for its mild reaction conditions; eco-friendliness; and high enantioselectivity, regioselectivity, and chemoselectivity. Furthermore, multi-enzymatic cascade reactions offer more advantages (Lopez-Gallego and Schmidt-Dannert 2010; Ricca et al. 2011). Tyramine could be produced by enzymatic decarboxylation of l-tyrosine, a reaction catalyzed by tyrosine decarboxylase (TDC, EC 4.1.1.25) (Panufnik and Kañska 2007; Thakur and Azmi 2009; Zhang and Ni 2014). Nevertheless, based on tyramine as the metabolite of l-tyrosine, this enzymatically synthetic route was more utilized to study the metabolic diversion of tyramine in plant (Lee et al. 2009) and the effect of tyramine on food safety and spoilage due to the health problem caused by the high quantity of tyramine (Buňková et al. 2012; Ladero et al. 2012; Toy et al. 2015). By far, there is no detailed reference to investigate the enzymatic synthesis of tyramine by TDC with recombinant whole cells, neither for the industrial production with the method. Furthermore, the cost of l-tyrosine and enzyme should be decreased in order to industrialize enzymatic synthesis of tyramine. KHW was therefore recycled to produce l-tyrosine, and recombinant whole cells were used to prepare tyramine since whole cells usually own higher operational stability and the isolated enzyme requires time- and money-consuming purification (Jakoblinnert and Rother 2014).
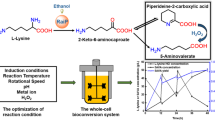
Here, we designed a three-step biocatalytic cascade reaction in which KHW was used as starting material and every step was catalyzed by recombinant whole cells in aqueous media (Scheme 1). In the first step, l-serine in KHW was deaminized by serine deaminase (SDA, EC 4.3.1.17) from Escherichia coli to yield pyruvate (Cicchillo et al. 2004; Zhang et al. 2010). Secondly, tyrosine phenol-lyase (TPL, EC 4.1.99.2) from Citrobacter koseri came into play (Chandel and Azmi 2009; Enei et al. 1973; Lütke-Eversloh et al. 2007; Milic et al. 2008); the corresponding substrates including phenol and ammonia chloride were added, and then, l-tyrosine precipitated at the optimal condition. At last, l-tyrosine was collected and used as the substrate to produce tyramine by TDC from Lactobacillus brevis. Pyridoxal phosphate (PLP) was the common coenzyme for these three enzymes. Though TPL could catalyze β-replacement of serine to give tyrosine (Fuganti et al. 1974; Sawada et al. 1975), it generally has very low conversion rate of KHW in comparison with forming pyruvate intermediate via SDA. Therefore, the three-step reaction was adopted.
The aim of this study is to explore the feasibility of KHW bioconversion and develop a green synthetic route of tyramine with high productivity. We have also studied the influence of various reaction parameters on the enzymatic reactions under aqueous conditions. At the same time, bioconversion efficiency and product yield are determined under the optimal conditions.
Materials and methods
Materials
The KHW used as a raw material was provided with Shine Star (Hubei) Biological Engineering Co. Ltd. (Hubei, China). The organic contents depended on the species of initial keratin used in the amino acid production, but most KHW contained ammonium chloride, pigment, suspended solids, and about nine amino acids. Physicochemical characteristics of KHW are shown in Table 1.
Lactose and pyridoxal-5′-phosphate (PLP) were from Sigma (St. Louis, Mo., USA). Silica gel GF254 plate (5.0 × 10.0 cm) was purchased from Haiyang Chemical Co. Ltd. (Qingdao, China). All other chemicals and reagents used in this work were of analytical grade and purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). Restriction enzymes and kits for genetic manipulation were purchased from Takara Bio (Dalian, China).
Microorganisms and shake flask fermentation
All the recombinant cells were constructed by cloning corresponding plasmids into E. coli BL21 (DE3). To amplify SDA gene (GenBank Gene ID: 946331), sda, from genomic DNA of E. coli str. K-12 substr. MG1655, two primers were designed based on the nucleotide sequence of sda. Forward primer sda-f is as follows: 5′-CCCAGATCTCATGATTAGTCTATTCGACAT-3′ (Bgl II site underlined); reverse primer sda-r is as follows: 5′-TTTCTCGAGTTAGTCACAC TGGACTTTG-3′ (Xho I site underlined). PCR amplification was performed using Taq DNA polymerase on a PTC-200 thermocycler (Bio-Rad, USA). The PCR fragment of sda gene was inserted into pMD18-T vector to create recombinant plasmid pMD18-sda, and the nucleotide sequence of sda was determined by Genscript Biotech Co. (Nanjing, China).
Both plasmids pMD18-sda and pETDuet were double-digested with Bgl II and Xho I, and then, the purified sda gene and linearized pETDuet fragments were ligated by T4 DNA ligase to generate plasmid pETDuet-sda, which was then transformed into E. coli BL21 (DE3). The positive clones were selected and confirmed by colony PCR and restriction enzyme digestion.
The gene encoding TPL (GenBank GeneID: 5581624), tpl, was from C. koseri ATCC BAA-895. The tpl gene was amplified by PCR using primer tpl-f: 5′-CCCAGATCTCATGAATTATCCGGCAGAACCCTT-3′ and primer tpl-r: 5′-CCCCTCGAGTTAAATATAATCAAAACGTGCAG-3′ with Bgl II and Xho I restriction sites, respectively.
The TDC gene (GenBank No. EU195891.1), tdc, was from genomic DNA of L. brevis CGMCC 1.2028 (Zhang and Ni 2014). The nucleotide sequence of tdc was PCR amplified with forward primer tdc-f: 5′- GGGGCTAGCATGGAAAAAAGTAATCGCTCACTT-3′ and reverse primer tdc-r: 5′-CCCCTCGAGTTAAACATTTTCCTTTTGATTAACCG-3′ and inserted into expression vector pET28a between Nhe I and Xho I restriction endonuclease sites.
Based on the protocols of constructing pETDuet-sda described above, the recombinant plasmid pETDuet-tpl and pET28a-tdc were separately constructed and transformed into E. coli BL21 for the expression of the corresponding enzyme.
All the media used for inocula preparation were adjusted to pH 7 before autoclaving. For TPL and SDA, a loopful of strain was inoculated in a 100-mL Erlenmeyer flask containing 30 mL of LB medium in which ampicillin (50 mg/L) was added in advance. For TDC, kanamycin (50 mg/L) was added into LB medium. These flasks were incubated on a rotary shaker at 220 rpm and 30 °C for 12 h.
The obtained bacterial strains in LB medium were inoculated in accordance with 3 % (v/v) and fermented in the sterilized fermentation media in which the ingredients included as follows: 0.5 % (m/v) peptone, 0.05 % (m/v) magnesium sulfate, 2 % (m/v) corn plasm, 0.5 % (m/v) lactose, 0.05 % (m/v) monopotassium phosphate, and 0.5 % (m/v) gourmet powder. These flasks were incubated in a shaker incubator for 15 h at 30 °C and 220 rpm. Fermented broth was collected at the end of fermentation process and centrifuged at 2800×g and 4 °C for 10 min to obtain wet cells. These cells were lyophilized for a minimum of 2 days (Martin Christ GmbH, Germany) and stored at −20 °C.
Optimization of SDA + TPL cascade reaction
For SDA + TPL cascade reaction, three modes were considered, including sequential cascade mode, simultaneous cascade mode, and no SDA mode. The reactions were operated as shown in Table 2. Phenol was added at intervals of 1 h, and total phenol reached 190 mM (3 × 50 mM + 40 mM). The reactions were incubated at 220 rpm, pH 8, and 37 °C for 4 h after phenol was completely added.
Optimization of TDC reaction
The concentration of l-tyrosine was optimized, and enzymatic activities were measured at 2, 4, 6, and 8 h. TDC cells (50 mg), l-tyrosine, 20 μL PLP (1 %, m/v), and 20 μL Triton X-100 (10 %, v/v) were added to 10 mL sodium acetate buffer (0.2 M, pH 5.5); the mixtures were shaken at 220 rpm and 40 °C for 8 h. Different amounts of l-tyrosine were mixed with 0.3, 0.5, 0.7, 0.9, 1.1, and 1.3 g, respectively.
Scaling up the fermentation, bioconversion, and isolation of l-tyrosine and tyramine hydrochloride
The fermentation of the enzymes was firstly validated in shake flasks with varying reaction volumes (0.25–1.0 L). Subsequently, fermentation was carried out in a stirring tank of a 10-L fermentor (East Biotech, Zhengjiang, China) which was filled with 7-L media containing 1 % (m/v) peptone, 0.05 % (m/v) magnesium sulfate, 3 % (m/v) corn plasm, 1 % (m/v) lactose, 0.05 % (m/v) monopotassium phosphate, 0.5 % (m/v) gourmet powder, and 0.1 % (v/v) polypropylene glycol (PPG, Sigma) solution as an anti-foam agent. The media temperature was maintained at 30 °C; fermentation pH was controlled automatically at 7 ± 0.1. At the end of fermentation process (15 h), fermentation broth was centrifuged at 5590×g and 4 °C for 10 min to obtain wet cells. The wet cells were lyophilized and then mixed with substrates under the optimum reaction conditions for bioconversion in a 20-L reactor (ZF-20, Wuxi, China).
The bioconversion of tyrosine was operated as follows: SDA cells (75 g) were mixed with 2 % (m/v) l-serine KHW under the optimum reaction conditions of pH 8, 37 °C, 30 mL PLP (1 %, m/v), and 30 mL Triton X-100 (10 %, v/v). The total reaction volume reached 15 L. Phenol (50 mM), TPL (75 g), and ammonia chloride (200 mM) were added after the reaction was carried out for 1 h. The remaining phenol (50, 50, and 40 mM) was added separately at intervals of 1 h. The reaction was stirred at 220 rpm for 12 h after all of the phenol was added. l-Tyrosine precipitated along with the l-tyrosine bioconversion proceeded. The reaction mixture was centrifuged, and the collected tyrosine was resuspended into water. The pH of the solution was adjusted to 11 using NaOH (10 %, m/v) to dissolve tyrosine. The solution was then heated to 80 °C, and 200 g activated charcoal was added for decoloration. After stirring at 80 °C for 30 min, the charcoal and the other impurities were removed by filtration. The filtrate was cooled and pH was adjusted to 6 with 6 M HCl. l-Tyrosine precipitated as white crystals and then was collected and dried in a vacuum.
In order to synthesize tyramine, tyrosine (1350 g) and TDC cells (75 g) were added to sodium acetate buffer (0.2 M, pH 5.5) and mixed with 30 mL PLP (1 %, m/v) and 30 mL Triton X-100 (10 %, v/v) at 40 °C for 15-L gross volume. Tyramine was monitored by TLC in a mobile phase composed of n-butyl alcohol/acetic acid/H2O/0.5 % (m/v) ninhydrin n-butyl alcohol solution (12:3:6:28, v/v). The R f value of tyramine is equal to 0.6. After 12 h, the conversion fluid cooled down and its pH was adjusted to 1 with 6 M HCl. The reaction solution was centrifuged at 5590×g, and the supernatant was concentrated to 5 L, and then, crystal was deposited, the crystal was filtrated and the filtrate was combined with the next centrifuged supernatant for further purification steps. Tyramine hydrochloride was obtained as white crystals.
Analytical methods
A Sykam S433D amino acid auto-analyzer (Munich, Germany) equipped with the cation separation column LCAK/06Na (4.6 × 150 mm) was employed for determination of serine in KHW. The samples were detected at a flow rate of 0.45 mL/min with the gradient elution of mobile phrase buffer A (pH 3.45, 11.8 g/L trisodium citrate, 6.0 g/L citric acid, 65 ml/L methanol, 5.6 ml/L HCl, and 0.1 ml/L octanoic acid) and buffer B (pH 10.85, 19.6 g/L trisodium citrate, 3.1 g/L NaOH, 5.0 g/L boric acid, and 0.1 ml/L octanoic acid). The column temperature was programmed to start at 58 °C for 20 min, heat to 74 °C at 3 °C/min, hold for 20 min at this temperature, and cool down to 58 °C again at 4 °C/min, then hold for another 8 min. The temperature of ninhydrin coloration was set at 130 °C. Serine was analyzed at 570 nm.
Pyruvate, tyrosine, and tyramine were all detected by HPLC. Pyruvate was monitored by HPLC at 215 nm using Shimadzu LC-3A chromatograph coupled with Shimadzu SPD-10A UV detector. The column ODS-2 HYPERSIL C18 (250 mm × 4.6 mm, 5-μm particle size; Thermo Fisher Scientific, USA) was operated with a chromatographic grade mobile phase of 5 % v/v acetonitrile and an analytical grade of 95 % v/v 0.05 M ammonium dihydrogen phosphate at a flow rate of 1 mL/min at 25 °C. The pH of mobile phrase was adjusted 2.6 with phosphoric acid. Approximate retention time was 3.4 min for pyruvate.
Tyramine and tyrosine formation was monitored by HPLC at 270 nm using the column Agilent ZORBAX SB-C18 (2.1 mm × 200 mm, 8-μm particle size; Agilent Technologies Inc., USA). The column was eluted with a chromatographic grade mobile phase of 5 % v/v acetonitrile and an analytical grade of 95 % v/v formic acid-ammonium formate buffer (50 mM, pH 5.8) at a flow rate of 0.5 mL/min at 25 °C. The approximate retention times were 2.2 min for tyrosine and 4.2 min for tyramine. All these samples were filtered with Millipore filtering membrane (0.45-μm pore size) before detection.
Results
Optimization of SDA + TPL cascades
As Fig. 1 shows, TPL and its substrates were added to the reaction mixture after 1, 2, and 3 h sequentially and resulted in the conversion rate of serine in KHW to tyrosine to be 92, 79, and 72 %. In sequential mode, the time point to add TPL, phenol, and ammonia chloride was investigated since pyruvate, as a metabolic intermediate of whole cells, could not stably exist in the reaction system (Lütke-Eversloh et al. 2007). The cascade reaction was initiated by the addition of TPL, phenol, and ammonia chloride after the mixture incubated for 1, 2, and 3 h, respectively. As shown in Fig. 1a, the conversion rate was the highest when TPL and its substrates were added after 1 h. When the substrates were added later (e.g., 2 and 3 h), tyrosine was produced less due to the loss of pyruvate. It was thus determined that 1 h was the best time to add in phenol, when approximately 40 % of pyruvate formed (Fig. 1).
Figure 2 shows the results of simultaneous mode and no SDA mode; neither of them achieved satisfactory enzymatic activities. In comparison with sequential mode, tyrosine was generated earlier, while the total conversion ratio of serine decreased in simultaneous mode. When phenol was concurrently added into KHW, phenol could not react completely since the concentration of pyruvate was very low in the beginning; the surplus phenol may result from the partial inhibition of SDA to make l-serine conversion rate decrease by 29 % (Fig. 2a).
When SDA cells were not added into the reaction system, tyrosine was produced so slowly that total bioconversion of serine to tyrosine was only 18 % after 7 h, and 76 % serine still remains in reaction solution (Fig. 2b). These experiments have indicated that sequential cascade was the most efficient reaction mode compared with simultaneous mode and no SDA mode.
Investigation of the influential factors for tyrosine production
Enzymatic activities can be affected by environmental factors, such as pH, surfactants, and temperature. The influential factors were investigated including ratio of SDA and TPL, pH, temperature, substrate concentration, and surfactants. Starting from the optimum 1-h incubation condition in sequential mode, the enzymatic activities were tested with varying each bioconversion condition one at a time. All assays were performed in triplicates.
When the substrate concentration was considered, 2 % serine (m/v) from KHW was utilized as the final concentration due to the low industrial cost. Lauchnor and Semprini (2013) have recently shown that phenol as substrate will result in the inhibition to enzyme to some extent. For TPL, the problem of inhibition has been successfully solved through adding phenol by fed batch in our lab (Feng et al. 2014). In this study, the inhibition to SDA and TPL was not observed and the conversion rate of serine to tyrosine could reach 92 % after adding phenol for four batches in sequential mode. With pyruvate being consumed during the reaction, the excessive phenol could not be used up and could even bring some trouble of posttreatment. Therefore, the molar ratio of l-serine and phenol substrate (1:1) was fixed.
As shown in Fig. 3a, 200 mM ammonium chloride is the most suitable concentration to achieve the highest conversion rate (92 %). When the concentration of ammonium chloride was higher than 200 mM, the conversion rate could not increase more. It is noteworthy that even though ammonia chloride was not added, SDA + TPL still could catalyze approximately 71 % serine to tyrosine (Fig. 3a) after reacting for 8 h. In fact, ammonium chloride is one of the essential ingredients of KHW in which the original concentration of ammonium chloride is approximately 400 mM. On the other hand, as described in Scheme 1, SDA produces pyruvate and ammonia simultaneously; that is, ammonia is not only the original material of TPL but also the product of SDA. The high concentration ammonium chloride will push TPL catalysis forward but hold SDA reaction back, so the ammonium chloride concentration needs to be optimized for maximum enzymatic activity.
Effects of ammonium chloride concentration (a), ratio of SDA/TPL (b), pH (c), temperature (d), and surfactants (e) on SDA + TPL activities. Different concentrations of ammonium chloride were investigated from 0 to 600 mM. TPL cells (50 mg) were fixed, and different quantities of SDA cells were added (20~80 mg) to inspect the influence of the ratio of SDA/TPL. Concentrations of Triton X-100, Tween-80, CTAB, and OP ranged from 0 to 0.05 % (v/v), respectively. The investigation of pH and temperature was carried out within 6~11 and 25~50 °C, respectively. The optimal conditions were 200 mM ammonium chloride, 1:1 ratio of SDA/TPL, pH 8, 37 °C, and 0.04 % Triton X-100, respectively
As indicated in Fig. 3b, the optimum ratio of SDA and TPL catalyst was found to be 1:1 and 1:1.2, and the relative activity showed an approximately 9 % decrease if SDA was added 1.6 times of the amount of TPL. In order to completely convert the 2 % l-serine, the ratio of SDA and TPL was optimized. When SDA catalyst is overdosed, pyruvate will be produced so fast that it produced tyrosine correspondingly faster (e.g., ratio >1:1.2 at 3 and 4 h), yet pyruvate could not be completely consumed in time by TPL, which could result in the loss of product due to the unstability of the pyruvate in the reaction system (e.g., ratio >1:1.2 at 6 and 8 h). However, when the released pyruvate is not enough, the added phenol could not be depleted in time, which would cause phenol to be accumulated and subsequently SDA to be inhibited (e.g., ratio <1:1). In order to maximize the use of the pyruvate, the SDA activity should be controlled by adjusting the catalyst load.
The union activities of SDA and TPL for production of tyrosine were maximal at pH 8 and 37 °C (Fig. 3c, d). High pH and temperature were not suitable for enzymatic catalysis, even causing the conversion rate of serine to tyrosine to approximate zero (e.g., pH 11 or 50 °C), which maybe resulted from the change of enzyme conformation and then deactivated enzymes at the conditions.
As depicted in Fig. 3e, the catalysts exhibited the highest activities with 0.04 % (v/v) Triton X-100 as the surfactant. In comparison with 0.04 % (v/v) Triton X-100, the cascade SDA and TPL activities were not improved more at the presence of Emulsifier OP-10 (OP), cetyltrimethyl ammonium bromide (CTAB), and Tween-80, respectively. Surfactant is important to the reactions catalyzed by whole cells because of its influence on substrates and products passing cell membrane. Interestingly, the cascade SDA and TPL activities were also significantly affected by different surfactants and concentrations.
Optimization of TDC-catalyzed reaction
It was relatively simple for the optimization of tyrosine decarboxylation. The optimum pH and temperature of l-tyrosine decarboxylation by TDC from L. brevis were 5.5 and 40 °C, respectively, according to the reference (Zhang and Ni 2014). As shown in Fig. 4, the optimum amount was 0.9 g tyrosine for 50 mg TDC cells in 10-mL reaction system with a 98 % conversion rate. Even though pH and temperature were all kept optimal, more tyramine would cause inhibition of TDC to cut down enzyme activity. When 1.3 g tyrosine was added, the conversion ratio was decreased to 84 % after 8 h.
Scaling up the fermentation, bioconversion, and identification of tyramine hydrochloride
The lyophilized whole cells of SDA, TPL, and TDC were collected after fermentation with the weight 47.6 ± 1.8, 43.8 ± 2.5, and 44.5 ± 2.1 g, respectively. The whole cells were utilized as a catalyst under the optimal conditions.
Under the catalysis of SDA and TPL, 460 g l-tyrosine was obtained for a reaction volume of 15 L. The yield of tyrosine was 89 % and its specific rotation was [α] 20 D = −11.8° (c = 5, 1 M HCl) which was detected by spectropolarimeter (WZZ-2B, Shanghai, China).
For tyrosine decarboxylation by TDC, tyramine hydrochloride crystal was dried in a vacuum for giving 1216 g in scale-up reaction. The yield of tyramine was 94 %. The identification of tyramine hydrochloride was as follows: MS (EI) (Micromass, Britain) m/z 137.1 (M+-HCl); IR (KBr pellet) (Nexus 870, US): 3092, 1614, 1518, 1494, 1255, 1220, 1141, and 836 cm−1; and 1HNMR (D2O) (Bruker DRX400, Germany) δ 7.12 (d, 2H), 6.81 (d, 2H), 3.15 (t, 2H), and 2.83 (t, 2H).
It is significant to maintain pH at 5.5 during the production of tyramine. Though l-tyrosine has a very poor solubility at pH 5.5, the decarboxylation reaction could be promoted because tyramine can dissolve in pH 5.5 aqueous system. However, tyramine is a weak base which will enhance the pH of reaction solution to go beyond buffer range and result in pH changing and deactivation of enzymes when tyramine was prepared in a large scale. Therefore, in order to study the reasonable industrial route, the buffer was utilized initially and 1 M HCl was continuously added to maintain the appropriate pH during the biotransformation. Additionally, due to mingling some impurities detected by HPLC, tyramine always was obtained as grey powder via direct precipitation at pH 11 from the conversion fluid by concentrating. Tyramine hydrochloride instead of tyramine was obtained owing to its superior stability and purity.
Discussion
Tyramine is mainly prepared through chemical synthesis and isolation from ergotine and rotted animals’ bodies, whereas it is desirable to obtain it by synthesis rather than by isolation. Chemical preparations of tyramine were widely published (Buck 1933; Wang and Xie 1994; Krysin et al. 2001; Li et al. 2009; Shimizu et al. 2012). For example, tyramine could be synthesized through the reduction of hydroxy mandelonitriles by hydrogen with platinum oxide as the catalyst (Buck 1933). Tyramine was afforded in a 98 % yield by deprotection of acetyl tyramine, but how to obtain acetyl tyramine is still a problem (Shimizu et al. 2012). Another improved method starting from phenol and acrylonitrile, through multistep reaction and a key Curtius rearrangement step in a 30.2 % total yield, was reported (Li et al. 2009). However, the disadvantages such as poor reduction selectivity, tedious operation, difficult separation, high toxic agents, and low total yield generally appear in tyramine chemical synthesis. Therefore, the method for synthesizing tyramine should be further improved.
In this study, we attempted to obtain a three-step enzymatic synthetic route for efficient production of tyramine and expected that it could be suitable for industrialization. In order to diminish the environment pollution and decrease the production cost, KHW was used as the original material to prepare tyrosine, and SDA was combined with TPL to convert serine in KHW to tyrosine, which was reported for the first time.
Since KHW, the mixture of many kinds of amino acids, was used as the starting material, it is very difficult to separate the product from this wastewater. However, fortunately, tyrosine could precipitate from the reaction solution for its poor solubility. On the other hand, pH condition of TDC was different from SDA + TPL cascade reaction. In order to conveniently produce tyramine, l-tyrosine was filtered firstly and then utilized as the substrate for TDC. The SDA and TPL reactions were consequently combined together to effectively transform serine in KHW to tyrosine, and the decarboxylation of tyrosine was discussed subsequently. When two-enzymatic cascade reactions were concerned, sequential cascade and simultaneous cascade modes always were investigated (Jakoblinnert and Rother 2014). As described in other works (Fuganti et al. 1974; Kumagai and Yamada 1977; Lütke-Eversloh et al. 2007; Sawada et al. 1975), TPL could catalyze α,β-elimination and β-replacement of serine to release pyruvate and synthesize tyrosine, respectively. Therefore, three catalysis modes including sequential cascade mode, simultaneous cascade mode, and no SDA mode were investigated. The experiment results have indicated that sequential cascade was the most efficient reaction mode. Although TPL from Erwinia herbicola (ATCC 21434) could transform dl-serine into l-tyrosine with a conversion ratio 78 % after reacting for 16 h (Enei et al. 1973; Lütke-Eversloh et al. 2007), 76 % serine still remains if SDA was not added to reaction solution in no SDA mode of this study. Considering TPL from different bacterium, the construction of TPL from E. herbicola and research on the difference between two kinds of TPL will be taken into consideration.
According to the optimal conditions, the three-step reaction was scaled up to 15 L to prepare tyrosine and tyramine. By scaling up the bioconversion, molar conversion yield of l-serine in KHW to tyrosine can reach 89 %, recovery rate of tyramine of 94 %, and the total yield of 84 %. The yield and purity of tyramine have been identified to satisfy the demand of production.
Although serine in KHW was completely converted, the treated KHW as the mixture of amino acids still contains other valuable amino acids, e.g., glutamic acid and proline; how to utilize the amino acids by enzyme catalysis is worthy studying. Additionally, the production efficiency needs to be improved. Our experiments have demonstrated that SDA could completely transform 6 % (m/v) purified serine into pyruvate, so it is a good idea to raise the content of l-serine from KHW and then use it as substrate. Unfortunately, KHW will become brown thickened liquid by simple evaporation at high temperature and turn into brown solid when cooled at room temperature, which was not appropriately employed as the substrate of enzyme. Ammonia chloride, the main salt in KHW, and the amino acid complex were confirmed to be the major factors for forming the sticky liquid. Electrodialysis is under way to remove the excessive ions to gain high concentration of serine in KHW (Xu et al. 2015).
Based on the foregoing study of synthesis of tyramine from KHW catalyzed by whole cells, enzymatic bioconversion proved to be an effective and low-cost method. In conclusion, a green and cost-efficient three-step reaction for production of tyramine at a high yield from industrial wastewater was developed and optimized. This study has indicated the feasibility of enzymatic synthesis tyramine from wastewater and provided a promising method for utilizing the wastewater containing l-serine from keratin acid hydrolysis, which has important environmental significance and economic value.
References
Buck JS (1933) Reduction of hydroxymandelonitriles—a new synthesis of tyramine. J Am Chem Soc 55:3388–3390
Buňková L, Buňka F, Dráb V, Krácmar S, Kubáň V (2012) Effects of NaCl, lactose and availability of oxygen on tyramine production by the Enterococcus durans CCDM 53. Eur Food Res Technol 234:973–979
Chandel M, Azmi W (2009) Optimization of process parameters for the production of tyrosine phenol lyase by Citrobacter freundii MTCC 2424. Bioresour Technol 100:1840–1846
Cheng S, Zhang YF, Zeng ZQ, Lin J, Zhang YW, Ni H, Li HH (2015) Screening, separating, and completely recovering polyphenol oxidases and other biochemicals from sweet potato wastewater in starch production. Appl Microbiol Biotechnol 99:1745–1753
Cicchillo RM, Baker MA, Schnitzer EJ, Newman EB, Krebs C, Booker SJ (2004) Escherichia coli L-serine deaminase requires a [4Fe-4S] cluster in catalysis. J Biol Chem 279:32418–32425
Coppens J, Decostere B, Hulle SV, Nopens I, Vlaeminck SE, Gelder LD, Boon N (2014) Kinetic exploration of nitrate-accumulating microalgae for nutrient recovery. Appl Microbiol Biotechnol 98:8377–8387
Enei H, Matsui H, Nakazawa H, Okumura S, Yamada H (1973) Synthesis of L-tyrosine or 3,4-dihydroxyphenyl-L-alanine from DL-serine and phenol or pyrocathechol. Agr Bioi Chem 37:493–499
Feng YY, Liu JZ, Zhang HJ, Liu Q, Jiao QC (2014) Biosynthesis of L-Tyrosine with whole cell coupled aspartate aminotransferase and tyrosine phenol-lyase. Fine Chem 31:1–6
Fuganti C, Ghiringhelli D, Giangrasso D, Grasselli P (1974) Stereochemical course of the enzymic synthesis of L-tyrosine from phenol and L-serine catalysed by tyrosine phenol lyase from Escherichia infermedia. J Chem Soc Chem Commun 726–727. doi:10.1039/C39740000726
Jakoblinnert A, Rother D (2014) A two-step biocatalytic cascade in micro-aqueous medium: using whole cells to obtain high concentrations of a vicinal diol. Green Chem 16:3472–3482
Khwanchuea R, Mulvany MJ, Jansakul C (2008) Cardiovascular effects of tyramine: adrenergic and cholinergic interactions. Eur J Pharmacol 579:308–317
Krysin AP, Egorova TG, Prosenko AE, Kobrin VS (2001) A method of preparation of 4-(2-aminoethyl)phenol (tyramine) from 4-(2-hydroxyethyl)-2,6-di-tert-butylphenol. RU 2003/2218326 C2
Kumagai H, Yamada H (1977) Stereochemistry of the conversion of serine and tyrosine into pyruvate by tyrosine phenol-lyase. J Chem Soc Chem Commun 85–86
Ladero V, Fernández M, Calles-Enríquez M, Sánchez-Llana E, Cañedo E, Martín MC, Alvarez MA (2012) Is the production of the biogenic amines tyramine and putrescine a species-level trait in enterococci? Food Microbiol 30:132–138
Lauchnor EG, Semprini L (2013) Inhibition of phenol on the rates of ammonia oxidation by Nitrosomonas europaea grown under batch, continuous fed, and biofilm conditions. Water Res 47:4692–4700
Lee K, Kang K, Park M, Park S, Back K (2009) Enhanced octopamine synthesis through the ectopic expression of tyrosine decarboxylase in rice plants. Plant Sci 176:46–50
Li Y, Yang F, Xu X, Pan S, Wang L, Xia C (2009) Improved preparation of tyramine by Curtius rearrangement. Chin J Chem 27:433–436
Lopez-Gallego F, Schmidt-Dannert C (2010) Multi-enzymatic synthesis. Curr Opin Chem Biol 14:174–183
Lütke-Eversloh T, Santos CNS, Stephanopoulos G (2007) Perspectives of biotechnological production of L-tyrosine and its applications. Appl Microbiol Biotechnol 77:751–762
Magnus NA, Astleford BA, Brennan J, Stout JR, Tharp-Taylor RW (2009) Syntheses of a selective peroxisome proliferator activated receptor modulator and practical new preparations of 2-(4-alkoxyphenyl) ethylamines. Org Process Res Dev 13:280–284
Milic D, Demidkina TV, Faleev NG, Matkovic-Calogovic D, Antson AA (2008) Insights into the catalytic mechanism of tyrosine phenol-lyase from X-ray structures of quinonoid intermediates. J Biol Chem 283:29206–29214
Panufnik E, Kañska M (2007) Enzymatic synthesis of isotopomers of tyramine labeled with deuterium and tritium. J Label Compd Radiopharm 50:85–89
Ricca E, Brucher B, Schrittwieserc JH (2011) Multi-enzymatic cascade reactions: overview and perspectives. Adv Synth Catal 353:2239–2262
Sawada S, Kumagai H, Yamada H, Hill RK (1975) Stereochemistry of β-replacement reactions catalyzed by tyrosine phenol-lyase. J Am Chem Soc 97:4334–4337
Sheldon RA (2014) Green and sustainable manufacture of chemicals from biomass: state of the art. Green Chem 16:950–963
Shimizu Y, Morimoto H, Zhang M, Ohshima T (2012) Microwave-assisted deacylation of unactivated amides using ammonium-salt-accelerated transamidation. Angew Chem Int Ed 51:8564–8567
Thakur M, Azmi W (2009) Biotransformation of L-tyrosine to tyramine by the growing cells of Lactococcus lactis. Acta Microbiologica Et Immunologica Hungarica 56:101–114
Toy N, Özogul F, Özogul Y, Ozogul F, Ozogul Y (2015) The influence of the cell free solution of lactic acid bacteria on tyramine production by food borne-pathogens in tyrosine decarboxylase broth. Food Chem 173:45–53
Vu KD, Yan S, Tyagi RD, Valéro JR, Surampalli RY (2009) Induced production of chitinase to enhance entomotoxicity of Bacillus thuringiensis employing starch industry wastewater as a substrate. Bioresour Technol 100:5260–5269
Wang Y, Xie D (1994) An improved synthetic method of tyramine. Chin J Med Chem 4:128–129
Wang W, Li L, Zhang X, Jiang L (2013) China amino acid production status and development of important amino acids. Amino Acids & Biotic Resour 35:68–70
Xu LS, Wang ZY, Mao PT, Liu JZ, Zhang HJ, Liu Q, Jiao QC (2013) Enzymatic synthesis of S-phenyl-L-cysteine from keratin hydrolysis industries wastewater with tryptophan synthase. Bioresour Technol 133:635–637
Xu J, Su XF, Bao JW, Chen YQ, Zhang HJ, Tang L, Wang K, Zhang JH, Chen XS, Mao ZG (2015) Cleaner production of citric acid by recycling its extraction wastewater treated with anaerobic digestion and electrodialysis in an integrated citric acid–methane production process. Bioresour Technol 189:186–194
Zhang K, Ni Y (2014) Tyrosine decarboxylase from Lactobacillus brevis: soluble expression and characterization. Protein Expres Purif 94:33–39
Zhang X, El-Hajj ZW, Newman E (2010) Deficiency in L-serine deaminase interferes with one-carbon metabolism and cell wall synthesis in Escherichia coli K-12. J Bacteriol 192:5515–5525
Zhao GH, Liu JZ, Dong K, Zhang F, Zhang HJ, Liu Q, Jiao QC (2011) Enzymatic synthesis of L-tryptophan from hair acid hydrolysis industries wastewater with tryptophan synthase. Bioresour Technol 102:3554–3557
Acknowledgments
We thank Xianbing Wang and GuoAn Xiao (Shine Star Biological Engineering Co. Ltd., Hubei, China) for their good cooperation. This work was supported by the National Natural Science Foundation of China (No. 21302100) and the Open Fund of State Key Laboratory of Pharmaceutical Biotechnology of Nanjing University, People’s Republic of China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical statement
We certify that this manuscript is original and has not been published previously (partly or in full) and will not be submitted elsewhere for publication while being considered by Applied Microbiology and Biotechnology. The study is not split up into several parts to increase the quantity of submissions and submitted to various journals or to one journal over time. No data have been fabricated or manipulated (including images) to support our conclusions. No data, text, or theories by others are presented as if they were our own. Consent to submit has been received explicitly from all co-authors, as well as from the responsible authorities—explicitly—at the institute where the work has been carried out, before the work is submitted. Authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results. We ensure the correct author group, corresponding author, and order of authors at submission. The research did not involve any studies with human participants or animals.
Funding
This study was funded by the National Natural Science Foundation of China (No.21302100).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, H., Wei, Y., Lu, Y. et al. Three-step biocatalytic reaction using whole cells for efficient production of tyramine from keratin acid hydrolysis wastewater. Appl Microbiol Biotechnol 100, 1691–1700 (2016). https://doi.org/10.1007/s00253-015-7054-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7054-7