Abstract
β-poly(L-malic acid) (PMLA) has attracted industrial interest for its potential applications in medicine and other industries. For a sustainable PMLA production, it requires replacing/reducing the CaCO3 usage, since the residual CaCO3 impeded the cells’ utilization, and a large amount of commercially useless gypsum was accumulated. In this study, it was found that more glucose was converted into CO2 using soluble alkalis compared with CaCO3 usage. Moreover, since the high ion strength and respiration effect of soluble alkalis also inhibited PMLA production, they could not effectively replace CaCO3. Furthermore, comparing the fermentations with different neutralizers (soluble alkali vs. CaCO3), it was found that the differential genes are mainly involved in the pathway of starch and sucrose metabolism, pentose and glucuronate interconversions, histidine metabolism, ascorbate and aldarate metabolism, and phagosome. In detail, in the case with CaCO3, 562 genes were downregulated and 262 genes were upregulated, and especially, those genes involved in energy production and conversion were downregulated by 26.7%. Therefore, the irreplaceability of CaCO3 was caused by its effect on the PMLA metabolic pathway rather than its usage as neutralizer. Finally, a combined pH shift control strategy with CaCO3 addition was developed. After the fermentation, 64.8 g/L PMLA and 38.9 g/L biomass were obtained with undetectable CaCO3 and less CO2 emission.
Key points
• The effect of CaCO3 on PMLA metabolic pathway resulted in its irreplaceability.
• A pH shift control strategy with CaCO 3 addition was developed.
• Undetectable CaCO 3 and less CO 2 emission were detected with the new strategy.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
β-poly(L-malic acid) (PMLA), composed of L-malic acid units linked by ester bonds, has attracted industrial interest for its potential application in medicine and other industries (Arif et al. 2018; Israel et al. 2019; West 2017). For its biosynthesis, high concentration of PMLA could be produced by most strains of Aureobasidium pullulans (Cao et al. 2019; Manitchotpisit et al. 2011; Zou et al. 2016; 2019). According to the pH control for PMLA biosynthesis (Table S1), there are two modes: without pH control, or using soluble alkali or insoluble CaCO3 for pH control.
Without pH control, both the PMLA production (6.9 g/L) and its production per cell mass (Yp/x) (0.53 g/g) were lowest (Liu and Steinbüchel 1997). Using soluble alkali for pH control, it was reported that pH 6.0 (Cao et al. 2013) and pH 4.0 (Liu and Steinbüchel 1996) were respectively suitable for A. pullulans ipe-1 and A. pullulans CBS591.75 to produce PMLA, wherein the highest Yp/x = 0.73 was obtained with 10% Na2CO3 as the neutralizer at pH 6.0. When 30 g/L CaCO3 was used for maintaining the culture pH around 6.5, the production of PMLA was strongly stimulated and the biomass slightly increased, and a Yp/x = 2.26 was achieved (Zhang et al. 2011). Similar results were also reported by Cao et al. (Cao et al. 2016). Further enhancing CaCO3 to 65 g/L, Wang et al. (Wang et al. 2015) obtained the highest Yp/x = 17.73. Thus, it seemed that CaCO3 was the best neutralizer for the PMLA production, which contributed to the highest Yp/x and PMLA concentration (152.5 g/L) (Wang et al. 2015). However, when CaCO3 was added, the residual CaCO3 could bond with the cells which impeded the cells’ utilization (Cao et al. 2016), and a large amount of commercially useless gypsum was accumulated in the PMLA fermentation and separation processes. Therefore, it needs to avoid using too much CaCO3 in large-scale production of PMLA.
To replace CaCO3 and reveal the mechanism of CaCO3 addition on PMLA production, Cao et al. (2016) found that pH and Ca2+ were responsible for promoting the PMLA concentration by CaCO3 addition. However, the Yp/x = 0.73 was still too low at pH 6.0 compared with that in Wang et al. (2015). Zou et al. (2014) reported that Na2CO3 as CO2 donor could obviously improve PMLA titer at pH 6.5 when compared with no CO2 supplier NaOH, which also exhibited more advantages than another donor CaCO3 because of its water-soluble characteristic. Later, Cao et al. (2016) indicated that there was no significant difference in PMLA production and cell growth with either NaOH or Na2CO3 as the neutralizer for keeping culture pH at 6.0. The different results between Zou et al. (2014) and Cao et al. (2016) may be caused by the different culture pHs, since pka1 of carbonic acid is 6.38. When pH was below 6.38, it was unfavorable for CO2 dissolution in the broth (Cao et al. 2018), which hindered Na2CO3 as CO2 donor for PMLA production at pH 6.0. Besides pH, the ionic strength may be another factor affecting PMLA production using soluble alkali, which was not studied so far. In fact, it was reported that a high conductivity was toxic to cells during succinic acid biosynthesis when a soluble alkali (i.e., NaOH) was used to replace sparingly soluble MgCO3 for pH control (Cao et al. 2018). Therefore, in order to further enhance PMLA production, it needed to study in depth the effect of pH and ionic strengths. In addition, it requires unraveling the molecular mechanisms of the effects of CaCO3 and soluble alkali on PMLA production, especially the use of genome-wide transcriptome analysis which could reveal significant changes in the expression profile of strain under different conditions (Li et al. 2019; Sun et al. 2019a; b). Thus, the analysis of transcriptome and fermentation characteristics of PMLA production with different neutralizers may give some enlightenments to improve PMLA production.
In the study reported here, in order to further enhance PMLA production and replace/reduce the consumption of CaCO3, the effect of pH control and ionic strengths on PMLA production was first studied. Moreover, to expound the effect of ionic strengths on PMLA biosynthesis, an integrated fermentation system was developed to couple PMLA fermentation with in situ separation using an expanded-bed adsorption (EBA) system. Then, the underlying molecular mechanisms of the effect of CaCO3 on PMLA production were studied. Finally, a favorable pH control strategy would be developed for high PMLA production to reduce the CaCO3 residues after the fermentation.
Materials and methods
Microorganism, media, cultivation conditions, and analytical methods
A. pullulans ipe-1 (CGMCC no. 3337), cultivation conditions, and the medium for PMLA production were the same as described in Cao et al. (2012). PMLA, biomass, and sugar were measured as recorded in Cao et al. (2019). The concentrations of oxygen and carbon dioxide were detected by using a precision gas analytics (HiTec Zang, Germany). The PMLA yield (Yp/s), cell yield (Yx/s), specific PMLA production per unit cell mass (Yp/x), sugar consumption rate (SCR), and PMLA productivity were the same as depicted in Cao et al. (2019). Genome information for A. pullulans ipe-1 was detected by GENEWIZ (Suzhou, China). The differential genes were analyzed using Bioconductor edgeR (V3.4.6). The annotation information was from GO (http://geneontology.org/) and KEGG (http://www.genome.jp/kegg/). Meanwhile, KEGG annotation results were derived from the KAAS (KEGG Automatic Annotation Server).
Expanded-bed adsorption system
The EBA system consisted of the 7.5 L bioreactor and EBA acrylic columns (height 35.5 cm and tubing ID 4.0 cm) connected through a recirculation loop with a peristaltic pump. Figure S1 shows the overall setup of the EBA system with two anion-exchange columns filled with D380 resin (Chemical plant of Nankai University, Tianjin, China) and one cation-exchange column filled with 0001 × 7 resin (Ningbo Zhengguang Resin co., Ltd, China). EBA, packaged with 30-cm-tall resins, was equilibrated with 1 mol/L NaOH solution for anion-exchange columns or HCl solution for cation-exchange column, and then rinsed using sterilized water before adsorption. After the system was connected in series and water was discharged by pressurizing sterile air, the broth was passed from the bioreactor to the EBA system, and back into the bioreactor.
For the in situ adsorption using two anion-exchange resin columns, it ran continuously for 24 h, and these two columns would be replaced by another two fresh columns at 48 h. Meanwhile, the elution and regeneration of EBA columns followed the standard unit operation as described above. The PMLA in the bioreactor was measured as mentioned above. The final PMLA concentration in the coupled process was calculated as below:
where C(PMLA, f) is the final PMLA concentration (g/L), C(PMLA, e) is the final PMLA concentration (g/L) in the bioreactor, C(PMLA, 24 h) (g/L) and C(PMLA, 48 h) (g/L) are the PMLA concentrations eluted from columns used at 24 h and 48 h respectively, V24 h and V48 h are the eluted solution volumes from the columns used at 24 h and 48 h respectively, and V(broth) is the fermentation volume.
For the in situ adsorption using two anion-exchange resin columns and one cation-exchange resin column, the operating period was controlled for 2 h at 40 h. The final PMLA concentration in the coupled process is calculated as below:
where C(PMLA, f) is the final PMLA concentration (g/L), C(PMLA, e) is the final PMLA concentration (g/L) in the bioreactor, C(PMLA, b) and C(PMLA, a) are the PMLA concentrations before the coupled process (g/L) respectively, and C(PMLA, a) is the PMLA concentration after the coupled process (g/L).
Results
Effect of pH control on PMLA production
In order to expound the effect of CaCO3 and soluble alkali (i.e., NaOH) on PMLA production, PMLA biosynthesis by the same strain ipe-1 with 8 M NaOH for pH control at 6.0 and 30 g/L CaCO3 respectively was compared (Fig. 1 a and b). Since PMLA productivity was high from the strain ipe-1, the culture pH could not be maintained at around 6.5 by 30 g/L CaCO3 (Fig. 1a). The culture pH decreased from about 7.0 at 0 h to 6.4 at 24 h, 6.0 at 40 h, and 5.8 at 60 h. After 60 h, the culture pH increased. As a result, 64.5 g/L PMLA with a Yp/x = 1.74 was obtained at the end of fermentation. However, when NaOH was used as the neutralizer (Fig. 1b), the culture pH could be kept at constant 6.0, and only 43.0 g/L PMLA with a Yp/x = 0.75 was obtained, which was decreased by 33.3% and 56.9% respectively compared with those with 30 g/L CaCO3. Thus, it seemed that the CaCO3 could not be simply replaced by NaOH as the neutralizer, and further study should be carried out in depth to reveal the effect mechanism of CaCO3 addition on PMLA production.
Effect of CaCO3 on PMLA production
Effect of pH control by insoluble carbonates on PMLA production
First, the concentration of CaCO3 used for pH control was decreased to 15 g/L (Fig. 1c). After culturing for 40 h, the broth pH went down below 6.0, and the lowest pH 3.62 was obtained at 58 h, then the broth pH began to increase after 58 h. Meanwhile, the PMLA production was similar to that in Fig. 1a, where pH was controlled with 30 g/L CaCO3 before 40 h, while little PMLA was produced after 40 h. As a result, only 38.7 g/L PMLA and 30.2 g/L biomass were obtained, which were decreased by 66.7% and 18.6% respectively compared with those in Fig. 1a. Secondly, the concentration of CaCO3 used for pH control was increased to 65 g/L (Fig. 1d). During this fermentation process, the pH was between 6.3 and 7.0, and the lowest pH 6.3 was obtained at about 55 h, then the broth pH increased after 55 h. As a consequence, the same PMLA concentration was obtained compared with that with 30 g/L CaCO3 in Fig. 1a, and the biomass was increased by 23.7%. It indicated that when CaCO3 above 30 g/L was added, the broth pH should not be strictly controlled for PMLA production, wherein we intended to know whether CaCO3 can be replaced by other insoluble carbonates. Then, 30 g/L of other insoluble carbonates (i.e., MnCO3 and MgCO3) was used for pH control. When MnCO3 with a lower solubility (i.e., 4.877 × 10−5-g/100 water, 20 °C) than CaCO3 (i.e., solubility, 6.17 × 10−4-g/100 water, 20 °C) was used for pH control (Fig. 1e), the broth pH went down below 6.0 at about 12 h and then kept between 4.9 and 5.7 during the fermentation. At the end of fermentation, 35.0 g/L PMLA was obtained, which was decreased by 45.7% compared with those in Fig. 1a, and the biomass was increased by 22.0%. Though a similar concentration of CO32− from MnCO3 was employed, the lower concentration of PMLA was obtained. Then, MgCO3 with a higher solubility (i.e., 3.9 × 10−2-g/100 water, 20 °C) than CaCO3 was used for pH control (Fig. 1f), the broth kept between 9.0 and 9.5 during the fermentation, and only 0.42 g/L PMLA and 6.3 g/L biomass were achieved. It meant that though a similar concentration of CO32− from MgCO3 was used, it was not suitable for PMLA biosynthesis since the high pH value appeared in the broth. Therefore, CaCO3 was more desirable to PMLA production. Moreover, Fig. 1 a and d also show that the culture pH was among 6.0 and 7.0 during the most period of fermentation when CaCO3 was used. Therefore, the effect of pH from 6.0 to 7.0 on PMLA production with NaOH as the neutralizer should be further studied, and NaOH may induce a high PMLA production at a suitable pH value.
Effect of pH control by NaOH on PMLA production
As shown in Table 1, there was a little difference between pH 6.0 and pH 6.25 on PMLA production and cell growth. However, as the pH went up above pH 6.25, the PMLA production, biomass, and SCR decreased. Thus, it meant that higher pH was not favorable for PMLA production from the strain ipe-1. Thus, by further comparing the pH values under 15, 30, and 65 g/L CaCO3 conditions, it could be found that PMLA production before 40 h was not significantly affected by the pH values (i.e., around 7.0 before 16 h, and among 6.11 and 6.5 from 16 to 40 h), while the pH value above 6.0 was necessary for higher PMLA production. Meanwhile, it seemed that there was no exact requirement of the pH value for PMLA biosynthesis under CaCO3 condition. Therefore, according to the results from the cases with NaOH (Table 1) and CaCO3 respectively (Fig. 1a, c, and d), pH shift (i.e., constant pH 7.0 before 16 h freely dropped to pH 6.4 and kept at constant pH 6.4 before 40 h, then freely dropped to pH 6.0 and kept at constant 6.0 until the end of fermentation) with NaOH as the neutralizer would be carried out for further understanding the effect of pH on PMLA production.
Effect of pH shift on PMLA production
When the pH shift strategy with NaOH as the neutralizer was carried out (Fig. 2a), it was found that PMLA production was increased by 26.0% with the same biomass compared with those without a pH shift strategy (Fig. 1b), while the PMLA production was still lower by 16.0% compared with the case with 30 g/L CaCO3. Meanwhile, the PMLA productivity and cell growth rate before 40 h in Fig. 2a were a little lower than those in Fig. 1b due to the higher pH value before 40 h, while the SCR was decreased by 27.8%. After 40 h, the PMLA productivity was increased sharply, and 54.2 g/L PMLA was obtained at the end of fermentation. It seems that the decreased sugar consumption before 40 h contributed to the increase of PMLA concentration, and the pH shift strategy mainly affected the glucose metabolism. When Na2CO3 was used as the neutralizer with the pH shift strategy (Fig. 2b), there was no significant difference in PMLA production and cell growth at the end of culture compared with the case with NaOH. It also showed that CO32− was not the main factor responsible for the positive effects of CaCO3 on PMLA production. When NH3·H2O was used as the neutralizer with the pH shift strategy (Fig. 2c), there was also no significant difference in PMLA production and cell growth at the end of culture compared with the case with NaOH. Furthermore, when KOH was used as the neutralizer with the pH shift strategy (Fig. 2d), we found that PMLA production, biomass, and the SCR were respectively decreased by 54.4%, 41.4%, and 26.6%. Therefore, with the same pH control strategy, different neutralizers showed much distinct results, and NaOH or Na2CO3 was better soluble neutralizer for PMLA production, although they cannot successfully replace CaCO3 for higher PMLA production. Thus, we firstly speculated that CaCO3 as a solid particle introducing other shear force on cells may positively affect PMLA production.
pH shift with the neutralizer of NaOH (a), Na2CO3 (b), NH3·H2O (c), and KOH (d) respectively. pH shift was carried as follow: constant pH 7.0 before 16 h, freely dropped to pH 6.4 and kept at constant pH 6.4 before 40 h, then freely dropped to pH 6.0 and kept at constant 6.0 until the end of fermentation. Data are given as mean ± SD, n = 2
Effect of shear force introduced by CaCO3 on PMLA production
In order to evaluate the effect of other shear force derived from CaCO3 addition on PMLA production, the shear force from sparingly soluble CaSO4 was first used to simulate that from CaCO3, where 30 g/L CaSO4 was added in the culture medium at the initial fermentation using the pH shift control strategy with Na2CO3 as the neutralizer. Figure 3a indicates that CaSO4 showed no significant effect on the PMLA production and cell growth compared with those in Fig. 2b. Thus, it was interesting to find what would happen if larger insoluble particles were added. Then, 30 g/L silica sand with a diameter around 0.75 mm was added under the same conditions as those in Fig. 3a. As a result in Fig. 3b, PMLA production was decreased by 40.0% with a similar cell growth compared with those in Fig. 3a. Therefore, shear force introduced by CaCO3 could not positively affect PMLA production. By further comparing the cases with NaOH and CaCO3 respectively, it can be concluded that it needs to offer two times of Na+ than Ca2+ in order to form charge balance at the same PMLA concentration. Thus, to synthesize 50 g/L PMLA, it required about 0.43 mol/L Na+, and it was speculated that the high ion strength from NaOH as the neutralizer may inhibit PMLA production, which should be evaluated.
Effect of ion strength on PMLA production
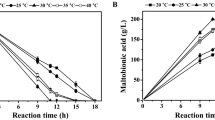
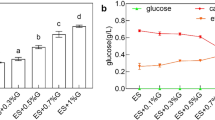
In order to evaluate the effect of ion strength on PMLA production, 33.3 g/L CaCl2 (Fig. 3c) was first added in the culture medium under the same conditions as those in Fig. 2b, where Ca2+ was the same as the case with 30 g/L CaCO3. It was found that the PMLA production was decreased by 34.2% with a similar cell growth compared with those in Fig. 2b. It indicated that the high ion strength from CaCl2 would inhibit PMLA concentration under pH shift condition, and such inhibition may also happen when NaCl was added in the case with CaCO3. Then, 25.0 g/L (i.e., 0.43 mol/L) NaCl was added in the culture medium at the initial fermentation with 30 g/L CaCO3 as the neutralizer (Table 2 and Fig. 4a). It was found that the PMLA production was decreased by 50.4%. Similarly, with the same ion strength, 31.7 g/L CaCl2, 31.9 g/L KCl, and 27.1 g/L MgCl2 were respectively added under the same conditions as the case with NaCl (Table 2), where PMLA production was respectively decreased by 36.7%, 11.6%, and 10.9%, while their effects on cell growth varied differently. Therefore, it can be concluded that the PMLA production was inhibited by a high ion strength, wherein the inhibiting effect was not merely caused by Na+.
In order to further evaluate the effect of ion strength on PMLA production when NaCl was used as the ion source, the concentration of NaCl added was first decreased to 18.75 g/L (i.e., 0.32 mol/L) with 30 g/L CaCO3 as neutralizer (Fig. 4b). Compared with the blank without NaCl addition (Fig. 1a), the PMLA concentration was decreased by 11.9%, while the cell growth was similar. Then, the NaCl concentration was further decreased to 12.5 g/L (i.e., 0.21 mol/L) (Fig. 4c); the PMLA concentration and cell growth were both similar to those in Fig. 1a. It meant that the low concentration of NaCl added showed no significant effect on PMLA production and cell growth. Thus, we speculated that the PMLA production could be improved in an EBA system, where both salt ions and synthesized PMLA could be removed from the broth, thus relieving the inhibiting effects of high ion strength and PMLA. Therefore, we considered that the PMLA production may be enhanced in the EBA system, which was more favorable to simultaneously eliminate the inhibiting effects of high PMLA concentration and ion strength.
In order to evaluate whether PMLA production could be improved by using EBA, an EBA system (Fig. S1), including two weak-base anion-exchange resin columns, was implemented with NaOH as the neutralizer using the pH shift strategy. The EBA system started to run at 24 h, and the two columns would be replaced by another two new columns at 40 h. As shown in Fig. 5a, PMLA concentration was maintained below 20 g/L in the whole fermentation process, and about 10.1 g/L PMLA in the fermentor was obtained. After eluting the fourth column, only 127 g PMLA was recovered. Based on the PMLA weight and the broth volume, only 41.9 g/L PMLA was produced, which was decreased by 22.7% compared with that under the sole pH shift strategy with NaOH as the neutralizer (Fig. 2a), while the biomass reached 64.9 g/L which was increased by 13.1%. It seemed that the cell growth was at the cost of PMLA concentration in this EBA system. Moreover, we also speculated that some ions were not effectively removed from the EBA system. Thus, we supplemented another EBA system including the same two weak-base anion-exchange resin columns and one strong-acid cation-exchange resin, but this EBA system started to run at 40 h and lasted for only 2 h (Fig. 5b). After 2 h, about 12.5 g/L PMLA was removed from the system, and 30.2 g/L PMLA was obtained at the end of fermentation. Thus, 42.7 g/L PMLA was roughly obtained by using this system, and the final biomass was about 62.5 g/L. These results were similar to those in Fig. 5a. It is confusing that the PMLA production could not be enhanced by removing the high ion strength. In addition, the reason why PMLA production could be enhanced by adding CaCO3 used for pH control is also not clear.
PMLA production in expanded-bed adsorption (EBA) systems with NaOH as the neutralizer using the pH shift strategy. a The EBA system included two weak-base anion-exchange resin columns which was run continuously for 24 h, and these two columns would be replaced by another two new columns at 40 h. b Besides the two weak-base anion-exchange resin columns above, the EBA system included another strong-acid cation-exchange resin which was only started at 40 h and lasted for 2 h. Data are given as mean ± SD, n = 2
Unraveling the effect of CaCO3 on PMLA production
In order to further unravel the effect of CaCO3 (used for pH control) on PMLA production, the difference of CTR and OTR in different fermentation strategies (Fig. 2a) was first evaluated. It was found that the oxygen transfer rate (OTR) and carbon dioxide transfer rate (CTR) with pH shift were both much higher than those with 30 g/L CaCO3 addition (Fig. 6). This is unexpected because CaCO3 could be broken down into CO2, and more CO2 appeared in the fermentation tail gas. Thus, from the results of CTR and OTR, we can draw a conclusion that the respiration under pH shift was more intense than that with CaCO3 addition, and more glucose was converted into CO2 which would decrease the PMLA yield. In addition, it also could expound why extra CO2 donor (i.e., Na2CO3) was not needed for the strain ipe-1, since much CO2 was released during PMLA production from the strain ipe-1.
Furthermore, the transcriptome bioinformatics analysis was studied. According to the KEGG Pathway Database, all reactions were involved in 10 metabolic subsystems (Fig. 7). Moreover, the differential genes were further analyzed at 48 h, since PMLA productivity was higher between 24 and 48 h. According to KEGG enrichment analysis, it was found that the differential genes are mainly involved in the pathway of starch and sucrose metabolism, pentose and glucuronate interconversions, histidine metabolism, ascorbate and aldarate metabolism, and phagosome (Table S2). Meanwhile, it was found that 562 genes were upregulated and 262 genes were downregulated in the case with pH shift compared with those with CaCO3, especially for those genes involved in energy production and conversion, they were upregulated by 73.3% (Table S3). In the “Effect of pH shift on PMLA production” section, it was verified that the decreased energy production and conversion by inhibiting glucose metabolism resulted in the increase of PMLA concentration for the pH shift strategy (the higher initial pH was the key). Therefore, the irreplaceability of CaCO3 on PMLA production was not caused by its simple utilization as neutralizer but from its effect on the PMLA metabolic pathway flux.
pH shift control strategy for PMLA production with CaCO3 addition
The above sections confirmed that CaCO3 as a neutralizer was irreplaceable for high PMLA production, and the high concentration of unutilized CaCO3 had a negative impact on the environment and PMLA extraction. However, if the CaCO3 addition is not high enough, it could not effectively maintain pH value, which reduced PMLA production (Fig. 1c). Thus, it was expected to produce PMLA by combining the pH shift control strategy and CaCO3 addition. First, the test under 15 g/L CaCO3 addition in Fig. 1c was carried out with pH shift controlled by NaOH, because there was no residual CaCO3 detected at the end of fermentation. The combined pH control strategy was as follows: uncontrolled pH at the first stage, followed by pH freely dropped to 6.4 and kept at constant 6.4 before 40 h at the second stage, then pH freely dropped to 6.0 and kept at constant 6.0 until the end of fermentation at the last stage. After the fermentation, 64.8 g/L PMLA and 38.9 g/L biomass were obtained (Fig. 8a), which were similar to those with 30 g/L CaCO3 addition. And at the end of fermentation, there was no CaCO3 residue, since the pH was lower than 6.4 from 40 to 66 h (below pka1, 6.38 of carbonic acid) (Cao et al. 2018). Furthermore, the required CaCO3 was decreased to 10 g/L under the combined pH control strategy (Fig. 8b). At the end of fermentation, PMLA concentration and biomass were respectively 62.7 g/L and 37.8 g/L, which were a little lower but were acceptable. Regarding OTR (Fig. 8c), the OTR with 10 g/L CaCO3 addition was lower than that with 15 g/L CaCO3 addition after 40 h. It may be caused by the pH decrease from 6.4 to 6.0 which affected the cell viability and the less CO32− in the broth. Thus, the addition of 15 g/L CaCO3 was more favorable in the combined pH control strategy. These findings in the present study not only offer an effective strategy to improve PMLA production but also provide a good reference for process development and optimization of PMLA production in industrial scale.
The combined pH shift strategy for PMLA production with initial 15 g/L CaCO3 (a) and 10 g/L CaCO3 (b) respectively. The OTR and CTR from a and b were shown in c. Finally, a combined pH shift control strategy with CaCO3 addition was developed as follows: uncontrolled pH with addition of CaCO3 at the first stage, then pH freely dropped to 6.4 and kept at constant 6.4 before 40 h at the second stage, then pH freely dropped to 6.0 and kept at constant 6.0 until the end of fermentation at the last stage. Data are given as mean ± SD, n = 2
Discussion
For PMLA biosynthesis, a large amount of PMLA was produced under around pH 6.5 with CaCO3 as the neutralizer by the strains A. pullulans ZD-3d (Zhang et al. 2011), Aureobasidium sp. P6 (Ma et al. 2013), Aureobasidium melanogenum GXZ-6 (Zeng et al. 2019), and A. pullulans var. pullulans MCW (Wang et al. 2015), wherein the highest Yp/x (17.73) and PMLA concentration (152.5 g/L) were obtained under 65 g/L CaCO3 (Wang et al. 2015). However, when high concentration of CaCO3 was appeared, the residual CaCO3 impeded the cells’ utilization, and a large amount of gypsum was accumulated in the extraction process. Thus, for a sustainable PMLA production, it requires replacing/reducing the CaCO3 usage. Meanwhile, with soluble alkali (i.e., NaOH or Na2CO3) as the neutralizer (Cao et al. 2013 and 2016) by the strain A. pullulans ipe-1 at pH 6.0, a much lower PMLA concentration (about 53.4 g/L) was obtained. Therefore, it needs to study in depth the effect of CaCO3 and soluble alkali on PMLA production, and a sustainable pH control strategy for efficient production of PMLA should be developed.
According to the literatures (Table S1), 30 g/L CaCO3 was first used for pH control using the strain A. pullulans ipe-1. The culture pH could not be maintained at around 6.5 by the 30 g/L CaCO3 (Fig. 1a), which was different from those reported by Zhang et al. (2011), Zou et al. (2014), and Xia et al. (2017a, b). One reason was that the PMLA productivity was much higher from the strain ipe-1, where the dissolution rate of CaCO3 could not meet the need. Another reason may be that some biosynthesized organic acids, such as acetic acid, succinic acid, and lactic acid (Zou et al. 2014), were assimilated by the strain ipe-1. Recently, it was already confirmed that acetic acid with low concentration was assimilated much faster than glucose (Cao et al. 2020), and the biosynthesized malic acid and pyruvic acid could also be assimilated by the strain ipe-1 (Yu et al. 2018). Then, the used CaCO3 concentration was increased to 65 g/L (Fig. 1d), which was the same as that used in Wang et al. (2015). During the fermentation process, the broth pH was much higher than that under 30 g/L CaCO3 (Fig. 1a), while the same PMLA concentration was obtained. Finally, when the used CaCO3 concentration was decreased to 15 g/L (Fig. 1c), a much lower pH value was detected during extensive PMLA biosynthesis, and thus a lower PMLA production was achieved. Meanwhile, it seemed that there was no exact requirement of the pH value for PMLA biosynthesis under CaCO3 above 30 g/L, though the pH value was important to PMLA production (Cao et al. 2013). In PMLA production by A. pullulans ipe-1 with soluble alkali (i.e., NaOH), PMLA production was not associated with cell growth (Table 1), which was proved by our previous study (Cao et al. 2012). In the study, it was found that PMLA production was associated with cell growth in the early exponential growth phase and dissociated from cell growth in the late exponential growth phase. Therefore, high cell growth could not contribute to high concentration of PMLA at the same glucose concentration and cultivation time.
Moreover, other insoluble carbonates used as neutralizing agents were investigated, but in all cases, decreased PMLA production was observed. Except for pH control, the similar concentration of CO32− gave different results, though CO2 donor was needed for enhancing PMLA concentration (Zou et al. 2014). In addition, owing to the presence of CaCO3 in the fermentation medium, a pH shift control strategy was not available. Thus, with soluble alkalis, our previous work indicated that the pH and Ca2+ were two factors for promoting the PMLA concentration by CaCO3 addition (Cao et al. 2016), and pH below 6.0 could inhibit PMLA production (Cao et al. 2013). In this work, to simulate the pH shift from CaCO3 usage, the pH shift strategy with soluble alkalis as the neutralizer was carried out. With the strategy controlled by NaOH or Na2CO3, there was no significant difference in PMLA production and cell growth at the end of culture, which was similar to those reported in Cao et al. (2016). However, the PMLA production under these two cases was still lowered by 16.0% compared with that in Fig. 1a. When NH3·H2O was used as the neutralizer with the pH shift strategy (Fig. 2c), there was also no significant difference in PMLA production and cell growth at the end of culture compared with the case with NaOH, which was much different from those in Cao et al. (2016). The reason may be that the cell growth and glucose metabolism were severely inhibited by the higher pH value before 40 h with NH3·H2O used as the neutralizer under the pH shift strategy. Meanwhile, after 40 h, PMLA productivity was much higher, where the PMLA metabolism was not significantly affected by the added NH4+. Therefore, with the similar pH shift process, the PMLA production was still much lower that from CaCO3, and there may be other unknown reason which affected PMLA production.
For further understanding the effect of CaCO3 on PMLA production, we intended to use soluble alkalis as the neutralizer and study other possible factors from CaCO3 on PMLA production. Firstly, it was found that high shear rates showed no significant effect on cell growth, and similar results of high shear force from stirring speed on cell growth were also reported by Cao et al. (2013) and Gibbs and Seviour (1996). Then, it was found that lower ion strength from CaCO3 than soluble alkalis could improve PMLA production. Meanwhile, it was speculated that PMLA production may be further enhanced by removing high ion strength under soluble alkalis with the bioprocess engineering technology. In fact, it has proved that bioprocess engineering can enhance the PMLA production by intensifying the metabolic pathway for PMLA biosynthesis. For example, it was reported that PMLA production could be increased by integrating in situ membrane filtration for removing the synthesized PMLA with NaOH as the neutralizer (Cao et al. 2012). Meanwhile, PMLA production was also enhanced in repeated batch fermentation with cell recycling after centrifugal separation of fermentation broth using CaCO3 as the neutralizer (Wei et al. 2017). Thus, under these conditions, the PMLA production was all enhanced by removing the biosynthesized PMLA from the fermentation system, which could alleviate feedback inhibition caused by the high concentration of PMLA. However, they could not explain why the PMLA production was increased by decreasing the concentration of PMLA or ionic strengths in the molecular level, since the PMLA and the ions were simultaneously removed. Alternatively, EBA is a coupled process that combines solid–liquid separation and primary product capture with relatively high product-specific selectivity into a single-unit operation (Frej and Hjorth 2018; Li and Chase 2009; Pathapati et al. 2018; Xue et al. 2003). In fact, by integrating EBA, the production of organic acids, such as succinic acid (Li et al. 2011), fumaric acid (Zhang and Yang 2015), and lactic acid (Ataei and Vasheghani-Farahani 2008), was highly enhanced. In addition, ion-exchange resins have been used as solid acid catalysts to provide protons in acid-promoted processes of organic synthesis (Gupta and Paul 2014; Zhu et al. 2018). However, with the constructed EBA systems, PMLA production was decreased with the increasing biomass. It is confusing that the PMLA production could not be enhanced by removing the high ion strength. In addition, the reason why PMLA production could be enhanced by adding CaCO3 used for pH control is also not clear.
To understand the molecular mechanism of CaCO3 on PMLA production, the transcriptome bioinformatics analysis was studied (Fig. 7 and Tables S2 and S3). According to the KEGG Pathway Database, all reactions were involved in 10 metabolic subsystems (Fig. 7), which was similar to those of A. pullulans CCTCC M2012223 (Feng et al. 2018). Under pH shift with soluble alkali (i.e., NaOH), the respiration was more intense than that with CaCO3 addition, and more glucose was converted into CO2 which would decrease the PMLA yield. In addition, it also could expound why extra CO2 donor was not needed for the strain ipe-1, since much CO2 was released during PMLA production from the strain ipe-1, which was different from the fermentation process from A. pullulans CCTCC M2012223 (Zou et al. 2014) and Physarum polycephalum (Lee et al. 1999), where CO2 donor was needed for higher PMLA production. This further interpreted why a high concentration of TFA (even up to 25 mM) would not inhibit PMLA biosynthesis in bioreactor (Yu et al. 2018), since the strain ipe-1 had more intense respiration. In fact, it was found that the pentose phosphate pathway (PPP) played a vital role in cell growth and PMLA biosynthesis, and the activities of PFK (a rate-limiting enzyme in the EMP) and G6PDH (the first step and crucial check point of the PPP) existed in A. pullulans ipe-1 (Yu et al. 2018). In addition, sucrose metabolism was also faster for the strain ipe-1 (Cao et al. 2019), which was similar to that for the strain A. pullulans CGMCC1234 (Sheng et al. 2016). Therefore, the irreplaceability of CaCO3 was caused by its effects on the PMLA metabolic pathway rather than its utilization as neutralizer. Finally, a combined pH shift control strategy with CaCO3 addition was developed. Using such strategy, 64.8 g/L PMLA and 38.9 g/L biomass were obtained without detectable CaCO3 residue after the fermentation, which were similar to those with 65 g/L CaCO3 addition. Moreover, since CaCO3 was undetectable at the end of the fermentation using this strategy, the cells could be used on other industries, and the useless gypsum was not accumulated. Meanwhile, CO2 emission was also reduced. Therefore, these findings in the present study not only offer an effective strategy to improve PMLA production but also provide a good reference for process development and optimization of PMLA production in industrial scale.
References
Arif M, Dong QJ, Raja MA, Zeenat S, Chi Z, Liu CG (2018) Development of novel pH-sensitive thiolated chitosan/PMLA nanoparticles for amoxicillin delivery to treat Helicobacter pylori. Mater Sci Eng C 83:17–24
Ataei SA, Vasheghani-Farahani E (2008) In situ separation of lactic acid from fermentation broth using ion exchange resins. J Ind Microbiol Biotechnol 35:1229–1233
Cao WF, Luo JQ, Zhao J, Qiao CS, Ding LH, Qi BK, Su Y, Wan YH (2012) Intensification of β-poly(L-malic acid) production by Aureobasidium pullulans ipe-1 in the late exponential growth phase. J Ind Microbiol Biotechnol 39:1073–1080
Cao W, Qi B, Zhao J, Qiao C, Su Y, Wan Y (2013) Control strategy of pH, dissolved oxygen concentration and stirring speed for enhancing β-poly(malic acid) production by Aureobasidium pullulans ipe-1. J Chem Technol Biotechnol 88:808–817
Cao W, Chen X, Luo J, Yin J, Qiao C, Wan Y (2016) High molecular weight β-poly (L-malic acid) produced by A. pullulans with Ca2+ added repeated batch culture. Int J Biol Macromol 85:192–199
Cao W, Wang Y, Luo J, Yin J, Xing J, Wan Y (2018) Effectively converting carbon dioxide into succinic acid under mild pressure with Actinobacillus succinogenes by an integrated fermentation and membrane separation process. Bioresour Technol 266:26–33
Cao W, Wang Y, Shen F, Luo J, Yin J, Qiao C, Wan Y (2019) Efficient β-poly(L-malic acid) production from Jerusalem artichoke by Aureobasidium pullulans ipe-1 immobilized in luffa sponge matrices. Bioresour Technol 288:121497
Cao W, Cao W, Shen F, Luo J, Yin J, Qiao C, Wan Y (2020) Membrane-assisted β-poly(L-malic acid) production from bagasse hydrolysates by Aureobasidium pullulans ipe-1. Bioresour Technol 295:122260
Feng J, Yang J, Yang W, Chen J, Jiang M, Zou X (2018) Metabolome- and genome-scale model analyses for engineering of Aureobasidium pullulans to enhance polymalic acid and malic acid production from sugarcane molasses. Biotechnol Biofuels 11:94
Frej KAK, Hjorth RA (2018) Expanded bed adsorption. Biopharmaceutical Processing, (Eds.), Elsevier, 269-277
Gibbs P, Seviour R (1996) Does the agitation rate and/or oxygen saturation influence exopolysaccharide production by Aureobasidium pullulans in batch culture? Appl Microbiol Biotechnol 46:503–510
Gupta P, Paul S (2014) Solid acids: green alternatives for acid catalysis. Catal Today 236:153–170
Israel LL, Braubach O, Galstyan A, Chiechi A, Shatalova ES, Grodzinski Z, Ding H, Black KL, Ljubimova JY, Holler E (2019) A combination of tri-leucine and angiopep-2 drives a polyanionic polymalic acid nanodrug platform across the blood–brain barrier. ACS Nano 13:1253–1271
Lee B, Maurer T, Kalbitzer H, Holler E (1999) β-Poly (L-malate) production by Physarum polycephalum. Appl Microbiol Biotechnol 52:415–420
Li J, Chase HA (2009) Use of expanded bed adsorption to purify flavonoids from Ginkgo biloba L. J Chromatog A 1216:8759–8770
Li Q, Wang D, Hu G, Xing J, Su Z (2011) Integrated bioprocess for high-efficiency production of succinic acid in an expanded-bed adsorption system. Biochem Eng J 56:150–157
Li YC, Xie CY, Yang BX, Tang YQ, Wu B, Sun ZY, Gou M, Xia ZY (2019) Comparative transcriptome analysis of recombinant industrial Saccharomyces cerevisiae strains with different xylose utilization pathways. Appl Biochem Biotechnol 189:1007–1019
Liu S, Steinbüchel A (1996) Investigation of poly (β-L-malic acid) production by strains of Aureobasidium pullulans. Appl Microbiol Biotechnol 46:273–278
Liu S, Steinbüchel A (1997) Production of polymalic acid by Aureobasidium pullulans CBS591175 and DSM2404 in 2L and 20L fermenters. Chin J Biotechnol 13:279–283
Ma Y, Wang GY, Liu GL, Wang ZP, Chi ZM (2013) Overproduction of poly(β-malic acid) (PMA) from glucose by a novel Aureobasidium sp. P6 strain isolated from mangrove system. Appl Microbiol Biotechnol 97:8931–8939
Manitchotpisit P, Skory CD, Peterson SW, Price NPJ, Vermillion KE, Leathers TD (2011) Poly (β-L-malic acid) production by diverse phylogenetic clades of Aureobasidium pullulans. J Ind Microbiol Biotechnol 39:125–132
Pathapati T, Rütze DN, den Boer P, de Wit P, Zaalberg M (2018) Expanded bed adsorption of γ-aminobutyric acid from E. coli broth by CS16GC and IRC747 resins. Chem Eng Technol 41:2427–2434
Sheng L, Tong Q, Ma M (2016) Why sucrose is the most suitable substrate for pullulan fermentation by Aureobasidium pullulans CGMCC1234? Enzym Microb Technol 92:49–55
Sun D, Li H, Song D, Zhang L, Zhao X, Xu X (2019a) Genome, transcriptome and fermentation analyses of Lactobacillus plantarum LY-78 provide new insights into the mechanism of phenyllactate biosynthesis in lactic acid bacteria. Biochem Bioph Res Co 519:351–357
Sun ZG, Wang MQ, Wang YP, Xing S, Hong KQ, Chen YF, Guo XW, Xiao DG (2019b) Identification by comparative transcriptomics of core regulatory genes for higher alcohol production in a top-fermenting yeast at different temperatures in beer fermentation. Appl Microbiol Biotechnol 103:4917–4929
Wang YK, Chi Z, Zhou HX, Liu GL, Chi ZM (2015) Enhanced production of Ca2+-polymalate (PMA) with high molecular mass by Aureobasidium pullulans var. pullulans MCW. Microb Cell Factories 14:115
Wei P, Cheng C, Lin M, Zhou Y, Yang ST (2017) Production of poly(malic acid) from sugarcane juice in fermentation by Aureobasidium pullulans: kinetics and process economics. Bioresour Technol 224:581–589
West TP (2017) Microbial production of malic acid from biofuel-related coproducts and biomass. Fermentation 3:14
Xia J, Liu X, Xu J, Xu J, Wang X, Li X (2017a) Simultaneously enhanced production of poly(β-malic acid) and pullulan using a dissolved oxygen shift (DO-shift) control strategy. J Chem Technol Biotechnol 92:1464–1471
Xia J, Xu J, Liu X, Xu J, Wang X, Li X (2017b) Economic co-production of poly(malic acid) and pullulan from Jerusalem artichoke tuber by Aureobasidium pullulans HA-4D. BMC Biotechnol 17:20
Xue B, Tong X, Sun Y (2003) Polydisperse model for the hydrodynamics of expanded-bed adsorption systems. AICHE J 49:2510–2518
Yu H, Liu B, Luo J, Cao W, Qiao C, Wan Y (2018) Toward understanding the key enzymes involved in β-poly (L-malic acid) biosynthesis by Aureobasidium pullulans ipe-1. Eng Life Sci 18:379–386
Zeng W, Zhang B, Li M, Ding S, Chen G, Liang Z (2019) Development and benefit evaluation of fermentation strategies for poly(malic acid) production from malt syrup by Aureobasidium melanogenum GXZ-6. Bioresour Technol 274:479–487
Zhang K, Yang ST (2015) In situ recovery of fumaric acid by intermittent adsorption with IRA-900 ion exchange resin for enhanced fumaric acid production by Rhizopus oryzae. Biochem Eng J 96:38–45
Zhang H, Cai J, Dong J, Zhang D, Huang L, Xu Z, Cen P (2011) High-level production of poly (β-L-malic acid) with a new isolated Aureobasidium pullulans strain. Appl Microbiol Biotechnol 92:295–303
Zhu H, Sadiq FA, Li Y, Yang S, Zhou F (2018) Application of ion-exchange resin as solid acid for buffer-free production of γ-aminobutyric acid using Enterococcus faecium cells. LWT-Food Sci Technol 98:341–348
Zou X, Tu G, Zan Z (2014) Cofactor and CO2 donor regulation involved in reductive routes for polymalic acid production by Aureobasidium pullulans CCTCC M2012223. Bioprocess Biosyst Eng 37:2131–2136
Zou X, Yang J, Tian X, Guo M, Li Z, Li Y (2016) Production of polymalic acid and malic acid from xylose and corncob hydrolysate by a novel Aureobasidium pullulans YJ 6–11 strain. Process Biochem 51:16–23
Zou X, Cheng C, Feng J, Song X, Lin M, Yang ST (2019) Biosynthesis of polymalic acid in fermentation: advances and prospects for industrial application. Crit Rev Biotechnol 39:408–421
Contributions
WC (Weifeng Cao) conceived and designed research. CQ and YW conducted experiments. FS contributed new reagents or analytical tools. WC analyzed data. WC (Weifeng Cao) and JL wrote the manuscript. All authors read and approved the manuscript.
Funding
The authors thank the Beijing Natural Science Foundation, China (No. 5182025); the National Natural Science Foundation of China, China (No. 21406240); and the National High Technology Research and Development Program of China (Nos. 2015AA021002 and 2014AA021005) for the financial supports.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 126 kb)
Rights and permissions
About this article
Cite this article
Cao, W., Cao, W., Shen, F. et al. A sustainable pH shift control strategy for efficient production of β-poly(L-malic acid) with CaCO3 addition by Aureobasidium pullulans ipe-1. Appl Microbiol Biotechnol 104, 8691–8703 (2020). https://doi.org/10.1007/s00253-020-10815-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10815-5