Abstract
Flavonoids possess diverse bioactivity and potential medicinal values. Glycosylation of flavonoids, coupling flavonoid aglycones and glycosyl groups in conjugated form, can change the biological activity of flavonoids, increase water solubility, reduce toxic and side effects, and improve specific targeting. Therefore, it is desirable to synthesize various flavonoid glycosides for further investigation on their medicinal values. Compared with chemical glycosylations, biotransformations catalyzed by uridine diphospho-glycosyltransferases provide an environmentally friendly way to construct glycosidic bonds without repetitive chemical synthetic steps of protection, activation, coupling, and deprotection. In this review, we will summarize the existing knowledge on the biotechnological glycosylation reactions either in vitro or in vivo for the synthesis of flavonoid O- and C-glycosides and other rare analogs.
Key points
• Flavonoid glycosides usually show improved properties compared with their flavonoid aglycones.
• Chemical glycosylation requires repetitive synthetic steps and purifications.
• Biotechnological glycosylation reactions either in vitro or in vivo were discussed.
• Provides representative synthetic examples in detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
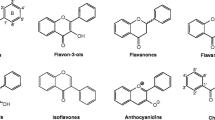
Flavonoids are a large number of small molecules with similar structure, 2-phenly ketone moiety, that can be found in the plants’ stems, flowers, leaves, or the fruits and exist as their glycosides like galactoside, rhamnoside, arabinoside, or rutinoside (Fang et al. 2013; Pugliese et al. 2013; Taheri et al. 2013; Leonard et al. 2008). Flavonoids possess diverse bioactivities and potential medicinal values, such as antibacterial (Cushnie and Lamb 2011), antimicrobial (Cushnie and Lamb 2005), anticancer (Liu et al. 2010), antiinflammation (González et al. 2011), antioxidant (Burda and Oleszek 2001), antidiabetes (Hii and Howell 1984), and influenza virus neuraminidase inhibition (Liu et al. 2008). Due to the broad medicinal prospect, flavonoids have been attracted considerable attention. To date, there are over 2500 varieties of identified flavonoids (Fig. 1), and the number is still rising sharply.
Flavonoid glycosides, which are conjugated through the linkage between flavonoid aglycones and glycosyl groups, usually show improved properties compared with their flavonoid aglycones, or the properties may be even different (Xiao 2017). Since sugar chain is involved in almost all life processes such as cell differentiation, development, immunity, aging, carcinogenesis, and information transmission, the glycosylation can change the biological activity of flavonoid, increase water solubility, reduce toxic and side effects, and improve specific targeting (Jiang et al. 2008). For instance, rutin (quercetin-3-O-rutinoside) has a higher stability in aqueous solution at 100 °C (Buchner et al. 2006) and a slower degradation rate in phosphate buffer containing Fe2+ and Cu2+ (Makris and Rossiter 2000) compared with quercetin.
Flavonoids share structures containing a benzene ring A linked with a pyrone ring C and a phenyl ring B in the 2 or 3 position, and glycosylations mostly occur in the positions of C-3, C-5, C-7, C-3′, C-4′, and C-5′ (Xiao 2017) (Fig. 1). Based on the linkage types, flavonoid glycosides are typically divided into two groups: O- and C-glycosides. However, their low content in nature as well as challenging purification process largely limit further investigation on their medicinal values (Qiu et al. 2013; Obmann et al. 2012). Thus, the synthesis, either chemical or biotransformation approach, offers potential alternative to obtain these various flavonoid glycosides, in which glycosylation reaction is the last but most important step and has been of major interest for biochemist to investigate.
However, glycosylations relying on purely synthetic chemical methods, based on repetitive steps of protection, activation, coupling, and deprotection, while highly desirable, are challenging (Nicolaou and Mitchell 2001; Douglas et al. 1998; Grice et al. 1995; Cheung et al. 1997). In addition, the undesirable isomers complicate the desired product isolation and decrease overall yields. Biotransformation, catalyzed by uridine diphospho-glycosyltransferase in the last step in flavonoid biosynthesis (Yonekura-Sakakibara and Hanada 2011), is an environmentally friendly approach that holds a great deal of promise for the bioengineering of glycoconjugates in both prokaryotes and eukaryotes. It offers the opportunity to prepare high-value sugar-based chemicals, biochemicals, and pharmaceuticals from simple feedstocks ranging from carbon dioxide, to glycerol and then glucose; thus, it is applicable in flavonoid glycoside production as well (Koffas and Linhardt 2018). Moreover, this method is capable of production of unnatural compounds which expands flavonoid glycoside library. To date, although hundreds of papers about flavonoid glycoside synthesis were published in the past 10 years, very few reviews paid attention to the summarization of the glycosylation approaches therein (Ati et al. 2017; Yang et al. 2018; Tapas et al. 2008). In this review, we will summarize the existing knowledge on the biotechnological glycosylation reactions either in vitro or in vivo for the synthesis of flavonoid O- and C-glycosides and other rare analogs.
Flavonoid O-glycosides
Flavonoid O-glycoside, in which the anomeric carbon of sugar and flavonoid skeleton are linked through a C–O bond (Roriz et al. 2014), is the most abundant type of flavonoid glycosides (Veitch and Grayer 2011). Glucose, rhamnose, galactose, xylose, arabinose, glucuronic acid, and apiose are the mainly sugar moieties, while glucosylation is the most frequent pattern and mostly presents as β-glucopyranose (Yang et al. 2018). The structures, sources, and activities of representative flavonoid O-glycosides are summarized in Table 1.
Enzymatic O-glycosylations in vitro
Enzymatic glycosylation in vitro is a very attractive strategy due to the enzyme’s specificity and the mild reaction condition. During enzymatic O-glycosylation, O-glycosyltransferase is utilized to transfer an activated sugar that containing a nucleoside phosphate or a lipid phosphate leaving group as donor to the flavonoid acceptor to form a glycosidic bond. 3-O-glycosyltransferase, 5-O-glycosyltransferase, and 7-O-glycosyltransferase are the most widely investigated enzymes as most glycosylations occur on 3-, 5-, and 7-OH positions of the flavonoids, and especially on the 3-OH (Xiao 2017). We will summarize the most common approaches for O-glycosylations in vitro and discuss the representative synthetic examples in detail.
Representative example: regioselective O-glycosylations
In traditional chemical synthesis of glycosides, repetitive protection and de-protection steps are unavoidable to achieve the regioselective glycosylations (Wang et al. 2013). In contrast, enzymes are capable of catalyzing the reactions with exquisite regioselectivity and stereoselectivity (α- or β-glycosidic linkages) directly (Chang et al. 2011). Park et al. (Rha et al. 2019) achieved the site-specific glycosylation of hydroxyflavones and hydroxyflavanones by Deinococcus geothermalis amylosucrase (DGAS), which shows unique transglycosylation activity on a wide range of acceptor-dependent flavonoids. They found that the enzyme could regioselectively catalyze glucose donors on the 6-OH and 4′-OH positions of hydroxyflavones and hydroxyflavanones while left 3-OH and 7-OH positions intact (Fig. 2a). This regioselectivity may attribute to the interaction between the substrate and enzyme, as the diphenyl propane backbone of flavonoids fits well into the active pocket, and 6-OH and 4′-OH axial positions are more readily accessible for transglycosylation by DGAS compared with 3-OH and 7-OH equatorial positions.
a Site-specific glycosylation of hydroxyflavones and hydroxyflavanones by amylosucrase from Deinococcus geothermalis. b α-Glucosylation of hesperetin catalyzed by cyclodextrin glucanotransferase. c β-Glycosylations between lactosyl fluoride and flavonoids. The glycosidase mutant showed regioselectivities as well, and position O6 was glycosylated instead when O4′ was absent
Representative example: stereoselective O-glycosylations
Stereoselective glycosylation can greatly increase the overall yields and facilitate the purifications. Hesperetin is a kind of flavanone extracted from fruits and shows potential antioxidant or anticancer activities. Plou et al. (González-Alfonso et al. 2018) successfully achieved stereoselective α-glucosylation of hesperetin (Fig. 2b), by a transglycosylation reaction catalyzed by free enzyme cyclodextrin glucanotransferase from Thermoanaerobacter sp. using soluble starch as glucosyl donor. This is the first report of direct glucosylation of hesperetin employing free enzymes instead of whole cells where the resulting products were glucosides with β-configuration. The synthesized monoglucoside could be of interest in the nutraceutical, cosmetic, and pharmaceutical industries. Davis et al. (Yang et al. 2007) successfully utilized Cel7B-E197S glycosynthase produced from Humicola insolens to transfer lactose from lactosyl fluoride to the 4′-OH position of flavonoid with only anomeric β-configuration (Fig. 2c). Interestedly, this enzyme showed no activity with the monosaccharide donor, α-glucosyl fluoride. The glycosylation stereoselectivity depends on the transition state of enzyme-substrate complex, which determines the reaction proceeds in either inversion or retention way at the anomeric position of the donor sugar (Unligil & Rini 2000).
Representative example: various O-glycosyltransferase exploration
Since enzymatic O-glycosylation provides a promising approach to construct O-glycosidic bond, significant efforts regarding O-glycosyltransferase exploration have been made in recent years. Ye et al. (Chen et al. 2019) have characterized 11 new O-glycosyltransferases (GuGTs) from Glycyrrhiza uralensis, including isoflavone 7-O-GTs, flavonol 3-O-GTs, and promiscuous O-GTs. Subsequently, they used 8 potential native substrates and 92 compounds of different structural types from a popular medicinal plant licorice to assess the functions of these enzymes (Fig. 3). These enzymes can be utilized to catalyze not only flavones, chalcones, and triterpenoids, but also main licorice compounds such as liquiritin, isoliquiritin, ononin, and 3-O-β-D-glucuronosyl glycyrrhetinic acid efficiently.
Other examples for enzymatic O-glycosylations in vitro
Seo et al. (Jang et al. 2018) successfully synthesized a series of α-flavone glucosides catalyzed by amylosucrase from Deinococcus geothermalis (DGAS) using sucrose as sugar donor. Ahn et al. (Ahn et al. 2009) expressed a glycosyltransferase BcGT-3 in Escherichia coli, which dominantly glycosylated with the 3-hydroxyl group of flavonols, or coupled with the 7-hydroxyl group if 3-hydroxyl group was not available. Pandey et al. (Pandey et al. 2013) discovered a glycosyltranferase YjiC from Bacillus licheniformis DSM-13 that can catalyze the O-glycosylation of five different flavonols, fisetin, quercetin, myricrtin, kaempferol, and 3-hydroxyflavone. Suzuki et al. (Suzuki et al. 2005) isolated and purified the flavonoid 3-O-glycosyltranferase from buckwheat cotyledons, which plays an important role in the quercetin-3-O-glycosylation to synthesize rutin.
Metabolic engineering O-glycosylations in vivo
Metabolic engineering of microorganisms enables productions of flavonoid glycosides through construction and optimization of different metabolic pathways via overexpressing specific gene pathways while suppressing competing pathways, in order to increase the desired product yield (Wang et al. 2011). The overall conversion efficiency of the biosynthetic pathway depends on various factors including precursors, cofactor demand, and optimal expression of the pathway enzymes.
Representative example: microorganisms as biocatalysts in O-glycosylations in vivo
Quercetin is a flavonoid possessing various bioactivities such as inhibition of cancer cells, antioxidative effect, and antiinflammatory activity. However, its glucoside has low content in nature and limit further biological studies. An entomopathogenic filamentous fungus Isaria fumosorosea typically used as pesticide was found as biocatalyst to achieve the O-glycosylation efficiently. Dymarska et al. (Dymarska et al. 2018a; Dymarska et al. 2018b) used these species to prepare O-methylglucosides of flavone, 5-hydroxyflavone, 6-hydroxyflavone, 7-hydroxyflavone, and daidzein (Fig. 4a). Furthermore, they utilized I. fumosorosea KCH J2 and I. farinosa from a spider’s carcass as combined catalyst to successfully prepare 4-O-methylglucopyranosides of 3-hydroxyflavone, 3-methoxyflavone, quercetin, and baicalein in one step. This research paves the way to enlarge the flavonoid glycoside library with compounds that have not been found in nature. Similarly, Xia and Eiteman (2017)) achieved quercetin glucoside formation with engineered E. coli in a shake flask culture.
Representative example: deuterated cyanidin 3-O-glucoside production
Cyanidin 3-O-glucoside (C3G) belongs to anthocyanin family that is applied as the colorants of flowers and natural food, such as vegetables and fruits. Although chemically unstable, C3G has received considerable attention due to its potential therapeutic applications, including antioxidant and anticancer properties and neuroprotective effect. Gupta et al. (2018)) reported the biosynthesis of deuterated C3G from recombinant E. coli cultured in the presence of deuterated glycerol and deuterated water (Fig. 4b). The in situ–formed deuterated uridine 5′-diphosphate glucose (UDP-glucose) was further incorporated into the deuterated C3G, catalyzed by the glycosyltransferase in the last stage of the anthocyanin biosynthetic pathway. Such deuterated anthocyanin showed improved stability at normal pH, thus will have longer residence in the human body and can be used for the further biological study. In addition, this technique provided an approach for the production of other deuterated anthocyanins using engineered E. coli (Jones et al. 2017; Zha et al. 2018).
Other examples for metabolic engineering O-glycosylations in vivo
Miyakoshi et al. (Miyakoshi et al. 2010) efficiently prepared O-glucosylated 3-hydroxyflavone and kaemferol by filamentous fungus Cunninghamella echinulata. Dymarska et al. (Dymarska et al. 2017) isolated fungus Isaria fumosorosea KCH J2 from a spider’s carcass which is an effective biocatalyst for glycosylation of 6-methylflavone to produce 6-methylflavone 8-O-β-D-(4″-O-methyl)-glucopyranoside and 6-methylflavone 4′-O-β-D-(4″-O-methyl)-glucopyranoside. Yoon et al. (Yoon et al. 2012) successfully produced a novel quercetin glycoside 3-O-(6-deoxytalose) in BgalU-rfbD-rffA E. coli, by which the production was increased 7-fold with significantly reduced by-products compared with the wild-type strain. Similarly, Ahn et al. (Kim et al. 2012) created two E. coli mutant strains deleted in phosphoglucomutase (pgm) or glucose-1-phosphate uridylyltransferase (galU), and the resulting galU mutant produced up to 3-fold more quercetin 3-O-N-acetylglucosamine than wild type. Sordon et al. (Sordon et al. 2019) found that fungi Beauveria bassiana, Absidia coerulea, and Absidia glauca could regioselectively catalyze O-glycosylation of flavonoids. For instance, the B. bassiana AM 278 could convert flavonoids to 4″-O-methyl-7-O-glucosyl derivatives, while A. coerulea AM 93 and A. glauca AM 177 could catalyze the mono-O-glucosylation reaction of flavonoids.
Flavonoid C-glycosides
Flavonoid C-glycoside is conjugated between aglycon like luteolin or apigenin, and sugar moiety, such as arerhamnoside, arabinoside, and amylaceum through a C-C bond catalyzed by C-glycosyltransferase (Yang et al. 2018). However, there is very limited research about the action pattern comparison between C-glycosyltransferase and O-glycosyltransferase. We speculate that the hydroxyl group from flavonoid is acted as a nucleophile to attack the anomeric position of sugar donor to form C-O bond, while the carbon atom from the aromatic ring of the flavonoid is played as nucleophile in the case of C-C bond formation. The types of glycosidic bonds (C-O or C-C), regioselectivity, and stereoselectivity are related to the inherent property of each glycosyltransferase as well as the transition state of the formed enzyme-substrate complex.
It has been well known that flavonoid C-glycosides typically possess better resistance ability to hydrolysis than O-glycosides due to the more stable C–C bond (Vanegas et al. 2018), thus making these compounds attractive targets in scientific research. Nevertheless, besides their potential therapeutic applications, additional efforts should be put in the future to investigate the function differences of the two kinds of glycosides in the biological process of the plant, which will be beneficial to better understand the whole pathway of flavonoid glycosides. Flavonoid C-glycosides are typically classified into two groups, mono-C-glycosylflavones and bis-C-glycosyflavones, where the glycosylations usually occur at C-6 and C-8 of the A-ring. The bis-C-glycosyl compounds have potential pharmaceutical activity and obvious stability in drug development though naturally occurring bis-C-glycosides are rare. The structures, sources, and activities of representative flavonoid C-glycosides are summarized in Table 2.
Enzymatic C-glycosylations in vitro
C-glycosyltransferases (CGT) can catalyze the glycosylations of flavonoids by formation of C–C bond in which anomeric carbon of the sugar is linked to the carbon of flavonoid aglycone directly (Yang et al. 2018). C-glycosyltransferases are the powerful tools used in C-glycosylation, especially for the bis-C-glycoside production, as chemical synthesis still encounters challenges in handling with two identical or different sugar moieties. However, compared with O-glycosyltransferases, fewer CGT has been characterized so far.
Representative example: 2-hydroxyflavanone as the most common substrate in C-glycosylations
2-Hydroxyflavanone, an intermediate formed by 2-hydroxylase (F2H), is a common substrate that can be glycosylated either directly or indirectly with the use of different types of C-glycosyltransferases. Ferreyra et al. (2013)) characterized a glycosyltranferase UGT708A6 from Oryza sativa, which is a bifunctional glycosyltranferase that can produce both O- and C-glycosidated flavonoids from 2-hydroxyflavanone. Vanegas et al. (2018)) have demonstrated a two-step indirect glycosylation to convert 2-hydroxyflavanone intermediates into the 6C-glucoside flavones (isovitexin and isoorientin) and the 8C-glucoside flavones (vitexin and orientin) with the combinations of F2H and C-glycosyltransferases. Furthermore, they established direct glycosylation of flavones with the use of recently identified GtUF6CGT1 from Gentiana triflora.
Representative example: regioselective C-glycosylations
He et al. (2019)) have found a brand new C-glycosyltransferase TcCGT1 from Trollius chinensis which is the first reported enzyme capable of regioselectively catalyzing the 8-C-glycosylation of flavones, flavonols, and other types of flavonoids (Fig. 5). The crystal structure of this enzyme was elucidated, and the catalytic multifunctional structural mechanism was investigated. Besides, TcCGT1 also can catalyze C-, O-, N-, and S-glycosylation reactions, which presents the possibility of catalyzing the N- and S-glycosylation of flavonoids with the use of TcCGT1.
Representative example: combined enzyme catalysis
Isoorientin and isovitexin are flavone C-glycosides that exhibit a number of bioactivities. Pei et al. (Pei et al. 2020) constructed a recombinant E. coli to produce isoorientin while the whole fermentation process took 116 h and the productivity was unsatisfactory. Thus, they chose in vitro enzymatic glycosylation instead. C-glucosyltransferase (Gt6CGT) was expressed from E. coli BL21 and combined with Glycine max sucrose synthase (GmSUS) to successfully produce isoorientin and isovitexin in an efficient and environmentally safe approach (Fig. 6a). The titer of isoorientin reached 3820 mg/L with a corresponding molar conversion of 94.7%, and isovitexin reached 3772 mg/L with a corresponding molar conversion of 97.1% through the optimizing coupled reaction conditions. This combined catalysis is a promising method that can form C-glycosides efficiently.
Representative example: one-pot C-glycosylations
In some instances, it might be more efficient to conduct multiple reaction sequences into one-pot fashion, in which the product is prepared without the required isolation or purification of intermediates (El Amrani et al. 2004). Nothofagin is a flavonoid C-glycoside separated from red-bush herbal tea and showed good antioxidant activity. Bungaruang et al. (2013)) reported a one-pot coupled glycosyltransferase catalysis to produce nothofagin efficiently. The transformation involves selective 3′-C-β-D-glucosylation of naturally abundant phloretin and applies sucrose as expedient glucosyl donor (Fig. 6b). C-glucosyltransferase from Oryza sativa (rice) was used for phloretin C-glucosylation from uridine 5′-diphosphate (UDP)-glucose, which was supplied continuously in situ through conversion of sucrose and UDP catalyzed by sucrose synthase from Glycine max (soybean).
Other examples for enzymatic C-glycosylations in vitro
Various efforts regarding enzymatic C-glycosylations in vitro were made. Hao et al. (2016)) chemically synthesized over 20 2-hydroxyflavanones as enzyme substrates and found that rice C-glycosyltransferase could produce novel C-glycosylflavones. Sasaki et al. (2015)) found a recombinant protein GtUF6CGT1 from Japanese gentian that could transfer a sugar group to the C6 position of flavone skeleton, which is the first reported C-glucosyltransferase that mediates direct C-glucosylation of the flavone skeleton.
Metabolic engineering C-glycosylations in vivo
Representative example: biosynthesis of flavone C-glucosides in engineered E. coli
The preparation of flavonoid C-glycosides in microbial cells is seldom reported in comparison with flavonoid O-glycosides. Shrestha et al. (2018)) have successfully produced chrysin 6-C-glucoside and luteolin 6-C-glucoside in E. coli with the use of a biotransformation approach (Fig. 7). They developed engineered strains to enhance the conversion rate by nearly 30% (1.5-fold). Subsequently, they significantly improved the conversion rate to 50% in a lab-scale fermentor at 3 L volume, demonstrating the potential of the system to biosynthesize different C-glucosides with the use of engineered E. coli.
Representative example: polyprotein expression technology
In recent years, the 2A-based polyprotein system has been used in a variety of eukaryotic systems for transgene co-expression, and in a huge range of different proteins, many with cotranslational and posttranslational subcellular localization signals, have been co-expressed together (El Amrani et al. 2004). Brazier-Hicks and Edwards (2013)) achieved the conversion from dihydrochalcone and flavanone precursors to their C-glycosides by the partially reconstruction of the biosynthetic pathway in tobacco and yeast using polyprotein expression technology (Fig. 8). The pathway includes a flavanone 2-hydroxylase (F2H) co-expressed with CGT, and the development of polyprotein system promoted this efficient co-expression. Though only very small amount of C-glycoside was produced via metabolic engineering in tobacco, by the treatment of flavanone naringenin, the yield of 2-hydroxynaringenin-C-glucoside was significantly improved in yeast with the use of the F2H-CGT polyprotein construct. This work can achieve the preparation of a series of flavonoid C-glycosides in sufficient amount, thus will facilitate future biological and dietary studies.
Other flavonoid glycosides
Flavonoid N-glycosides
Flavonoid N-glycosides are formed when a sugar donor is attached to an aglycon through a nitrogen atom, thus establish a C-N-C linkage. Unfortunately, the flavonoid N-glycosides are very rare in nature. It was reported that N-glycosides occur on the nucleosides and peptides occasionally (Brito-Arias 2007) and a few N-glycosyltransferases have been characterized and utilized to perform N-linked glycosylation of proteins. For instance, N-glycosyltransferase ApNGT from Actinobacillus pleuropneumoniae is an effective post-translational modification enzyme capable of catalyzing glycosylation with complex proteins (Naegeli et al. 2014), while UGT71E5 is another enzyme extracted from Carthamus tinctorius to generate the rare N-glycoside (Xie et al. 2017).
Flavonoid S-glycosides
Similar to flavonoid N-glycosides, there are very few naturally occurring flavone S-glycosides thus very rare S-glycosylations reported as well. However, S-glycosyltransferases have been utilized in the glycosylations of glycopeptide, particularly in the post-translational modifications of proteins. For example, S-glycosyltransferase SunS expressed in Escherichia coli was reported to catalyze the conjugation of carbohydrates to the cysteine thiol of proteins selectively (Oman et al. 2011). ThuS, another S-glycosyltransferase can catalyze both S-glycosylation of the thiol of cysteine and O-glycosylation of the hydroxyl group of serine in peptide substrates (Wang et al. 2014). To date, though the glycosylation in the synthesis of flavonoid S/N-glycoside has not been reported, we anticipate that metabolic engineering and cell-based technique will one day be developed to solve these problems.
Conclusions
Flavonoid glycosides are a family of important natural products that perform numerous physiological and pharmacological functions. Besides chemical synthesis that has been well developed over decades, biotechnological techniques, especially enzymatic catalysis and metabolic engineering approaches, mimicing the biosynthetic pathway of flavonoid glycosides, represent an alternative strategy to prepare these biological compounds. Direct enzymatic catalysis in vitro might be more concise and faster than metabolic engineering approach in vivo but requires equimolar quantities of expensive uridine diphosphate (UDP)-sugar. In addition, it is still challenging to elucidate the mechanism of regioselectivity and stereoselectivity of enzymatic glycosylations, which blocks its further applications. We expect the regeneration strategy to prepare various sugar donors in situ (Feng et al. 2020), immobilized enzyme technique, as well as more profound structure analysis to reveal the mechanism of enzymatic reactions, will be developed and significantly reduce the costs and facilitate further rational design for more novel flavonoid glycosides in the future.
Metabolic engineering in vivo may be an alternate way to produce glycosides, as cell metabolism can provide a continual supply of UDP-glucose (De Bruyn et al. 2015). However, this approach sometimes suffers from poor product yields due to redox imbalance and excess metabolic burden, and thus requires compartmentalization of the pathway for optimal function. Fortunately, over the past 5 years, there has been a steady improvement in this area, including better enzyme expression, better enzymes resulting from protein engineering, and better engineered microorganisms used as the carriers. For instance, efforts on co-cultivation of more than one engineered microbial strains to distribute metabolic burden between the co-cultivation partners significantly improved the product yields. Many challenges remain in this young field, for example, large-scale preparation is still challenging and very few work could achieve even milligram scale. However, the promises of the biotechnology are great, offering an alternative approach to meet industrial needs.
References
Ahn BC, Kim BG, Jeon YM, Lee EJ, Lim Y, Ahn JH (2009) Formation of flavone di-O-glucosides using a glycosyltransferase from Bacillus cereus. J Microbiol Biotechnol 19(4):387–390. https://doi.org/10.4014/jmb.0802.116
Ati J, Lafite P, Daniellou R (2017) Enzymatic synthesis of glycosides: from natural O-and N-glycosides to rare C-and S-glycosides. Beilstein J Org Chem 13(1):1857–1865. https://doi.org/10.3762/bjoc.13.180
Brazier-Hicks M, Edwards R (2013) Metabolic engineering of the flavone-C-glycoside pathway using polyprotein technology. Metab Eng 16:11–20. https://doi.org/10.1016/j.ymben.2012.11.004
Brito-Arias M (2007) Synthesis and characterization of glycosides, vol 352. Springer
Buchner N, Krumbein A, Rohn S, Kroh LW (2006) Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun Mass Spectrom 20(21):3229–3235. https://doi.org/10.1002/rcm.2720
Bungaruang L, Gutmann A, Nidetzky B (2013) Leloir glycosyltransferases and natural product glycosylation: biocatalytic synthesis of the C-glucoside nothofagin, a major antioxidant of redbush herbal tea. Adv Synth Catal 355(14–15):2757–2763. https://doi.org/10.1002/adsc.201300251
Burda S, Oleszek W (2001) Antioxidant and antiradical activities of flavonoids. J Agr Food Chem 49(6):2774–2779. https://doi.org/10.1021/jf001413m
Carte BK, Carr S, DeBrosse C, Hemling ME, MacKenzie L, Offen P, Berry DE (1991) Aciculatin, a novel flavone-C-glycoside with DNA binding activity from Chrysopogon aciculatis. Tetrahedron 47(10–11):1815–1822. https://doi.org/10.1021/jf001413m
Chang A, Singh S, Phillips GN, Thorson JS (2011) Glycosyltransferase structural biology and its role in the design of catalysts for glycosylation. Curr Opin Biotech 22(6):800–808. https://doi.org/10.1016/j.copbio.2011.04.013
Chen WQ, Song ZJ, Xu HH (2012) A new antifungal and cytotoxic C-methylated flavone glycoside from Picea neoveitchii. Bioorg Med Chem Lett 22(18):5819–5822. https://doi.org/10.1016/j.bmcl.2012.07.089
Chen K, Hu ZM, Song W, Wang ZL, He J-b, Shi XM, Cui QH, Qiao X, Ye M (2019) Diversity of O-glycosyltransferases contributes to the biosynthesis of flavonoid and triterpenoid glycosides in Glycyrrhiza uralensis. ACS Synth Biol 8(8):1858–1866. https://doi.org/10.1021/acssynbio.9b00171
Cheung MK, Douglas NL, Hinzen B, Ley SV, Pannecoucke X (1997) One-pot synthesis of tetra-and pentasaccharides from monomeric building blocks using the principles of orthogonality and reactivity tuning. Synlett 1997(03):257–260. https://doi.org/10.1055/s-1997-765
Conrad J, Förster-Fromme B, Constantin MA, Ondrus V, Mika S, Mert-Balci F, Klaiber I, Pfannstiel J, Möller W, Rösner H (2009) Flavonoid glucuronides and a chromone from the aquatic macrophyte Stratiotes aloides. J Nat Prod 72(5):835–840. https://doi.org/10.1021/np800769g
Cushnie TPT, Lamb AJ (2005) Antimicrobial activity of flavonoids. Int J Antimicrob Ag 26(5):343–356. https://doi.org/10.1016/j.ijantimicag.2005.09.002
Cushnie TPT, Lamb AJ (2011) Recent advances in understanding the antibacterial properties of flavonoids. Int J Antimicrob Ag 38(2):99–107. https://doi.org/10.1016/j.ijantimicag.2011.02.014
De Bruyn F, Maertens J, Beauprez J, Soetaert W, De Mey M (2015) Biotechnological advances in UDP-sugar based glycosylation of small molecules. Biotechnol Adv 33(2):288–302. https://doi.org/10.1016/j.biotechadv.2015.02.005
Douglas N, Ley S, Warriner S (1998) Tuning glycoside reactivity: new tool for efficient oligosaccharide synthesis. J Chem Soc Perkin Trans 1(1):51–66. https://doi.org/10.1039/A705275H
Duarte-Almeida JM, Negri G, Salatino A, De Carvalho JE, Lajolo FM (2007) Antiproliferative and antioxidant activities of a tricin acylated glycoside from sugarcane (Saccharum officinarum) juice. Phytochemistry 68(8):1165–1171. https://doi.org/10.1016/j.phytochem.2007.01.015
Dymarska M, Grzeszczuk J, Urbaniak M, Janeczko T, Pląskowska E, Stępień Ł, Kostrzewa-Susłow E (2017) Glycosylation of 6-methylflavone by the strain Isaria fumosorosea KCH J2. PLoS One 12(10):e0184885. https://doi.org/10.1371/journal.pone.0184885
Dymarska M, Janeczko T, Kostrzewa-Susłow E (2018a) Biotransformations of flavones and an isoflavone (daidzein) in cultures of entomopathogenic filamentous fungi. Molecules 23(6):1356. https://doi.org/10.3390/molecules23061356
Dymarska M, Janeczko T, Kostrzewa-Susłow E (2018b) Glycosylation of 3-hydroxyflavone, 3-methoxyflavone, quercetin and baicalein in fungal cultures of the genus Isaria. Molecules 23(10):2477. https://doi.org/10.3390/molecules23102477
El Amrani A, Barakate A, Askari BM, Li X, Roberts AG, Ryan MD, Halpin C (2004) Coordinate expression and independent subcellular targeting of multiple proteins from a single transgene. Plant Physiol 135(1):16–24. https://doi.org/10.1104/pp.103.032649
El-Desouky SK, Ryu SY, Kim YK (2008) A new cytotoxic acylated apigenin glucoside from Phyllanthus emblica L. Nat Prod Res 22(1):91–95. https://doi.org/10.1080/14786410701590236
Fang JB, Jia W, Gao WY, Yao Z, Teng J, Zhao AH, Duan HQ (2007) Antitumor constituents from Alternanthera philoxeroides. J Asian Nat Prod Res 9(6):511–515. https://doi.org/10.1080/10286020600782231
Fang R, Veitch NC, Kite GC, Porter EA, Simmonds MSJ (2013) Enhanced profiling of flavonol glycosides in the fruits of sea buckthorn (Hippophae rhamnoides). J Agr Food Chem 61(16):3868–3875. https://doi.org/10.1021/jf304604v
Feng Y, Yao M, Wang Y, Ding M, Zha J, Xiao W, Yuan Y (2020) Advances in engineering UDP-sugar supply for recombinant biosynthesis of glycosides in microbes. In press Biotechnol Adv https://doi.org/10.1016/j.biotechadv.2020.107538, 107538
Fernandes DC, Regasini LO, Vellosa JCR, Pauletti PM, Castro-Gamboa I, Bolzani VS, Oliveira OMM, Silva DHS (2008) Myeloperoxidase inhibitory and radical scavenging activities of flavones from Pterogyne nitens. Chem Pharm Bull 56(5):723–726. https://doi.org/10.1248/cpb.56.723
Ferreyra MLF, Rodriguez E, Casas MI, Labadie G, Grotewold E, Casati P (2013) Identification of a bifunctional maize C-and O-glucosyltransferase. J Biol Chem 288(44):31678–31688. https://doi.org/10.1074/jbc.M113.510040
Fu P, Zhao CC, Tang J, Shen YH, Xu X, Zhang WD (2009) New flavonoid glycosides and cyanogenic glycosides from Dracocephalum peregrinum. Chem Pharm Bull 57(2):207–210. https://doi.org/10.1248/cpb.57.207
González R, Ballester I, López-Posadas R, Suárez MD, Zarzuelo A, Martínez-Augustin O, Medina FSD (2011) Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci 51(4):331–362. https://doi.org/10.1080/10408390903584094
González-Alfonso JL, Míguez N, Padilla JD, Leemans L, Poveda A, Jiménez-Barbero J, Ballesteros AO, Sandoval G, Plou FJ (2018) Optimization of regioselective α-glucosylation of hesperetin catalyzed by cyclodextrin glucanotransferase. Molecules 23(11):2885. https://doi.org/10.3390/molecules23112885
Grice P, Ley SV, Pietruszka J, Priepke HWM, Walther EPE (1995) Tuning the reactivity of glycosides: efficient one-pot oligosaccharide synthesis1. Synlett 1995(07):781–784. https://doi.org/10.1055/s-1995-5052
Gupta M, Zha J, Zhang X, Jung GY, Linhardt RJ, Koffas MAG (2018) Production of deuterated cyanidin 3-O-glucoside from recombinant Escherichia coli. ACS Omega 3(9):11643–11648. https://doi.org/10.1021/acsomega.8b01134
Hao B, Caulfield JC, Hamilton ML, Pickett JA, Midega CAO, Khan ZR, Wang J, Hooper AM (2016) Biosynthesis of natural and novel C-glycosylflavones utilising recombinant Oryza sativa C-glycosyltransferase (OsCGT) and Desmodium incanum root proteins. Phytochemistry 125:73–87. https://doi.org/10.1016/j.phytochem.2016.02.013
Hawas UW, Abou El-Kassem LT (2017) Thalassiolin D: a new flavone O-glucoside sulphate from the seagrass Thalassia hemprichii. Nat Prod Res 31(20):2369–2374. https://doi.org/10.1080/14786419.2017.1308367
He JB, Zhao P, Hu ZM, Liu S, Kuang Y, Zhang M, Li B, Yun CH, Qiao X, Ye M (2019) Molecular and structural characterization of a promiscuous C-glycosyltransferase from Trollius chinensis. Angew Chem 131(33):11637–11644. https://doi.org/10.1002/ange.201905505
Hii CST, Howell SL (1984) Effects of epicatechin on rat islets of langerhans. Diabetes 33(3):291–296. https://doi.org/10.2337/diab.33.3.291
Hussain A, Perveen S, Malik A, Afza N, Iqbal L, Tareen RB (2009) Urease inhibitiory flavone glucosides from Marrubium anisodon. Pol J Chem 83(7):1329–1335
Ishida H, Wakimoto T, Kitao Y, Tanaka S, Miyase T, Nukaya H (2009) Quantitation of chafurosides a and B in tea leaves and isolation of prechafurosides a and B from oolong tea leaves. J Agr Food Chem 57(15):6779–6786. https://doi.org/10.1021/jf900032z
Jang SW, Cho CH, Jung YS, Rha C, Nam TG, Kim DO, Lee YG, Baek NI, Park CS, Lee BH (2018) Enzymatic synthesis of α-flavone glucoside via regioselective transglucosylation by amylosucrase from Deinococcus geothermalis. PLoS One 13(11):e0207466. https://doi.org/10.1371/journal.pone.0207466
Jiang JR, Yuan S, Ding JF, Zhu SC, Xu HD, Chen T, Cong XD, Xu WP, Ye H, Dai YJ (2008) Conversion of puerarin into its 7-O-glycoside derivatives by Microbacterium oxydans (CGMCC 1788) to improve its water solubility and pharmacokinetic properties. Appl Microbiol Biot 81(4):647–657. https://doi.org/10.1007/s00253-008-1683-z
Jiang XL, Wang L, Wang EJ, Zhang GL, Chen B, Wang MK, Li F (2018) Flavonoid glycosides and alkaloids from the embryos of Nelumbo nucifera seeds and their antioxidant activity. Fitoterapia 125:184–190. https://doi.org/10.1016/j.fitote.2018.01.009
Jones JA, Vernacchio VR, Collins SM, Shirke AN, Xiu Y, Englaender JA, Cress BF, McCutcheon CC, Linhardt RJ, Gross RA (2017) Complete biosynthesis of anthocyanins using E. coli polycultures. Mbio 8(3):e00621–e00617. https://doi.org/10.1128/mBio.00621-17
Kim BG, Sung SH, Ahn JH (2012) Biological synthesis of quercetin 3-O-N-acetylglucosamine conjugate using engineered Escherichia coli expressing UGT78D2. Appl Microbiol Biotechnol 93(6):2447–2453. https://doi.org/10.1007/s00253-011-3747-8
Koffas MAG, Linhardt RJ (2018) Metabolic bioengineering: glycans and glycoconjugates. Emerg Top Life Sci 2(3):333–335. https://doi.org/10.1042/ETLS20180091
Leonard E, Yan Y, Fowler ZL, Li Z, Lim C, Lim KH, Koffas MAG (2008) Strain improvement of recombinant Escherichia coli for efficient production of plant flavonoids. Mol Pharm 5(2):257–265. https://doi.org/10.1021/mp7001472
Liu AL, Wang HD, Lee SM, Wang YT, Du GH (2008) Structure-activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorgan Med Chem 16(15):7141–7147. https://doi.org/10.1016/j.bmc.2008.06.049
Liu HL, Jiang WB, Xie MX (2010) Flavonoids: recent advances as anticancer drugs. Recent Pat Anti-Canc 5(2):152–164. https://doi.org/10.2174/157489210790936261
Makris DP, Rossiter JT (2000) Heat-induced, metal-catalyzed oxidative degradation of quercetin and rutin (quercetin 3-Orhamnosylglucoside) in aqueous model systems. J Agric Food Chem 48(9):3830–3838. https://doi.org/10.1021/jf0001280
Mendiratta A, Dayal R, Bartley JP (2007) Two new apigenin glycosides from Cephalotaxus harringtonia var. harringtonia. Nat Prod Commun 2(11):1113–1116. https://doi.org/10.1177/1934578X0700201113
Mishra BB, Yadav SB, Singh RK, Tripathi V (2007) A novel flavonoid C-glycoside from Sphaeranthus indicus L. (family compositae). Molecules 12(10):2288–2291. https://doi.org/10.3390/12102288
Miyakoshi S, Azami S, Kuzuyama T (2010) Microbial glucosylation of flavonols by Cunninghamella echinulata. J Biosci Bioeng 110(3):320–321. https://doi.org/10.1016/j.jbiosc.2010.02.015
Montenegro H, Gonzalez J, Ortega-Barria E, Cubilla-Rios L (2007) Antiprotozoal activity of flavonoid glycosides isolated from Clidemia sericea. And Mosquitoxylon jamaicense. Pharm Biol 45(5):376–380. https://doi.org/10.1080/13880200701214821
Naegeli A, Neupert C, Fan YY, Lin CW, Poljak K, Papini AM, Schwarz F, Aebi M (2014) Molecular analysis of an alternative N-glycosylation machinery by functional transfer from Actinobacillus pleuropneumoniae to Escherichia coli. J Biol Chem 289(4):2170–2179. https://doi.org/10.1074/jbc.M113.524462
Naso L, Martínez VR, Lezama L, Salado C, Valcarcel M, Ferrer EG, Williams PAM (2016) Antioxidant, anticancer activities and mechanistic studies of the flavone glycoside diosmin and its oxidovanadium (IV) complex. Interactions with bovine serum albumin. Bioorgan Med Chem 24(18):4108–4119. https://doi.org/10.1016/j.bmc.2016.06.053
Nazemiyeh H, Bahadori F, Delazar A, Ay M, Topcu G, Kolak U, Nahar L, Auzie AA, Sarker SD (2008) Tricetin 4′-O-α-L-rhamnopyranoside: a new flavonoid from the aerial parts of Erica arborea. Chem Nat Compd+ 44(2):174. https://doi.org/10.1007/s10600-008-9007-1
Nicolaou KC, Mitchell HJ (2001) Adventures in carbohydrate chemistry: new synthetic technologies, chemical synthesis, molecular design, and chemical biology. Angew Chem Int Edit 40(9):1576–1624. https://doi.org/10.1002/1521-3773(20010504)40:9<1576::AID-ANIE15760>3.0.CO;2-G
Obmann A, Purevsuren S, Zehl M, Kletter C, Reznicek G, Narantuya S, Glasl S (2012) HPLC determination of flavonoid glycosides in Mongolian Dianthus versicolor Fisch. (Caryophyllaceae) compared with quantification by UV spectrophotometry. Phytochem Analysis 23(3):254–259. https://doi.org/10.1002/pca.1351
Oman TJ, Boettcher JM, Wang H, Okalibe XN, Van Der Donk WA (2011) Sublancin is not a lantibiotic but an S-linked glycopeptide. Nat Chem Biol 7(2):78–80. https://doi.org/10.1038/nchembio.509
Pandey RP, Parajuli P, Koirala N, Park JW, Sohng JK (2013) Probing 3-hydroxyflavone for in vitro glycorandomization of flavonols by YjiC. Appl Environ Microb 79(21):6833–6838. https://doi.org/10.1128/AEM.02057-13
Pei J, Sun Q, Gu N, Zhao L, Fang X, Tang F, Cao F (2020) Production of isoorientin and isovitexin from luteolin and apigenin using coupled catalysis of glycosyltransferase and sucrose synthase. Appl Biochem Biotech 190(2):601–615. https://doi.org/10.1007/s12010-019-03112-z
Pugliese AG, Tomas-Barberan FA, Truchado P, Genovese MI (2013) Flavonoids, proanthocyanidins, vitamin C, and antioxidant activity of Theobroma grandiflorum (Cupuassu) pulp and seeds. J Agr Food Chem 61(11):2720–2728. https://doi.org/10.1021/jf304349u
Qiu L, Jiao Y, Xie JZ, Huang GK, Qiu SL, Miao JH, Yao XS (2013) Five new flavonoid glycosides from Nervilia fordii. J Asian Nat Prod Res 15(6):589–599. https://doi.org/10.1080/10286020.2013.790377
Rao KS, Babu GV, Ramnareddy YV (2007) Acylated flavone glycosides from the roots of Saussurea lappa and their antifungal activity. Molecules 12(3):328–344. https://doi.org/10.3390/12030328
Rha CS, Jung YS, Seo DH, Kim DO, Park CS (2019) Site-specific α-glycosylation of hydroxyflavones and hydroxyflavanones by amylosucrase from Deinococcus geothermalis. Enzyme Microb Tech 129:109361. https://doi.org/10.1016/j.enzmictec.2019.109361
Rigano D, Formisano C, Grassia A, Grassia G, Perrone A, Piacente S, Vuotto ML, Senatore F (2007) Antioxidant flavonoids and isoflavonoids from rhizomes of Iris pseudopumila. Planta Med 73(01):93–96. https://doi.org/10.1055/s-2006-957071
Rivière C, Pieters L, Dejaegher B, Vander Heyden Y, Van MC, Quetin-Leclercq J (2009) Polyphenols isolated from antiradical extracts of Mallotus metcalfianus. Phytochemistry 70(1):86–94. https://doi.org/10.1016/j.phytochem.2008.10.008
Roriz CL, Barros L, Carvalho AM, Santos-Buelga C, Ferreira ICFR (2014) Pterospartum tridentatum, Gomphrena globosa and Cymbopogon citratus: a phytochemical study focused on antioxidant compounds. Food Res Int 62:684–693. https://doi.org/10.1016/j.foodres.2014.04.036
Sasaki N, Nishizaki Y, Yamada E, Tatsuzawa F, Nakatsuka T, Takahashi H, Nishihara M (2015) Identification of the glucosyltransferase that mediates direct flavone C-glucosylation in Gentiana triflora. FEBS Lett 589(1):182–187. https://doi.org/10.1016/j.febslet.2014.11.045
Sathiamoorthy B, Gupta P, Kumar M, Chaturvedi AK, Shukla PK, Maurya R (2007) New antifungal flavonoid glycoside from Vitex negundo. Bioorg Med Chem Lett 17(1):239–242. https://doi.org/10.1016/j.bmcl.2006.09.051
Shrestha A, Pandey RP, Dhakal D, Parajuli P, Sohng JK (2018) Biosynthesis of flavone C-glucosides in engineered Escherichia coli. Appl Microbiol Biot 102(3):1251–1267. https://doi.org/10.1007/s00253-017-8694-6
Sordon S, Popłoński J, Tronina T, Huszcza E (2019) Regioselective O-glycosylation of flavonoids by fungi Beauveria bassiana, Absidia coerulea and Absidia glauca. Bioorg Chem 93:102750. https://doi.org/10.1016/j.bioorg.2019.01.046
Suzuki T, Kim SJ, Yamauchi H, Takigawa S, Honda Y, Mukasa Y (2005) Characterization of a flavonoid 3-O-glucosyltransferase and its activity during cotyledon growth in buckwheat (Fagopyrum esculentum). Plant Sci 169(5):943–948. https://doi.org/10.1016/j.plantsci.2005.06.014
Taheri R, Connolly BA, Brand MH, Bolling BW (2013) Underutilized chokeberry (Aronia melanocarpa, Aronia arbutifolia, Aronia prunifolia) accessions are rich sources of anthocyanins, flavonoids, hydroxycinnamic acids, and proanthocyanidins. J Agr Food Chem 61(36):8581–8588. https://doi.org/10.1021/jf402449q
Tapas AR, Sakarkar DM, Kakde RB (2008) Flavonoids as nutraceuticals: a review. Trop J Pharm Res 7(3):1089–1099. https://doi.org/10.4314/tjpr.v7i3.14693
Unligil UM, Rini JM (2000) Glycosyltransferase structure and mechanism. Curr Opin Struc Biol 10(5):510–517. https://doi.org/10.1016/S0959-440X(00)00124-X
Vanegas KG, Larsen AB, Eichenberger M, Fischer D, Mortensen UH, Naesby M (2018) Indirect and direct routes to C-glycosylated flavones in Saccharomyces cerevisiae. Microb Cell Factories 17(1):107. https://doi.org/10.1186/s12934-018-0952-5
Veitch NC, Grayer RJ (2011) Flavonoids and their glycosides, including anthocyanins. Nat Prod Rep 28(10):1626–1695. https://doi.org/10.1039/C1NP00044F
Wang Y, Chen S, Yu O (2011) Metabolic engineering of flavonoids in plants and microorganisms. Appl Microbiol Biot 91(4):949–956. https://doi.org/10.1007/s00253-011-3449-2
Wang Z, Yang L, Yang X, Zhang X (2013) Advances in the first total synthesis of natural flavonoids. Synthetic Commun 43(23):3093–3114. https://doi.org/10.1080/00397911.2013.820835
Wang H, Oman TJ, Zhang R, Garcia De Gonzalo CV, Zhang Q, van der Donk WA (2014) The glycosyltransferase involved in thurandacin biosynthesis catalyzes both O- and S-glycosylation. J Am Chem Soc 136(1):84–87. https://doi.org/10.1021/ja411159k
Wen L, Zhao Y, Jiang Y, Yu L, Zeng X, Yang J, Tian M, Liu H, Yang B (2017) Identification of a flavonoid C-glycoside as potent antioxidant. Free Radical Bio Med 110:92–101. https://doi.org/10.1016/j.freeradbiomed.2017.05.027
Xia T, Eiteman MA (2017) Quercetin glucoside production by engineered Escherichia coli. Appl Biochem Biotech 182(4):1358–1370. https://doi.org/10.1007/s12010-017-2403-x
Xiao J (2017) Dietary flavonoid aglycones and their glycosides: which show better biological significance? Crit Rev Food Sci 57(9):1874–1905. https://doi.org/10.1080/10408398.2015.1032400
Xie K, Chen R, Chen D, Li J, Wang R, Yang L, Dai J (2017) Enzymatic N-glycosylation of diverse arylamine aglycones by a promiscuous glycosyltransferase from Carthamus tinctorius. Adv Synth Catal 359(4):603–608. https://doi.org/10.1002/adsc.201601128
Yang M, Davies GJ, Davis BG (2007) A glycosynthase catalyst for the synthesis of flavonoid glycosides. Angew Chem Int Edit 46(21):3885–3888. https://doi.org/10.1002/anie.200604177
Yang B, Liu H, Yang J, Gupta VK, Jiang Y (2018) New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci Tech 79:116–124. https://doi.org/10.1016/j.tifs.2018.07.006
Yonekura-Sakakibara K, Hanada K (2011) An evolutionary view of functional diversity in family 1 glycosyltransferases. Plant J 66(1):182–193. https://doi.org/10.1111/j.1365-313X.2011.04493.x
Yoon KD, Jeong DG, Hwang YH, Ryu JM, Kim J (2007) Inhibitors of osteoclast differentiation from Cephalotaxus koreana. J Nat Prod 70(12):2029–2032. https://doi.org/10.1021/np070327e
Yoon JA, Kim BG, Lee WJ, Lim Y, Chong Y, Ahn JH (2012) Production of a novel quercetin glycoside through metabolic engineering of Escherichia coli. Appl Environ Microb 78(12):4256–4262. https://doi.org/10.1128/AEM.00275-12
Zha J, Zang Y, Mattozzi M, Plassmeier J, Gupta M, Wu X, Clarkson S, Koffas MAG (2018) Metabolic engineering of Corynebacterium glutamicum for anthocyanin production. Microb Cell Factories 17(1):1–13. https://doi.org/10.1186/s12934-018-0990-z
Funding
This study was supported by grants from National Key Research and Development Project (no. 2019YFC1605801) and Natural Science Research Project of Jiangsu Higher Education Institutions (no. 19KJB150013 to X.Z. and 19KJB150012 to L.L.).
Author information
Authors and Affiliations
Contributions
All authors wrote the manuscript, read, and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ji, Y., Li, B., Qiao, M. et al. Advances on the in vivo and in vitro glycosylations of flavonoids. Appl Microbiol Biotechnol 104, 6587–6600 (2020). https://doi.org/10.1007/s00253-020-10667-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10667-z