Abstract
Flavonoids are widely distributed secondary metabolites in plants that are known to be present in different vegetables and fruits. The flavonoid groups include various subclasses of compounds such as flavanones, flavones, dihydroflavonols, flavonols, flavan-3-ols, anthocyanidins, isoflavones, proanthocyanidins, and chalcones with a C6-C3-C6 structure. These compounds are considered to be imperative structural motifs owing to their distinctive way of physiological action and biochemical effects in humans as well as in plants. Keeping in view of their immense importance and physiological roles, the summation of the structural features is quintessential for their therapeutic applications. Furthermore, the structure-activity relationship together with their chemical and biochemical synthetic preview could help the scientific community to explore and design novel drugs. Therefore, structural features of flavonoids may prompt a great deal of attention in the field of organic synthesis and biology to study innovative methods of synthesis and therapeutic applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Natural products have been used as food products and for therapeutic benefits for thousands of years. Flavonoids are the class of natural polyphenolic compounds derived as secondary metabolites from plants and fungus, and their direct association has been reportedly found with the human health. They have been known to play multiple roles in plants including UV filtration, detoxifying agents, symbiotic nitrogen fixation, self-healing agents, and floral pigmentation. Besides, they also act as chemical messengers, antimicrobial defensive agents, auxin transport inhibitors, physiological regulators, photoreceptors, and cell cycle inhibitors. They are being used as health-benefited and disease-averting dietary supplements because they possess a wide range of biochemical and pharmacological activities in the containment of various diseases including oxidative damage, chronic diseases, cardiovascular diseases, cancer, neurodegenerative diseases, gastrointestinal disorders, and others. Flavonoids are supposed to interact with receptive sites or receptors of the cells. Molecular structures, physical and chemical properties of the receptor largely determine what moieties are essential for affinity with the receptors (de la Rosa et al. 2010; Andersen and Markham 2006). The goal of this chapter is to highlight the structural features, classification, and their common food sources along with brief chemical and biosynthetic methods of flavonoids. In addition to this, the structure-activity relationship facilitates the relationship between their molecular structure and biological or physicochemical activities. It will be helpful in the improvement of the effect or the potency of flavonoids by altering its chemical structural functionalities and development of the new flavonoid derivatives of therapeutic values.
2 Structural Features and Classification
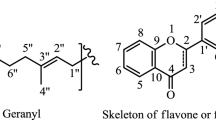
The word “flavonoid” originates from Latin word flavus which means “yellow.” Flavonoids are widely distributed in plant kingdom especially in fruits, flowers, vegetables, and herbs included as pigment color from yellow to red to blue. They are responsible for vivid coloration, taste properties, prevention of fat oxidation, and vitamin as well as enzyme protection in foods and plants. More than 8000 flavonoids of which several hundred are found in edible plants have been reported and characterized (Bone and Mills 2013). Chemically, flavonoids have 2-phenylchromane nucleus (C6-C3-C6) which consists of a heterocyclic pyrane ring (C) fused with the ring (A) and linked to the benzene ring (B). The various groupings of multiple hydroxyls (-OH), methoxyl (-OCH3), and glycoside group substituents along with oxo group at position 4 of ring C are present on the basic skeleton of flavonoids. They can be categorized into a variety of subclasses on the basis of the different oxidation level, unsaturation and substitution pattern of the C ring, as well as the bonding position of ring B to either C2/C3/C4 carbon of ring C. Among the subclasses of flavonoids, each compound differs in the substituent’s position on the rings A and B from others. The flavonoids further classified into other subclasses by the account of the ring B bonding position either at C2/C3/C4 position of ring C as well as the structural features of ring C. In addition of the ring B position at C2 position, flavones have C2–C3 double bond along with oxo group (=O) at C4 position of ring C while flavonols have a hydroxyl group (OH) at C3 position of ring C as well as a double bond between C2 and C3 along with oxo group (=O) at C4 position of ring C. Besides these subclasses, flavanones are also known as dihydroflavones because they have saturated C rings, i.e., lacking C2–C3 double bond. In a similar way, flavanonols have also saturated C rings, i.e., lacking C2–C3 double bond, but the hydroxyl group is present at position C3 of the same ring. Therefore they are also known as dihydroflavonols or 3-hydroxy derivatives of flavanones. In case of flavan-3-ol, the hydroxyl group (-OH) presents at position C3 of ring C with two chiral centers at positions C2 and C3 of ring C and absence of oxo group (=O) at position C4 of the same ring. The presence of chiral centers may result in the possibility of four diastereoisomers. They have the ability to form polymers, resulting in the formation of proanthocyanidins which undergo acid-catalyzed cleavage, and to form the anthocyanidins. When the ring B is attached to the C3 position of the ring C in flavonoids, they are called as isoflavones, whereas the same ring presents at the C4 position of ring C in the case of neoflavonoids. The next subclass of flavonoids is the plant pigments which are commonly called as anthocyanidins responsible for plant color. These are flavylium cations while counterions are mostly chloride. Chalcones and dihydrochalcones also belong to flavonoids because of their synthetic pathways being similar to that of flavonoids despite having an open structure. Aurones is a rarely occurring subclass of flavonoids in nature and contains a benzofuran ring system which is linked at position 2 of benzylidene. They exist in two isomeric forms, i.e., (E) and (Z) configurations. The information about different subclasses of flavonoids and their derivatives along with common sources is summarized in Figs. 3.1 and 3.2 (Bhagwat et al. 2013; Kozłowska and Szostak-Węgierek 2014; Kumar and Pandey 2013; Erdman et al. 2007; de la Rosa et al. 2010; Andersen and Markham 2006). These flavonoids may exist as aglycones which are basic structures of these compounds and their methylated, acetylated, sulfonated congener and glycoside derivatives. All the structural features or configurations of flavonoids such as the number of hydroxyl (OH) functional groups and substitution pattern of functional groups in nucleus edifice determines the bioavailability, metabolism, biochemical, and pharmacological activities (Kumar and Pandey 2013; Erdman et al. 2007; Teles et al. 2018; Correia-da-Silva et al. 2013; Wen et al. 2017; Bone and Mills 2013; Sharma et al. 2018a, b).
3 Structure-Activity Relationship
The chemical structure along with substituent’s nature and their positions affects the apparent potency of the bioactive compound. It facilitates the determination of chemical groups which is accountable for evoking a remarkable biological effect in the organism. The chemical structure and functionalities of flavonoids, that is, the presence and positions of hydroxyl groups and substitution pattern of functional groups and C2–C3 double bond, are responsible to interact with receptive sites or receptors in the tissue that are accountable for their biochemical and pharmacological properties. In general, the structure-activity relationship of flavonoids among various therapeutic applications has been summarized as below:
The favorable health effects of flavonoids have been reported due to various proposed mechanisms such as antioxidant effects, enzyme inhibition, gene regulation, and metal chelation (Erlejman et al. 2004). The free radicals cause various injuries that can be prevented by flavonoids through the following mechanisms: (a) direct reactive oxygen species (ROS) scavenging; (b) antioxidant enzyme activation; (c) metal chelation property; (d) α-tocopheryl radical reduction; (e) oxidase inhibition; (f) mitigation of oxidative stress instigated by nitric oxide; (g) rise in uric acid levels; and (h) rise of antioxidant activities of low molecular antioxidants (Prochazkova et al. 2011). The major structural features of flavonoids for antioxidant activity are given below:
-
(i)
The number of hydroxyl groups and their substitution patterns on B ring affects the antioxidant activity. These parameters confer the formation of phenoxyl radical after the hydrogen atom donation and result in the high stability of the flavonoid due to the electron delocalization. The following parameters about a number of hydroxyl groups and their substitution pattern on B ring are:
-
(ia)
1,2-Benzenediol (catechol moiety)
-
(ib)
1,4-Benzenediol (hydroquinone moiety)
-
(ic)
1,2,3-Benzenetriol (galloyl moiety)
-
(ia)
-
(ii)
The presence of a 4-oxo group with C2–C3 double bond on the C ring causes the movement of the electron to the C ring from phenoxyl radicals of the B ring.
-
(iii)
The 3-OH group present in combination with C2–C3 double bond in flavonoids increases the resonance stabilization for electron movement across the molecule.
-
(iv)
The presence of 3- and 5-OH groups in ring A with the 4-oxo functionality in C rings is a crucial factor for maximum radical scavenging ability.
-
(v)
The presence of hydroxyl group at C3 position of ring C is also vital for antioxidant activity because it enhances the stability of the flavonoid radical.
Due to the occurrence of 3-OH, flavonols and flavan-3-ols are planar, whereas the flavones and dihydroflavones are slightly twisted. The planarity factor is responsible for conjugation and electron dislocation which are further responsive to increase flavonoid phenoxyl radical stability. Removal of the 3-OH group abolishes the planarity and conjugation which lower down the desired antioxidant properties. The glycosylation at 3-OH group also decreases their activity in comparison with their corresponding aglycones because of the steric effect having pronounced effect on activity (Sharma et al. 2018a; Dai and Mumper 2010; Heim et al. 2002).
The prooxidant activity of flavonoids, as well as their electrophilic coupling reactions with biological molecules, has also been proposed for their anticancer and anti-inflammatory effects. This includes the oxidation of flavonoids into electrophilic quinones (o-quinones or p-quinones), and these quinones are very reactive toward nucleophilic natured thiols and amino groups of proteins and glutathione. These reactions lead to the formation of different addition products that are responsible for their valuable biological effects. The presence of functionalities on B ring like either catechol moiety, hydroquinone moiety, or galloyl moiety in flavonoids is significant, and, on oxidation, these lead the formation of electrophilic quinones while resorcinol (1,3-benzenedol) cannot readily undergo oxidization. The presence of a C2–C3 double bond and hydroxyl groups at 5 and 7 positions of ring A with 4′ position at ring B are the requisite basic structural features for anti-inflammatory activity. The presence of hydroxyl group at either 2′ or 3′-position of ring B reduced the activity, while the 5′-OH group or 4′-OCH3 on ring B abolished the activity. The hydroxy derivatives have more potency than their corresponding methoxy derivatives. The glycosides also possess lower potency than their corresponding aglycones (Sharma et al. 2018a; Nambi et al. 1996; Ravishankar et al. 2013, Batra and Sharma 2013; Chen et al. 2016; Lopez-Lazaro 2002). The cytotoxicity and apoptosis induction effect of flavonoids on human leukemia cells were reported by Chang et al. The structure-activity analysis displayed that the presence of the C2–C3 double bond may be crucial for effective cytotoxicity. In addition to this, the hydroxyl group at positions 3 (ring C) and 6 (ring A), as well as the catechol moiety in ring B, may enhance the cytotoxic activity, while 5-OH and resorcinol moiety in ring B may reduce the cytotoxic activity (Chang et al. 2010).
Flavonoids may show a defensive role in the fight against cancer, cardiovascular diseases, and age-linked degenerative diseases. They could interact with several effluxes like P-gp (P-glycoprotein), MRP1 and MRP2 (multidrug resistance proteins), BCRP (breast cancer resistance protein), and uptake transporters including OATP (organic anion-transporting polypeptide), OAT (organic anion transporter), and MCTs (monocarboxylate transporters) (Wang and Morris 2014).
P-gp is supposed to act as an energy-dependent pump to effluence the anticancer agents from the tumor cells. The flavonoids have the ability to inhibit P-gp activity and act as the possible candidates to modulate multidrug resistance revealed by Kitagawa 2006. The structure-activity relationship studies suggested (1) the presence of C2–C3 double bond as well as the linkage of ring B at C2 position of ring C; (2) the number of double-bond (planar structure), i.e., 2–3; (3) the number of hydroxyl groups (at positions 3 and 5); and (4) the substitution of either 6-, 7-, 8-, or 4′-hydroxyl group of the A or B rings with hydrophobic groups. These features are responsible for high P-gp-modulating activities, while the glycosylation would dramatically decrease the activity of flavonoids (Kitagawa 2006; Zandena et al. 2005; Wang and Morris 2014).
Flavonoids have modulating activity toward the efflux transport protein MRP1 in the treatment of infectious diseases and cancer. The SAR studies specified that flavones and flavonols possessed more potency than flavanols, flavanonols, flavanones, and isoflavones. The inhibitory action of flavonoids decreases in case of glycosylation. For high MRP1 inhibitory activity, the following structural features are responsible: (1) the presence of two to three double bonds for planar molecular structure, (2) the presence of hydroxyl at both 3′ and 4′ positions on the B ring, and (3) the substitution of 4′-hydroxyl group on the B ring with hydrophobic group. The pyrogallol group on the B ring is a vital structural characteristic for inhibition of MRP2 by the flavonoids (Wang and Morris 2014).
The structural traits of flavonoids for maximal inhibitory BCRP activity (breast cancer resistance protein) include the following: (1) the number of double bonds, i.e., two to three for the planar molecular structure; (2) the presence of 5-OH group and absence of 3-OH group; (3) the position of ring B at C2 carbon of ring C; and (4) the substitution of hydroxyl group at 6-, 7-, 8-, or 4′-positions with hydrophobic substituents. The lower BCRP-inhibiting activities are observed in glycosides (Wang and Morris 2014).
4 Biosynthesis of Flavonoids
Flavonoids are one of the categories of products from plant aromatic pathway. The biosynthesis pathway of plant aromatics generally consists of three sections, i.e., the shikimate, phenylpropanoid, and flavonoid route. The shikimate pathway produces phenylalanine aromatic amino acids, while phenylpropanoid segment produces the cinnamic acid derivatives such as 4-coumaroyl-CoA, caffeoyl-CoA, and cinnamoyl-CoA, building blocks of flavonoids. In the flavonoid pathway, various flavonoid compounds are produced by the action of a variety of enzymes (Hrazdina 1992). The CHS (chalcone synthase) is a key enzyme which carried out the condensation of cinnamic acid derivatives with three molecules of malonyl-CoA, resulting in the formation of chalcone intermediates such as naringenin chalcone, eriodictyol chalcone, and pinocembrin chalcone. In some cases, the CHR (chalcone reductase) with CHS results in the formation of isoliquiritigenin chalcone. These chalcones are common intermediates which stereospecifically cyclized into respective flavanones by the action of CHI (chalcone isomerase). The chalcones undergo an oxidative cyclization by the action of AUS (aureusidin synthase) to form a five-member heterocycle fused to the A ring of the aurone. The isoflavone synthase (IFS) carried out the conversion of flavanones into isoflavones through the 1,2-aryl migration of ring B, while the conversion of flavanones to flavones is carried out through the sequential removal of the vicinal hydrogen atoms from C2 and C3 and generation of C2–C3 double bond in the ring C by the action of FNS (flavone synthase). The hydroxylation of flavanones at C3 position of ring C is carried out by F3H (flavanone 3-hydroxylase) which results the formation of flavanonols. The flavanonols are intermediate in the biosynthesis of flavonols, catechins, and anthocyanidins. FLS (flavonol synthase) is responsible for the conversion of flavanonols into flavonols by introducing C2–C3 double bond between ring C. The hydroxyl group is generated at 4 position in place of oxo group of flavanonols by the action of DFR (dihydroflavonol 4-reductase). The flavan-3,4-diols are substrates for formation of flavan-3-ols and anthocyanidins by the action of leucoanthocyanidin reductase (LCR) and leucoanthocyanidin dioxygenase (LDOX), respectively (Fig. 3.3) (Morreel et al. 2006; Miranda et al. 2012; Ferreyra et al. 2012).
The plausible schematic pathway of flavonoid biosynthesis in plants, starting with general phenylpropanoid metabolism and illustrating the major subclasses such as chalcones, aurones, isoflavonoids, flavones, flavonols, flavandiols, proanthocyanidins, and anthocyanidins. The names of common enzymes involved have been abbreviated as follows: aureusidin synthase (AUS); cinnamate-4-hydroxylase (C4H), chalcone isomerase (CHI), chalcone reductase (CHR), chalcone synthase (CHS), 4-coumaroyl:CoA-ligase (4CL), p-coumarate 3-hydroxylase (C3H); dihydroflavonol 4-reductase (DFR), flavanone 3-hydroxylase (F3H), flavone synthase (FNSI and FNSII), flavonoid 3′ hydroxylase (F3′H); flavonoid 3′5′ hydroxylase (F3′5′H); p-hydroxycinnamoyl-CoA:nshikimate/quinate p-hydroxycinnamoyltransferase (HCT); isoflavone synthase (IFS), leucoanthocyanidin dioxygenase (LDOX); leucoanthocyanidin reductase (LCR); phenylalanine ammonia-lyase (PAL)
5 Chemical Synthetic Methods of Flavonoids
A large group of natural products is known to contain usually a heterocyclic ring which can also be prepared by chemical synthesis through semi-synthesis and total synthesis approaches. These approaches play a central role in the field of organic chemistry by resolving even challenging synthetic targets in the easy and cost-effective way (Sharma et al. 2014, 2015; Khare et al. 2016). However, there is still a wide scope of research to achieve the desired structural features from a biological point of vision such as the arrangements of the functional groups, rings with respect to one another and the number of carbon atoms along with other atoms including their stereochemical elements, etc. In the last few decades, several attractive developments of methods and approaches related to the synthesis of flavonoids have been reported in the literature (Wagner and Farkas 1975; Kshatriya et al. 2018, Sharma et al. 2018a, b). In this instance, the various reports regarding chalcones’ synthesis, which belong to the flavonoid family, have been elucidated previously (Cazarolli et al. 2013; Zhuang et al. 2017; Gomes et al. 2017). More specifically, 2′-hydroxy chalcones are valuable synthon for the synthesis of other flavonoid subclasses. The synthesis of 2′-hydroxy chalcones (3) is generally achieved either by (a) Claisen-Schmidt reaction which involves a base-catalyzed reaction of substituted acetophenones (1) and aromatic aldehyde (2) through conventional methods and greener methods or (b) condensation of phenols (4) with cinnamoyl chloride (5) through Friedel-Crafts reaction and (c) Heck coupling reaction which involves the action of iodobenzene (7) on aryl vinyl ketone (6) (Fig. 3.4) (Sharma et al. 2018a, b; Kakati and Sarma 2011; Stoyanov et al. 2002; Kumar et al. 2008; Qian et al. 2013; Bianco et al. 2003, 2004). The most commonly used synthetic approaches are (a) Algar-Flynn-Oyamada approach, (b) Allan-Robinson approach, (c) Baker-Venkataraman approach, d) Claisen-Schmidt approach, (e) Karl von Auwers approach, (f) Kostanecki approach, (g) Mentzer Pyrone approach, and (h) Suzuki-Miyaura approach (Fig. 3.5).
The plausible approaches toward the synthesis of 2′-hydroxy chalcones; (a) Claisen-Schmidt reaction of substituted acetophenones and aromatic aldehyde; (b) Friedel-Crafts condensation of phenols and cinnamoyl chloride; (c) Heck coupling reaction of aryl vinyl ketone and iodobenzene [(ai) NaOH/KOH, EtOH, or acid catalyst/bronsted acidic ionic liquid, MW or grindstone method or ultrasound accelerated method:(ci) Pd(OAc)2, Ph3P, CH3CN, Et3N; (cii) MeONa, THF/MeOH; (ciii) EtSNa, DMF]
5.1 Algar-Flynn-Oyamada Approach
Algar-Flynn-Oyamada synthetic approach of flavonoid synthesis involved the oxidative cyclization of 2′-hydroxychalcones (3) with methoxy groups at different positions in the two aromatic nuclei in the presence of hydrogen peroxide under alkaline conditions. If a methoxy group is present at 6′position in 2′-hydroxychalcone, the aurone (10) will be the main product rather than flavonol (scheme 1). This reaction has wide application for the preparation of flavanonols (8) and flavonols (9) (Wang 2010a; Li 2009a).
5.2 Allan-Robinson Approach
This approach established the synthesis of flavone or isoflavone derivatives (13) by means of the condensation between 2-hydroxyacetophenones (11) and aromatic acid anhydride (12) using the sodium salt of corresponding aryl carboxylic acid anhydride. An aryloxy or alkoxy group is present at ω position of the acetophenone which is a favorable condition for the reaction (Wang 2010b; Li 2009b; Kshatriya et al. 2018; Kashyap et al. 2017, 2018; Sharma et al. 2018a, b).
5.3 Baker-Venkataraman Approach
Baker-Venkataraman approach was reported for the synthesis of flavone by rearranging the o-acyloxyketones (15) into β-diketones (16) under basic conditions via intramolecular acyl transfer. The ring closure of the dibenzoylmethane (16) is effected by the treatment with the acid catalyst so as to form flavone whereas direct conversion of o-acyloxyketones (15) into flavone by heating in the solvent (Wang 2010c; Li 2009c; Kshatriya et al. 2018).
5.4 Claisen-Schmidt Approach
Claisen-Schmidt approach is one of the well-known methods for the production of chalcones (3). The oxidative cyclization of chalcones using various acidic or basic catalysts (Lewis and Bronsted acid/base) resulted in the flavones (13) (Kshatriya et al. 2018; Kashyap et al. 2017, 2018; Sharma et al. 2018a, b).
5.5 Karl von Auwers Approach
Karl von Auwers approach involves the formation of 1,2-dibromo addition product (17) by the reaction of aurone (10) with bromine. In alkaline conditions, the attack of hydroxyl ion on 1,2-dibromo adducts that undergo dehydrohalogenation and result in the formation of flavonols (9) (Li 2005).
5.6 Kostanecki Approach
This synthetic approach utilizes Claisen condensation between benzaldehydes (1) and 2-hydroy acetophenones (2). The flavanones (8) are obtained in acidic conditions as a condensation product, which reacts with isoamyl nitrite and subsequent upon hydrolysis gives flavonols (9) (Kashyap et al. 2017, 2018; Sharma et al. 2018a, b).
5.7 Mentzer Pyrone Approach
This approach involves the synthesis of flavone derivatives (13) by the reaction between phenols (4) and β-ketoesters (18) at high temperature for a longer period or in a microwave irradiation (Wang 2010d).
5.8 Suzuki-Miyaura Approach
The Suzuki-Miyaura approach utilizes the reaction of compounds (19, 22, 23) containing sp2-hybridized carbon and halogen bond with boronic acids/esters (20a, b) in the presence of palladium compounds (Selepe and Heerden 2013).
6 Conclusion
Flavonoids have been abundantly present in human diet such as in fruits, vegetables, and beverages (tea, wine) owing to their wide spread in the plant kingdom. It is a wide class of polyphenolic compounds with 2-phenylchromane nucleus. This class of compounds is being intensively investigated because of their health-associated therapeutic, biochemical, and pharmacological benefits. The structural features, their classification, and structure-activity relationships are extremely helpful to understand the relations between their molecular structures and biological and physicochemical activities. The existence of hydroxyl groups on ring A, double bonds, and oxo group with ring B hydroxyl group substitution pattern are the requisite structural feature for their activity toward the health benefits. Chemically, they are synthetically accessed by various methods. Among these, the Baker-Venkataraman approach (β-diketones formation) or the Claisen-Schmidt condensation (chalcones formation) and their successive cyclisation pathways to 2-phenylchromane heterocycles are mostly adopted by different studies. The development of the new flavonoid derivatives with improved therapeutic values would be useful for chemists, biologist, and biochemist to understand, design, and insert new functionalities into these biomedical compounds and further test the modified compounds for their biological effects.
References
Andersen QM, Markham KR (2006) Flavonoids: chemistry, biochemistry and applications. CRC Press, Taylor & Francis Group, Boca Raton
Batra P, Sharma AK (2013) Anti-cancer potential of flavonoids: recent trends and future perspectives. 3 Biotech 3(6):439
Bhagwat S, Haytowits DB, Holden JM (2013) Usda database for the flavonoid content of selected foods. Release 3.1. Nutrient Data Laboratory, Beltsville Human Nutrition Research Center Agricultural Research Service U.S. Department of Agriculture, Beltsville, pp 1–155
Bianco A, Cavarischia C, Guiso M et al (2003) A new synthesis of flavonoids via Heck reaction. Tetrahedron Lett 44:9107–9109
Bianco A, Cavarischia C, Guiso M (2004) The Heck coupling reaction using aryl vinyl ketones: synthesis of flavonoids. Eur J Org Chem 2004:2894–2898. https://doi.org/10.1002/ejoc.200400032
Bone K, Mills S (2013) Chapter 2 – principles of herbal pharmacology principles and practice of phytotherapy. In: Modern herbal medicine, 2nd edn. Elsevier, Amsterdam, pp 17–82. https://doi.org/10.1016/B978-0-443-06992-5.00002-5
Cazarolli LH, Kappel VD, Zanatta AP et al (2013) Natural and synthetic chalcones: tools for the study of targets of action – insulin secretagogue or insulin mimetic? In: Atta-ur-Rahman (ed) Studies in natural products chemistry, vol 39. Elsevier, Amsterdam, pp 47–89
Chang H, Mi M, Ling W et al (2010) Structurally related anticancer activity Of flavonoids: involvement of reactive oxygen species generation. J Food Biochem 34:1–14. https://doi.org/10.1111/j.1745-4514.2009.00282.x
Chen L, Teng H, Xie Z et al (2016) Modifications of dietary flavonoids towards improved bioactivity: an update on structure–activity relationship. Crit Rev Food Sci Nutr 58:513–527. https://doi.org/10.1080/10408398.2016.1196334
Correia-da-Silva M, Sousa E, Pinto MMM (2013) Emerging sulfated flavonoids and other polyphenols as drugs: nature as an inspiration. Med Res Rev 34(2):1–57. https://doi.org/10.1002/med
Dai J, Mumper RJ (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15:7313–7352. https://doi.org/10.3390/molecules15107313
de la Rosa LA, Alvarez-Parrilla E, Gonzalez-Aguilar GA (2010) Fruit and vegetable phytochemicals: chemistry, nutritional value, and stability, 1st edn. Wiley, Ames
Erdman JW Jr, Balentine D, Arab L et al (2007) Flavonoids and heart health: proceedings of the ILSI North America flavonoids workshop, May 31–June 1, 2005, Washington, DC. J Nutr 137:718S–737S
Erlejman AG, Verstraeten SV, Fraga CG et al (2004) The interaction of flavonoids with membranes: potential determinant of flavonoid antioxidant effects. Free Radic Res 38:1311–1320
Ferreyra MLF, Rius SP, Casati P (2012) Flavonoids: biosynthesis, biological functions, and biotechnological applications Front. Plant Sci 3:222. https://doi.org/10.3389/fpls.2012.00222
Gomes MN, Muratov EN, Pereira M et al (2017) Chalcone derivatives: promising starting points for drug design. Molecules 22(8):1210. https://doi.org/10.3390/molecules22081210
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13:572–584
Hrazdina G (1992) Biosynthesis of flavonoids. In: Hemingway RW, Laks PE (eds) Plant polyphenols. Basic life sciences, vol 59. Springer, Boston
Kakati D, Sarma JC (2011) Microwave assisted solvent free synthesis of 1,3-diphenylpropenones. Chem Cent J 5:8. https://doi.org/10.1186/1752-153X-5-8
Kashyap D, Sharma A, Tuli HS et al (2017) Kaempferol – a dietary anticancer molecule with multiple mechanisms of action: recent trends and advancements. J Funct Foods 30:203–219
Kashyap D, Sharma A, Sak K et al (2018) Fisetin: a bioactive phytochemical with potential for cancer prevention and pharmacotherapy. Life Sci 194:75–87
Khare R, Sharma J, Sharma A (2016) Russ J Gen Chem 86:702. https://doi.org/10.1134/S1070363216030312
Kitagawa S (2006) Inhibitory effects of polyphenols on P-glycoprotein-mediated transport. Biol Pharm Bull 29(1):1–6
Kozłowska A, Szostak-Węgierek D (2014) Flavonoids – food sources and health benefits. Rocz Panstw Zakl Hig 65(2):79–85
Kshatriya R, Jejurkar VP, Saha S (2018) In memory of Prof. Venkataraman: recent advances in the synthetic methodologies of flavones. Tetrahedron 74:811–833
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: an overview. Sci World J 2013:162750, 16 pages. https://doi.org/10.1155/2013/162750
Kumar S, Lamba MS, Makrandi JK (2008) An efficient green procedure for the synthesis of chalcones using C-200 as solid support under grinding conditions. Green Chem Lett Rev 1(2):123–125. https://doi.org/10.1080/17518250802325993
Li J (ed) (2005) Name reactions in heterocyclic Chemistry. Wiley, Hoboken, pp 262–265
Li JJ (2009a) Algar— Flynn— Oyamada reaction. In: Li JJ (ed) Name reactions. Springer, Berlin/Heidelberg. https://doi.org/10.1007/978-3-642-01053-8_3
Li JJ (2009b) Allan–Robinson reaction. In: Li JJ (ed) Name reactions. Springer, Berlin/Heidelberg. https://doi.org/10.1007/978-3-642-01053-8_4
Li JJ (2009c) Baker–Venkataraman rearrangement. In: Li JJ (ed) Name reactions. Springer, Berlin/Heidelberg. https://doi.org/10.1007/978-3-642-01053-8_7
Lopez-Lazaro M (2002) Flavonoids as anticancer agents: structure-activity relationship study. Curr Med Chem Anticancer Agents 2(6):691–714
Miranda CL, Maier CS, Stevens JF (2012) Flavonoids. In: eLS. https://doi.org/10.1002/9780470015902.a0003068.pub2
Morreel K, Goeminne G, Storme V et al (2006) Genetical metabolomics of flavonoid biosynthesis in Populus: a case study. Plant J 47:224–237. https://doi.org/10.1111/j.1365-313X.2006.02786.x
Nambi RA, Viswanathan S, Thirugnanasambantham P et al (1996) Anti-inflammatory activity of flavone and its Hydroxy derivatives-a structure activity study. Indian J Pharm Sci 58(1):18
Prochazkova D, Bousova I, Wilhelmova N (2011) Antioxidant and prooxidant properties of flavonoids. Fitoterapia 82(4):513–523
Qian H, Wang Y, Liu D (2013) Ultrasound-accelerated synthesis of substituted 2′-hydroxychalcones by reusable ionic liquids. Ind Eng Chem Res 52(37):13272–13275. https://doi.org/10.1021/ie401557j
Ravishankar D, Rajora AK, Greco F, Osborn HM (2013) Flavonoids as prospective compounds for anti-cancer therapy. Int J Biochem Cell Biol 45(12):2821–2831
Selepe MA, Heerden FRV (2013) Application of the Suzuki-Miyaura reaction in the synthesis of flavonoids. Molecules 18:4739–4765. https://doi.org/10.3390/molecules18044739
Sharma A, Khare R, Kumar V et al (2014) 1-(Substituted)-4, 4, 6-trimethyl-3, 4-dihydropyrimidine-2(1H)-thione: green synthesis, antibacterial activity and DNA photocleavage activity. Int J Pharm Pharm Sci 6(3):171–175
Sharma A, Kumar V, Khare R et al (2015) Synthesis, docking study, and DNA photocleavage activity of some pyrimidinylhydrazones and 3-(quinolin-3-yl)-5, 7-dimethyl-1, 2, 4-triazolo [4, 3-a] pyrimidine derivatives. Med Chem Res 24(5):1830–1841
Sharma A, Sharma P, Singh HT et al (2018a) Phytochemical and pharmacological properties of flavonols. In: eLS. Wiley. https://doi.org/10.1002/9780470015902.a0027666
Sharma A, Kashyap D, Sak K et al (2018b) Therapeutic charm of quercetin and its derivatives: a review of research and patents. Pharm Pat Anal 7(1):15–32. https://doi.org/10.4155/ppa-2017-0030
Stoyanov EV, Champavier Y, Simon A et al (2002) Efficient liquid-phase synthesis of 2′-hydroxychalcones. Bioorg Med Chem Lett 12(19):2685–2687
Teles YCF, Souza MSR, Vanderlei de Souza MF (2018) Sulphated flavonoids: biosynthesis, structures, and biological activities. Molecules 23:480. https://doi.org/10.3390/molecules23020480
Wagner H, Farkas L (1975) Synthesis of flavonoids. In: Harborne JB, Mabry TJ, Mabry H (eds) The flavonoids. Springer, Boston
Wang Z (2010a) Algar-Flynn-Oyamada (AFO) reaction. In: Wang Z (ed) Comprehensive organic name reactions and reagents. doi:https://doi.org/10.1002/9780470638859.conrr013
Wang Z (2010b) Allan-Robinson condensation. In: Wang Z (ed) Comprehensive organic name reactions and reagents. https://doi.org/10.1002/9780470638859.conrr015
Wang Z (2010c) Baker-Venkataraman rearrangement. In: Wang Z (ed) Comprehensive organic name reactions and reagents. https://doi.org/10.1002/9780470638859.conrr040
Wang Z (2010d) Mentzer Pyrone synthesis. In: Wang Z (ed) Comprehensive organic name reactions and reagents. https://doi.org/10.1002/9780470638859.conrr427
Wang X, Morris ME (2014) Diet/nutrient interactions with drug transporters. In: You G, Morris ME (eds) Drug transporters, 2nd edn. John, Hoboken, pp 409–427. https://doi.org/10.1002/9781118705308.ch21
Wen L, Jiang Y, Yang J et al (2017) Structure, bioactivity, and synthesis of methylated flavonoids. Ann NY Acad Sci 1398:120–129. https://doi.org/10.1111/nyas.13350
Zandena JJV, Wortelboerb HM, Bijlsmab S et al (2005) Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochem Pharmacol 69:699–708
Zhuang C, Zhang W, Sheng C et al (2017) Chalcone: a privileged structure in medicinal chemistry. Chem Rev 117(12):7762–7810. https://doi.org/10.1021/acs.chemrev.7b00020
Acknowledgments
The authors would like to acknowledge the assistance of Career Point University, in Tikker – kharwarian, Hamirpur, Himachal Pradesh, and Maharishi Markandeshwar (deemed to be university) in Mullana, Ambala, Haryana, for providing the required facilities to complete this study.
Conflict of Interest
There exists no conflict of interest among authors regarding the publication of this book chapter.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sharma, A., Singh Tuli, H., Sharma, A.K. (2019). Chemistry and Synthetic Overview of Flavonoids. In: Singh Tuli, H. (eds) Current Aspects of Flavonoids: Their Role in Cancer Treatment . Springer, Singapore. https://doi.org/10.1007/978-981-13-5874-6_3
Download citation
DOI: https://doi.org/10.1007/978-981-13-5874-6_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-5873-9
Online ISBN: 978-981-13-5874-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)