Abstract
The co-culturing of Pleurotus eryngii var. ferulae and Rhodotorula mucilaginosa was confirmed in our previous studies to be an efficient strategy to improve laccase production by submerged fermentation. To determine the possible regulation principles underlying this behaviour, comparative transcriptomic analysis was performed on P. eryngii var. ferulae to investigate the differential expression of genes in co-culture. RNA-seq analysis showed that genes concerning xenobiotic biodegradation and expenditure of energy were upregulated. However, genes related to oxidative stress were downregulated. In addition, the transcription levels of laccase isoenzymes were not consistent in the co-culture system: 3 laccase genes (lacc1, lacc2, lacc12) were upregulated, and 3 laccase genes (lacc4, lacc6, lacc9) were downregulated. The enhancement in laccase activity can be due to upregulation of a laccase heterodimer encoded by the genes lacc2 and ssPOXA3a (or ssPOXA3b), whose expression levels were increased by 459% and 769% (or 585% for ssPOXA3b) compared with those of a control, respectively. β-Carotene produced by R. mucilaginosa upregulated the transcription of lacc2 only. Combining these results with an analysis of cis-acting responsive elements indicated that four transcription factors (TFs) had potential regulatory effects on the transcription of laccase genes. It was supposed that TFa regulated lacc transcription by binding with methyl jasmonate and heat shock response elements. The expression of TFb, TFc, and TFd was regulated by β-carotene. However, β-carotene had no effect on TFa expression. These results provide a possible mechanism for the regulation of laccase gene transcription in the co-culture system and are also beneficial for the future intensification of fungal laccase production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laccases (EC 1.10.3.2) are blue multicopper-containing phenol oxidases that are widely distributed in various basidiomycetous and ascomycetous fungi (Asina et al. 2016; Giardina and Sannia 2015; Hoegger et al. 2006). Research shows that laccases are involved in physiological processes, such as morphogenesis, sporulation, pigment production, the formation of fruiting bodies, and plant pathogenesis (Kües and Rühl 2011). Laccases are also useful biocatalysts for a wide range of biotechnological applications, including lignin degradation, dye decolorization, effluent detoxification, and food or beverage processing (Sharma et al. 2018). However, fungal laccases do usually not give enough yield in cultivation to supply industrial production, which increases the industrial cost. The industry of filamentous fungi for producing laccase needs to be further expanded and improved (Sharma et al. 2018). This deficiency has led to gradually rising interest in co-culture, which can effectively enhance the production of laccase in liquid-state fermentation.
Fungal co-culture can cooperatively utilize the metabolic pathways and products of two or more species, which offers a novel, economical, and environmentally friendly strategy for enhancing enzymatic and decolourising activity (Copete-Pertuz et al. 2019). Meanwhile, co-culture processes vary widely among microorganisms. Gongronella sp. w5 and Coprinopsis cinerea, Leptosphaerulina sp. and Trichoderma viride, and Pleurotus ostreatus and Trametes versicolor were all confirmed to elevate laccase production as co-cultured pair strains (Copete-Pertuz et al. 2019; Hu et al. 2019; Luo et al. 2017). In these processes, the transcription of laccase genes was altered, and several silent laccase genes were activated (Flores et al. 2009; Lv et al. 2014). However, the specific mechanisms governing laccase overproduction during co-culture have not been clarified. It was speculated that synergism by lignin-degrading enzymes and responses to nutritional and oxidative stresses were all possible mechanisms (Wang et al. 2009; Hailei et al. 2009; Qi-He et al. 2011; Zhong et al. 2017). In addition, several secondary metabolites (usually low-molecular-weight molecules) released by one strain can stimulate laccase expression by another (Fischer et al. 2017; Piscitelli et al. 2011). The metabolites involved are p-hydroxybenzoic acid and glycerol (Ramírez et al. 2018; Hu et al. 2019; Wei et al. 2010). However, there is still no research illuminating the intrinsic causes of beneficial co-culture behaviour from the perspective of transcriptional regulation. The transcription levels of laccase isoenzymes and transcription factors (TFs) have not been explored in Pleurotus eryngii var. ferulae.
In a previous study of ours, co-culture with Rhodotorula mucilaginosa was an effective strategy to enhance the laccase production of P. eryngii var. ferulae (Wang et al. 2015). β-Carotene from R. mucilaginosa was confirmed as a key inducing compound (Guo et al. 2017). To obtain a further understanding of the interactions between the two strains, it is necessary to determine the molecular mechanism governing the interactions and the factors affecting transcriptional regulation of laccases. In this work, RNA sequencing (RNA-seq) experiments were carried out to inspect the changes in gene expression in co-culture and β-carotene culture. Cis-element analysis was performed on the promoter sequences of upregulated laccase isoenzymes and combined with the TFs of differentially expressed genes (DEGs) for comprehensive analysis. These studies lay a foundation for further exploring the regulation mechanism of laccase in co-culture.
Materials and methods
Strain and culture conditions
The laccase-producing fungal strain P. eryngii var. ferulae JM301 (CCTCC AF 2019009) and the co-culture yeast strain R. mucilaginosa JM401 (CCTCC M 2013088) were collected at the China Center for Type Culture Collection. The liquid medium for laccase production from P. eryngii var. ferulae consisted of 20 g/L glucose, 20 g/L corn powder, 20 g/L wheat bran, and 0.1742 g/L K2SO4 (pH 9.0). Fungal mycelia were inoculated in the culture medium and incubated at 30 °C and 150 rpm. The culture medium for R. mucilaginosa was YPD (10 g/L yeast extract, 20 g/L peptone, and 20 g/L glucose), and the yeast was incubated at 30 °C and 200 rpm.
Preparation of transcriptome samples
Four kinds of samples were prepared (all containing three biological replicates): monoculture, co-culture, β-carotene culture, and oil culture (as a negative control for β-carotene culture). Since β-carotene is a fat-soluble substance, it cannot dissolve directly in the liquid medium. Peanut oil was selected as a solvent for β-carotene. After monoculture of P. eryngii var. ferulae for 2 days, 5.0 × 109 yeast cells, 10 mg of β-carotene (dissolved in 1 mL of plant oil), and 1 mL of peanut oil were added into 150 mL of the fermentation broth respectively and incubated for another 5 days. The samples on the 5th day of cultivation were immediately collected by centrifugation and transferred to liquid nitrogen for preservation before RNA extraction.
RNA extraction, cDNA library construction, and sequencing
Total RNA was isolated from prepared samples using the MiniBEST Universal RNA Extraction Kit (TaKaRa, Dalian, China) following the manufacturer’s instructions. The RNA concentration was measured using a Quawell UV-Vis Spectrophotometer Q5000. RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, USA). A total amount of 1.5 μg of RNA per sample was used as input material for RNA sample preparations. Sequencing libraries were generated using the NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (NEB, Ipswich, MA, USA), and index codes were added to attribute sequences to each sample. The raw transcriptome sequencing data have been deposited in NCBI Sequence Read Archive (SRA) database (SRA study: SRP212144, bioproject ID: PRJNA551351).
The libraries were then size selected for the cDNA target fragments, and the selected cDNA was amplified via polymerase chain reaction (PCR) using adaptor-specific primers. The cDNA library was quantified using the KAPA library quantification kit (Roche, Basel, Switzerland) according to the manufacturer’s recommendations. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using the TruSeq PE Cluster Kit v3-cBot-HS (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina HiSeq platform (Grabherr et al. 2011). At the same time, the Phred quality score Q20, GC content, and sequence duplication level of the clean data were calculated. All downstream analyses were based on clean data of high quality.
Transcriptome assembly and functional annotation
Transcriptome assembly was accomplished based on the clean reads using Trinity (Grabherr et al. 2011) with min_kmer_cov set to 2 by default and all other parameters set to their default values. To obtain comprehensive gene information, the functional annotations of all assembled transcripts were based on six databases, including non-redundant protein sequences (Nr), nucleotide sequences (Nt), Protein family (Pfam), euKaryotic Orthologous Groups (KOG), Swiss-Prot, and the Kyoto Encyclopedia of Genes and Genomes (KEGG) (e value ≤ 1e−10).
Differential expression analysis
RNA-seq by expectation maximization (RSEM) was used to quantify gene and isoform abundances for each sample (Li and Dewey 2011). The read count for each gene was performed using the R statistical package software EdgeR (Empirical analysis of Digital Gene Expression in R) (Robinson et al. 2010). The expression level of each transcript was calculated according to the fragments per kilobase of exon per million mapped reads (FPKM). The index of the P value was optimized to adjust the P value (padj) to control the false discovery rate. Restrictive thresholds (padj < 0.05 and |log2(foldchange)| > 1) were used to screen the genes for DEGs.
Gene expression analysis by RT-qPCR
Reverse transcription was performed using the PrimeScript1RT reagent Kit with gDNA Eraser (TaKaRa, Dalian, China). To examine the transcription level of target genes and internal controls, real-time quantitative polymerase chain reactions (RT-qPCR) were carried out using an Analytik Jena qTOWER2.0 thermal cycler (Analytik Jena, Jena, Germany). Each RT-qPCR contained 30 ng of first-strand cDNA and 0.2 μM each of forward and reverse primer in a total reaction volume of 20 μL. The product can be detected at 497 nm owing to SYBR Green fluorescent dye (Genscript, Nanjing, China). The primers were designed using NCBI Primer Blast and referred to previous research (Supplemental Table S1) (Castanera et al. 2012). Sar1 was used as internal reference genes for normalization (Castanera et al. 2015). The relative abundances of target genes were calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001). For each sample, at least three independent technical and biological repeats were carried out.
Mycelium ROS level
The reactive oxygen species (ROS) levels of P. eryngii var. ferulae mycelia were examined under four culture conditions for 2–7 days by a tissue ROS detection kit according to the instruction manual (BestBio Biotechnology, Shanghai, China). Briefly, fresh testicular tissues were ground with a glass homogenizer, and an O11 probe was added to the supernatant of the homogenate. After incubation in the dark for 30 min at 37 °C, fluorescence intensity was detected at Ex/Em wavelengths of 488/530 nm with a multimode plate reader (PerkinElmer, Waltham, MA, USA). In contrast, the protein concentration of the homogenate supernatant was measured with a BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The mycelium ROS intensity was represented as the fluorescence intensity divided by milligrams of protein.
Gene-walking approach for acquiring promoters
Based on the partial sequence of lacc2, lacc6, ssPOXA3a, and ssPOXA3b (GenBank numbers of MN017367, MN017366, MN058987, and MN058986, respectively), which were all obtained by RNA-seq, specific primers with similar annealing temperatures were designed (Supplemental Table S1), and the target laccase complete fragments and promoter sequences were obtained by the Genome Walking Kit (TaKaRa, Dalian, China).
Enzymatic activity assay
Extracellular culture supernatant was collected for enzymatic activity assay under four culture conditions for 2–7 days. Laccase activity was measured in accordance with a previous study (Hou et al. 2004). The resulting 1-mL reaction mixtures contained 880 μL of 100 mM sodium acetate buffer (pH 4.5), 100 μL of 10 mM 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) at 420 nm (ε420 = 36,000 M−1 cm−1). One unit of activity was defined as the amount of enzyme required to oxidize 1 mmol of ABTS per minute.
Determination the content of β-carotene
The R. mucilaginosa was separated by centrifugation and then ground in liquid nitrogen under condition of co-culture for 3-7 days. Then, β-carotene was extracted with methenyl trichloride. An ultra-performance liquid chromatography (UPLC) system (Thermo Scientific, Waltham, MA, USA) was applied to separate and determine the β-carotene with a reverse-phase C18 column (Waters, Milford, MA, USA). Mobile phases of acetonitrile–isopropanol (10:90, v/v) (a) and acetonitrile (b) were eluted at a flow rate of 0.3 mL/min in a gradient program (Supplemental Table S2). A standard of β-carotene was also assayed by the same method.
Native polyacrylamide gel electrophoresis and identification by mass spectrometry
To further analyse the relative composition and activity of laccase isoenzymes, SurePAGE (4–12%) from GenScript Company (Nanjing, China) was employed. Sample buffer consisted of 5 mL glycerol, 25 mg of bromophenol blue, 150 mg of Tris base, and ddH2O (pH 6.8, constant volume to 10 mL). Extracellular culture supernatant (10 μL) was taken from samples of four treatments at 5 days and analysed according to the manufacturer’s instructions. Separated laccase bands were visualized by a reaction with 1.0 mM ABTS in 0.1 M sodium acetate buffer (pH 4.5) at room temperature. Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) was applied to identify the protein hydrolysate for each of native polyacrylamide gel electrophoresis (Native-PAGE) bands. Mascot 2.2 software (Matrix Science, London, UK) was employed to analyse the original file of mass spectrometry by retrieving the corresponding database. The specific method of MALDI-TOF-MS analysis was introduced at full length in the previous study (Kang et al. 2013).
Statistical analysis
All experiments were performed in triplicate. The data were analysed with SPSS 11.5 software and were expressed as the mean ± standard deviation. Trends were considered significant when the mean values of the compared sets were different at *P < 0.05.
Results
RNA-seq analysis
Sequencing results, de novo assembled transcriptome and annotation
Transcriptome analysis was performed according to the workflow shown (Supplemental Fig. S1). High-quality RNA samples (OD260/280 = 1.8~2.1, OD260/230 ≥ 2.0, 28S:18S ≥ 1.0, > 10 μg) were reserved and applied for high-throughput sequencing by Illumina paired-end sequencing technology. The sequencing data were largely eligible for further use (Supplemental Table S3). The sequencing error rate of a single base was less than 0.03%, and low-quality reads had been removed cleanly (Q20 > 95%). Since R. mucilaginosa JM401 and IIPL32 (INSDC: NHTJ00000000.1) genome homology reached as high as 92.21% (> 90%), the yeast reads of co-culture samples were cleaned up by referring to the known genome. Considering the effect of sequencing depth and gene length on the count of fragments, the read count was converted to FPKM, which is the most common method for estimating the expression level of genes (Trapnell et al. 2010). A total of 103,637 unigenes were obtained from Trinity de novo assembly. For this transcriptome analysis without a reference genome, assembly first (de novo), which uses reads to assemble transcripts directly, is a common and valid method. This method always leads to a number of unigenes that far exceeds those from the reference transcriptome approach (Grabherr et al. 2011). Different transcripts of the same gene are obtained simultaneously, which causes multiple unigenes to map to the same gene. After the unigenes were compared and annotated by Nr database (Supplemental Fig. S2), 73.9% of them were found to be annotated to P. ostreatus PC15 (accession number: PRJNA521560), which indicates that the transcriptomes of P. ostreatus and P. eryngii var. ferulae were very similar. In addition, 14.6% of unigenes were annotated to yeast, whose data were removed again. For correlation analyses among samples (Supplemental Fig. S3), the correlation coefficient for three biological replicates was relatively close. The correlation coefficient of gene expression levels among the samples other than co-cultured samples was higher than 0.60 in all cases, which indicates similar expression patterns among samples. The raw data quality control ensured that all samples exhibited superior sequencing quality and that the data can fulfil the requirements of subsequent analysis.
Analysis of DEGs
Compared with that of monoculture, the RNA-seq analysis of co-culture revealed 5799 DEGs in the transcriptome of P. eryngii var. ferulae, including 2867 upregulated genes and 2932 downregulated genes. The RNA-seq analysis of the β-carotene culture revealed 1717 genes whose transcription level changed significantly, including 674 upregulated genes and 1043 downregulated genes.
According to Venn diagrams (Fig. 1a, b), 262 genes were upregulated and 548 genes were downregulated under conditions of monoculture and co-culture. The specific transcription level of DEGs was drawn as a volcano based on the indexes of -log10(padj) and log2(foldchange) (Fig. 1c, d). The distribution of laccase and its isoenzymes in DEGs is also shown in the Figures. Among these isoenzymes, the transcription levels of lacc2 and lacc6 had similar variation tendencies under both conditions. However, co-culture, which contained additional complex biological processes, caused changes in the other six laccase isoenzymes. This result implied that β-carotene may be one of the key factors promoting high expression of laccase in co-culture, though it is not the only factor.
Venn diagrams and volcano plot of DEGs in two culture conditions. a Genes upregulated. b Genes downregulated. c Co-culture vs. monoculture. d β-Carotene culture vs. monoculture. C, co-culture; B, β-carotene culture; M, monoculture. Cluster-1041.48984: lacc2; Cluster-1041.29201: lacc6; Cluster-1041.58782: ssPOXA3a; Cluster-1041.22317: ssPOXA3b. Genes above the dotted line exhibit significant differences (padj < 0.05)
Cluster analysis of DEGs
Gene function groups
To analyse the function of DEGs in co-culture and β-carotene culture compared to the control, we acquired 11 and 10 main functional groups annotated against Nr database, respectively (Fig. 2a, b). Each functional group contained at least two DEGs. The most notable finding was that the genes concerning xenobiotic biodegradation and energy expenditure were upregulated as a whole, and the genes concerning oxidative stress were downregulated in both cultivation modes.
In co-culture, xenobiotic biodegradation and metabolism terms, which included polysaccharide lyase, glutathione transporter, laccase, and peroxidase, involved the degradation of polysaccharide, polypeptide, and lignin. There were six upregulated genes of polysaccharide lyases belonging to family 1 protein, which contains pectate lyase (EC 4.2.2.2), exo-pectate lyase (EC 4.2.2.9), and pectin lyase (EC 4.2.2.10). The glutathione transporter group, which functioned as a detoxifier, contained 4 upregulated genes and 2 downregulated genes (Zhong et al. 2019). The laccase group had 5 upregulated genes and 3 downregulated genes. The expression levels of 2 DyP-type peroxidase genes and one manganese peroxidase gene were enhanced in the peroxidase group. In β-carotene culture, the group of xenobiotic biodegradation and metabolism except for the group of glutathione transporters was still notably upregulated.
NADH dehydrogenase was essential for ATP synthesis, and ATPase was used as a pivotal enzyme for catalysing the hydrolysis of ATP. The upregulation of the carbohydrate-binding module family in the co-culture system showed that the co-culture process had a distinct impact on the carbon metabolic flux of P. eryngii var. ferulae, which may be related to competition for environmental nutrients (Zhong et al. 2019). The upregulation of its transcriptional level indicated that under these two cultivation modes, the demand for and consumption of ATP in P. eryngii var. ferulae cells had increased.
Oxidative stress and elimination of free radicals may enhance expression of laccase in co-culture (Du et al. 2017). However, in this study, the expression of genes responding to oxidative stress, which included catalase, oxidase, reductase, oxidoreductase, and cytochrome P450, showed different degrees of downregulation under both cultural conditions. Among these proteins, the transcriptional levels of glucose oxidase and quinone oxidoreductase were almost downregulated. R. mucilaginosa did not induce oxidative stress in P. eryngii var. ferulae and even inhibited the expression of the abovementioned functional groups. In addition to cytochrome P450, other groups of proteins upregulated by oxidative stress caused by the addition of β-carotene exhibited activities consistent with the trend of the co-culture system. The mycelium ROS level is shown in Supplemental Fig. S4. The ROS level in monoculture was higher than that in co-culture throughout the cultivation process, and the gap between the two rapidly increased in 5–7 days.
The 25 upregulated genes with the greatest fold change in monoculture and co-culture are presented in Supplemental Table S4, where genes of unknown function were not included. Most highly upregulated genes were noted in functional groups. Of these genes, many significantly upregulated genes were involved in xenobiotic biodegradation and the expenditure of energy, including laccase, mitochondrial external NADH dehydrogenase, phosphodiesterase, and NAD-aldehyde dehydrogenase. Upregulation of ribosomal proteins indicated a demand for an increased transcription frequency. In addition, GH16 (endoglucanase) and GH47 (α-mannosidase) were involved in hydrolysis of the cell wall and glycosylation in the endoplasmic reticulum, respectively (Alfaro et al. 2016; Xiang et al. 2016). In β-carotene culture, energy storage and consumption were additionally manifested in endoplasmic reticulum calcium ATPase, acetyl-CoA synthetase-like protein, and carbohydrate-binding module family 50 protein.
KEGG analysis
KEGG pathway enrichment of DEGs upregulated in co-culture and β-carotene culture compared with the control is shown in Fig. 3. The pathway enrichment of energy consumption was also observably upregulated in both cultivation modes. One energy metabolism pathway of “glycolysis/gluconeogenesis,” one chromosome repair group of “non-homologous end joining,” and two amino acid metabolism pathways of “tryptophan metabolism” and “tyrosine metabolism” were significantly enriched pathways of DEGs (padj < 0.05) in co-culture. In addition, the “endocytosis” metabolism pathway contained 25 genes, which indicated that there was a large amount of xenobiotics that was consumed by P. eryngii var. ferulae cells. In β-carotene culture, in addition to “glycolysis/gluconeogenesis,” “citrate cycle” and “pyruvate metabolism” were also part of the group of energy storage and consumption groups. There were 11 and 9 basal TFs in co-culture and β-carotene culture, respectively. Moreover, the upregulation of the ribosome metabolic pathway further indicated that the transcription and expression levels of some enzymes were enhanced in P. eryngii var. ferulae.
KEGG analysis of upregulated genes in P. eryngii var. ferulae transcriptome in co-culture and β-carotene culture. a Co-culture vs. monoculture. b β-Carotene culture vs. monoculture. The size of circles represents the number of genes. The saturation of the circle color represents the significance of the pathway
Enzyme activity and transcription level of laccase isoenzymes
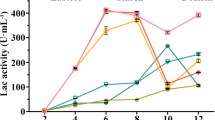
When P. eryngii var. ferulae was co-cultured with R. mucilaginosa, the laccase activity was markedly increased in the supernatant of the fermentation solution, reaching 3.27 U/mL on the 6th day (Fig. 4a), which was as much as 218% greater than the control activity. Adding β-carotene also caused a distinct increase in the laccase activity, which reached 2.15 U/mL on the 6th day. Interestingly, in the whole culture process, the trend of laccase activity remained completely consistent in co-culture and β-carotene culture. The β-carotene produced by R. mucilaginosa was increased in co-culture during the cultivation process (Supplemental Fig. S5). On the 5th day, the content of β-carotene reached 40.54 μg/L. In addition, oil did not induce over-expression of laccase throughout the fermentation process.
Enzyme activity and transcription level of laccase isoenzymes in four kinds of culture conditions. a Laccase activity from the 2nd to 7th day. b Heat map of laccase isoenzymes. The significantly analysis used the monoculture group as a reference (***P < 0.001, 0.001 < **P < 0.01, 0.01 < *P < 0.05). c The transcription levels of laccase isozyme genes on the 5th day were validated by RT-qPCR. The fold change is denoted as the ratio of the transcription level in the other three cultural conditions to that in monoculture
Based on the RNA-seq analysis, there were 3 upregulated and 3 downregulated laccase isoenzyme genes and 2 upregulated small subunits of laccase (ssPOXA3a and ssPOXA3b) in co-culture (Fig. 5b). Among these genes, the upregulated genes were lacc1 (Cluster-1041.14150), lacc2 (Cluster-1041.48984), lacc12 (Cluster-1041.55007), ssPOXA3a (Cluster-1041.58782), and ssPOXA3b (Cluster-1041.22317). The downregulated genes were lacc4 (Cluster-1041.23876), lacc6 (Cluster-1041.29201), and lacc9 (Cluster-1041.62755). The tendencies of changes in the transcription level of laccase isoenzymes were similar in β-carotene culture and co-culture, but the degree of change was generally lower in the former. In general, the composition of laccase isoenzymes in co-culture and β-carotene culture differed from that observed in monoculture.
The RT-qPCR analysis verified and supplemented the transcription data. The relative expression values of 8 laccase genes in three cultural conditions were compared to those in the control sample (Fig. 4c). Under co-culture conditions, lacc2 was upregulated by 459.19% and lacc6 was downregulated by 37.87%. In addition, the small subunits of POXA3a and POXA3b were upregulated by 769.39% and 585.27%, respectively. In β-carotene culture, lacc2 was upregulated by 340.76% and lacc6 was downregulated by 20.63%. Meanwhile, ssPOXA3a and ssPOXA3b were upregulated by 70.53% and 70.13%, respectively. The upregulation extent of ssPOXA3b measured by RNA-seq differed from that measured by qPCR. As mentioned above, the de novo transcriptome approach caused multiple unigene map to the same gene. It would result in numerical differences between transcriptome analysis and qPCR, but the variation trend of genes was basically consistent. The other results measured by RT-qPCR were mainly in accordance with the RNA-seq data, which indicated that the laccase analysis and RNA-seq data in all cultivation modes were reliable. The transcription level of laccase isoenzymes in oil culture changed slightly. This finding indicated that oil was a good negative control for the addition of β-carotene samples. The Native-PAGE showed that the production of Lacc2 was enhanced significantly and Lacc6 was decreased in co-culture and β-carotene culture compared with the monoculture, which was consistent with the concerned results of RT-qPCR (Supplemental Fig. S6).
Cis-acting responsive element analysis
Under different biotic and abiotic stress conditions, laccase isoenzymes exhibited varying regulation efficiency, which was related to the cis-acting responsive elements in their promoters. By using gene-walking technology, we obtained the promoters of lacc2, lacc6, ssPOXA3a, and ssPOXA3b. Among them, verified by sequence alignment, the promoter sequences of ssPOXA3b and ssPOXA3a were completely identical. Thus, only one of them was studied in the following experiments.
It was meaningful to perform cis-acting responsive element analysis on a laccase gene promoter with high expression and significant variation in different culture conditions. Online analysis was performed by the Berkeley Drosophila Genome Project (http://www.fruitfly.org/seq_tools/promoter.html) and PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). Three promoters (upstream 1000 bp of genes) of laccases (lacc2, ssPOXA3b, and lacc6) were determined to contain TATA box, CAAT box, xenobiotic response element (XRE), heat shock element (HSE), methyl jasmonate (MeJA) response element, stress response element (STRE), light response element (LRE), and many other functional elements (Table 1). These results indicated that all three promoters possessed inducible promoter characteristics. The transcription level of the promoter can be sensitively regulated by TFs under the influence of external stress or environmental changes (Piscitelli et al. 2011). Three promoters contained at least one TATA box and six CAAT boxes, which was a basic guarantee that the promoters were strong and efficient. Therefore, in monoculture, both lacc2 and lacc6 had high transcription levels.
The numbers of XRE (T/GCG/AT/CGC/G), MeJA response element (CGTCA-motif and TGACG-motif), MYBHv1 binding site (CCAAT-box), and circadian control element (CAAAGATATC) motifs in the promoters of lacc2 and ssPOXA3b were clearly greater than those in the lacc6 promoter. Among these elements, the most obvious difference focused on the XRE and the MeJA response elements. There were 6 XREs in the promoter of lacc2. Moreover, two promoters of upregulated laccases shared significant homology with TCGTGC/G, which provided a valuable binding motif for future research. The ssPOXA3b promoter was rich in MeJA response elements, having 400% as many as the lacc6 promoter. In addition, the Unnamed_4 response elements were also abundantly present in the promoters of two upregulated genes compared to the lacc6 promoter. However, the function of these elements remains unclear. Not all of the exogenous substance response elements in the promoters of two upregulated genes were abundant; these elements include a metal response element (MRE, TGCRCNC) and a gibberellin response element (GARE motif and TATC box). The amounts of these two elements did not have obvious differences in the three promoters.
Among the three promoters, the cis-acting response elements related to environmental factors, such as STRE (CCCCT) and LRE (Sp1, chs-Unit 1 ml, G-box, and Box 4), were not notably different quantitatively. Regarding these response elements, both lacc2 and lacc6 contained two STREs and contained no or only one LRE. This result indicated that lacc2 and lacc6 were relatively sensitive to environmental stress and that ssPOXA3b was relatively insensitive. In addition, the promoters of lacc2 and ssPOXA3b had few antioxidant response elements (ARE, TGACNNNGC). These results indicated that these two laccases did not have the ability to respond to oxidative stress. All three promoters contained at least eleven HSEs (TCNNGAAN), which meant that the laccase isoenzymes were sensitive to temperature changes with slight differences. With regard to competition between carbon and nitrogen sources, three promoters all had a certain number of Cre-A (SYGGRG) and NIT2 (TATCDH).
In summary, a relatively large number of XREs were found in the promoter of lacc2, which may be related to the metabolism and degradation of β-carotene, and MeJA response elements in the promoter of ssPOXA3b may be able to recruit TFs induced by co-culture. The other cis-acting responsive elements presented slight dissimilarity in quantity among the three promoters.
Transcription factors of DEGs
Some useful data for the P. ostreatus PC15 genomic information were obtained by bioinformatics analysis using Fungal Transcription Factor Database (http://ftfd.snu.ac.kr/intro.php). There are 379 TFs classified into 33 TF families in the genome of P. ostreatus PC15. The nucleic acid sequences encoding these 379 TFs were blasted with DEGs record by record, and finally, four TFs with significantly different expressions were obtained, namely, estExt_Genemark1. C_110352 (Cluster-1041.76581), estExt_Genemark1. C_020710 (Cluster-1041.20044), estExt_Genemark1. C_040625 (Cluster-1041.40661), and estExt_Genemark1. C_070839 (Cluster-1041.39592), abbreviated TFa, TFb, TFc, and TFd. Regarding the transcription levels of these four TFs (Fig. 5a), TFa and TFb were upregulated genes, and TFc and TFd were downregulated genes in co-culture.
Two kinds of domains (a) DNA binding domain and a transcriptional activation domain) of TFs were analysed by using GenomeNet Database Resources (https://www.genome.jp/) and JASPAR (http://jaspar.genereg.net/) (Fig. 5b–f). There are three zinc finger proteins (TFa, TFb, and TFd) and one basic region-leucine zipper protein (TFc). Among these proteins, TFa and TFd were C2H2-type, and TFb was CCHC-type.
TFa contained three C2H2-type zinc finger domains, one C3HC4-type zinc finger domain, and one double-stranded RNA-binding domain. TFb contained structural features of a CCHC-type zinc knuckle, a retrotransposon gag protein, and an aspartyl protease. TFc was a basic region-leucine zipper (bZIP). TFd contained two C2H2-type zinc finger domains. The discovery of these TFs enabled the regulation mechanism underlying the enhanced laccase transcription level in co-culture to be analysed.
Discussion
Fungal co-culture has gradually become an effective method for producing enzymes and metabolites. To favour laccase expression, many fungi were tentatively composed in pairs, and the laccase output showed notable improvement (Copete-Pertuz et al. 2019; Hu et al. 2019). However, determining the mechanism-inducing fungi interaction was exceedingly difficult. In this study, comparative transcriptomic analysis was performed to reveal the mechanisms for the enhancement of laccase production in co-culture of P. eryngii var. ferulae and R. mucilaginosa from the perspective of transcriptional regulation.
According to the transcriptomic analysis, the upregulated DEGs were mainly distributed in xenobiotic biodegradation and expenditure of energy groups in both co-culture and β-carotene culture. Similarly, the laccase activity and transcription levels were completely consistent in both cultivation modes. This result indicated that β-carotene was indeed a crucial xenobiotic produced by R. mucilaginosa. Clearly, the effect of co-culture cannot be fully achieved by simply adding β-carotene to the medium. There was more than one inducing factor, and there may be other compounds, such as amino acids and vitamins (Zengler and Zaramela 2018). The KEGG analysis indicated that the metabolic pathways of two aromatic amino acids (tyrosine and tryptophan) were both enhanced. Tryptophan was confirmed to be a significant inducer for promoting laccase expression in different fungi species (Zhong et al. 2019). Moreover, some mycotoxins, chemical signals, and other secondary metabolites have also been confirmed. For example, Aspergillus nidulans induces Streptomyces hygroscopicus to produce toxic aromatic polyketides during interspecific fungal interactions (Schroeckh et al. 2009). This finding also indicated that enhanced laccase activity may play an essential role in determining the detoxification of compounds transferred from another strain. Hence, co-culture of two fungi was a complex, interactive process, and β-carotene was one of the key factors that induced laccase production in P. eryngii var. ferulae.
Clearly, xenobiotic stimulation, nutrient competition, and oxidative stress were commonly recognized as the main reasons for promoting laccase expression in co-culture. However, in this co-culture system, the genes related to oxidative stress were generally downregulated. β-Carotene may play a crucial role in this downregulation. β-Carotene, which was metabolized by R. mucilaginosa, is an intracellular singlet oxygen quencher and has a strong antioxidation effect. It can effectively scavenge oxygen free radicals, reduce lipid peroxide damage, and maintain cell membrane structure and membranous fluidity (Darwish et al. 2018; Wang et al. 2014; Zhou et al. 2018). These functions are not available in other co-cultured paired strains. Therefore, β-carotene was observed to notably reduce the oxidative stress in P. eryngii var. ferulae cells. Meanwhile, the relatively loose structure of hyphal entanglement was another essential reason for the high laccase expression observed with this co-culture. The competition for nutrients and space was more intense for the co-culture of P. ostreatus and Trametes versicolor than for the co-culture in this study, as the hyphae of the former were closely intertwined and in contact, which increased the number of intracellular oxidation reactions (Zhong et al. 2017). However, for P. eryngii var. ferulae and R. mucilaginosa, the yeast did not send forth hyphae, and hyphal entanglement was improved, which probably resulted in downregulation of most genes related to oxidative stress. Moreover, the decrease of the mycelium ROS level in co-culture directly verified that oxidative stress was not responsible for enhancing laccase production in this co-culture system.
Most white rot fungi contain different kinds of laccase isoenzymes that have multiple functions to cope with a wide variety of environmental changes throughout the life cycle of fungi (Giardina and Sannia 2015). Thus, in response to the influence of yeast or xenobiotics, different laccase isoenzymes could exhibit varying regulation efficiency. Using the enzymatic activity and transcriptome data, it was not difficult to find that in the co-culture, the expression level of laccase was markedly improved. However, the changes in the transcription levels of these isoenzymes were not consistent. Lacc2 and two small subunits of laccase were upregulated synchronously, and lacc6 was downregulated, which substantially changed the main laccase isoenzyme. In β-carotene culture, the upregulation of transcription of two small subunits of laccase was not as significant as that of lacc2. This result indicated that β-carotene may regulate the transcription of only lacc2 and not ssPOXA3a and ssPOXA3b, which can explain why the addition of β-carotene to the medium did not achieve the increased level of laccase activity during co-culture.
Almost all laccase isoenzymes were monomeric or homodimeric proteins. However, a large subunit (lacc2, also named POXA3) and a small subunit (ssPOXA3a or ssPOXA3b) can constitute a heterodimer protein (Giardina et al. 2007). The amino acid sequence of lacc2 shared high homology with other reported laccase sequences, and the protein product formed disulfide bridges with four putative copper-binding residues and four Cys residues (Goudopoulou et al. 2010). Two small subunits were produced by the allelic gene as a result of the dikaryotic state of P. eryngii var. ferulae (Rivera-Hoyos et al. 2015). According to qPCR results, the transcription level of the two genes was distinctly upregulated under the condition of co-culture. In fact, the large subunit would lose its native structure quickly in the absence of the small subunit, which decreased laccase activity and stability (Faraco et al. 2008). In general, a significant enhancement in the secretion of ssPOXA3a and ssPOXA3b played a crucial role in the increase in laccase activity in fermentation broth.
From the perspective of transcriptional regulation, in co-culture, the different transcriptional levels of lacc2, ssPOXA3a, and lacc6 were related to cis-acting responsive elements contained in their promoters. There are 6 XREs in the promoter of lacc2 and 4 MeJA response elements in the promoter of ssPOXA3a. XREs are responsible for recognition of exogenous environmental pollutants in eukaryotic cells (Fan et al. 2011; Janusz et al. 2013; Kuramoto et al. 2002). In Trametes sp., 7 XREs and 2 XREs were identified in the promoter of lacA and lacB, respectively, and can respond to different aromatic compounds (Xiao et al. 2006). MeJA acts as a signal molecule to activate plant defence mechanisms for resistance to plant disease (Rivas-San Vicente and Plasencia 2011). These elements often require a combination of TFs to regulate the transcriptional level of laccase.
Referring to the P. ostreatus PC15 genomic information, 4 TFs were obtained by blasting all DEGs with 379 TFs. In addition to TFs in Saccharomyces cerevisiae, few TFs in fungi have been verified experimentally. MYB genes were determined to be indispensable in spore formation and development in P. ostreatus (Wang et al. 2018). The transcription levels of TFb, TFc, and TFd showed the same trend in co-culture and β-carotene culture compared with the control. These results indicated that these three TFs may be related to the induction by β-carotene, although TFb and TFc had no homologous proteins in JASPAR database.
According to JASPAR database, TFa is homologous to the blmp-1 protein from Caenorhabditis elegans. Blmp-1 acts as a transcriptional activator and directly and positively regulates the bed-3 gene (Yang et al. 2015). Blmp-1 also cooperates with lin-40 to regulate stress-specific developmental adaptations (Hyun et al. 2016). The expected conserved binding site for TFa is A(G)A(G)A(G)A(G)G(A/T)T(A/G/C)GAA(G/T)A(G/T)A(G/C/T) (Niu et al. 2011). The promoter of lacc2 had two potential binding motifs, AAGGCTGATGA (− 850, − 840) and AGAACGGATGC (− 754, − 744), containing a part of the MeJA response element (− 842, − 838) and a HSE (− 754, − 750), respectively. These results indicated that TFa may bind with these two specific elements, leading to the enhancement of lacc2 transcription levels. Clearly, TFa was significantly upregulated in only co-culture, which revealed that some inducing factors other than β-carotene can elevate laccase production.
TFd is homologous with the MIG1 protein from Saccharomyces cerevisiae. The expected conserved binding site is C(A/T/G)CCCCG(A/C/T)C(A/TG) (Badis et al. 2008). The transcription factor MIG1 in yeast is related to the strength of biofilms. The deletion of MIG1 weakened biofilms during immobilized fermentation, resulting in a decline in cell-to-cell adhesion (flocculation ability) and a slight enhancement of cell-substrate adhesion (invasive growth ability) (Yang et al. 2018). For filamentous fungi in submerged fermentation, loose hyphae are beneficial to the expression of laccase in P. eryngii var. ferulae (Morin et al. 2012; Pollegioni et al. 2015). Meanwhile, MIG1 in yeast can improve tolerance towards weak monocarboxylic acids from lignocellulosic hydrolysates and formation of weakened biofilms during immobilized fermentation (Balderas-Hernandez et al. 2018).
In this study, by comparing the transcriptome data of monoculture, co-culture, and β-carotene culture, β-carotene was confirmed to be an essential substance for promoting laccase expression, possibly functioning through stimulating TFb, TFc, and TFd to regulate the transcription level of laccase in P. eryngii var. ferulae. Clearly, there is not only one inducing factor, and there may be other substances related to TFa, such as amino acids, vitamins, and MeJA (Zengler and Zaramela 2018). In addition, the regulatory mechanism of TFd revealed a loose adhesion of fungus to fungus, which was also one of the important methods for enhancing the yield of laccase.
Complicated interactions are almost universal among microorganisms in nature. This co-culture system of P. eryngii var. ferulae and R. mucilaginosa used two mechanisms for enhancing the expression of laccase, mainly about xenobiotics and energy expenditure, rather than oxidation stress. Meanwhile, the transcription levels of laccase isoenzymes were all determined by RT-qPCR. Two small subunits of laccase (ssPOXA3a and ssPOXA3b), which enhanced laccase activity and stability, were markedly upregulated. As the study of TFs in white rot fungi is still in its infancy, an in vitro electrophoretic mobility shift assay (EMSA) experiment will need to be applied to further reveal the regulatory mechanism. These studies have great significance for elucidating the regulation mechanism of laccase, optimizing the co-culture system, expanding laccase production, and improving lignin biorefinery efficiency.
References
Alfaro M, Castanera R, Lavín JL, Grigoriev IV, Oguiza JA, Ramírez L, Pisabarro AG (2016) Comparative and transcriptional analysis of the predicted secretome in the lignocellulose-degrading basidiomycete fungus Pleurotus ostreatus. Environ Microbiol 18(12):4710–4726. https://doi.org/10.1111/1462-2920.13360
Asina F, Brzonova I, Voeller K, Kozliak E, Kubatova A, Yao B, Ji Y (2016) Biodegradation of lignin by fungi, bacteria and laccases. Bioresour Technol 220:414–424. https://doi.org/10.1016/j.biortech.2016.08.016
Badis G, Chan ET, van Bakel H, Pena-Castillo L, Tillo D, Tsui K, Carlson CD, Gossett AJ, Hasinoff MJ, Warren CL, Gebbia M, Talukder S, Yang A, Mnaimneh S, Terterov D, Coburn D, Li Yeo A, Yeo ZX, Clarke ND, Lieb JD, Ansari AZ, Nislow C, Hughes TR (2008) A library of yeast transcription factor motifs reveals a widespread function for Rsc3 in targeting nucleosome exclusion at promoters. Mol Cell 32(6):878–887. https://doi.org/10.1016/j.molcel.2008.11.020
Balderas-Hernandez VE, Correia K, Mahadevan R (2018) Inactivation of the transcription factor mig1 (YGL035C) in Saccharomyces cerevisiae improves tolerance towards monocarboxylic weak acids: acetic, formic and levulinic acid. J Ind Microbiol Biotechnol 45(8):735–751. https://doi.org/10.1007/s10295-018-2053-1
Castanera R, Perez G, Omarini A, Alfaro M, Pisabarro AG, Faraco V, Amore A, Ramírez L (2012) Transcriptional and enzymatic profiling of Pleurotus ostreatus laccase genes in submerged and solid-state fermentation cultures. Appl Environ Microbiol 78(11):4037–4045. https://doi.org/10.1128/AEM.07880-11
Castanera R, López-Varas L, Pisabarro AG, Ramírez L (2015) Validation of reference genes for transcriptional analyses in Pleurotus ostreatus by using reverse transcription-quantitative PCR. Appl Environ Microbiol 81(12):4120–4129. https://doi.org/10.1128/AEM.00402-15
Copete-Pertuz LS, Alandete-Novoa F, Placido J, Correa-Londono GA, Mora-Martinez AL (2019) Enhancement of ligninolytic enzymes production and decolourising activity in Leptosphaerulina sp. by co-cultivation with Trichoderma viride and Aspergillus terreus. Sci Total Environ 646:1536–1545. https://doi.org/10.1016/j.scitotenv.2018.07.387
Darwish WS, Ikenaka Y, Nakayama S, Mizukawa H, Thompson LA, Ishizuka M (2018) beta-Carotene and retinol reduce benzo[a]pyrene-induced mutagenicity and oxidative stress via transcriptional modulation of xenobiotic metabolizing enzymes in human HepG2 cell line. Environ Sci Pollut Res Int 25(7):6320–6328. https://doi.org/10.1007/s11356-017-0977-z
Du W, Sun C, Wang J, Xie W, Wang B, Liu X, Zhang Y, Fan Y (2017) Conditions and regulation of mixed culture to promote Shiraia bambusicola and Phoma sp. BZJ6 for laccase production. Sci Rep 7(1):17801. https://doi.org/10.1038/s41598-017-17895-w
Fan F, Zhuo R, Sun S, Wan X, Jiang M, Zhang X, Yang Y (2011) Cloning and functional analysis of a new laccase gene from Trametes sp. 48424 which had the high yield of laccase and strong ability for decolorizing different dyes. Bioresour Technol 102(3):3126–3137. https://doi.org/10.1016/j.biortech.2010.10.079
Faraco V, Ercole C, Festa G, Giardina P, Piscitelli A, Sannia G (2008) Heterologous expression of heterodimeric laccase from Pleurotus ostreatus in Kluyveromyces lactis. Appl Microbiol Biotechnol 77(6):1329–1335. https://doi.org/10.1007/s00253-007-1265-5
Fischer CN, Trautman EP, Crawford JM, Stabb EV, Handelsman J, Broderick NA (2017) Metabolite exchange between microbiome members produces compounds that influence Drosophila behavior. Elife 6:e18855. https://doi.org/10.7554/eLife.18855
Flores C, Vidal C, Trejo-Hernandez MR, Galindo E, Serrano-Carreon L (2009) Selection of Trichoderma strains capable of increasing laccase production by Pleurotus ostreatus and Agaricus bisporus in dual cultures. J Appl Microbiol 106(1):249–257. https://doi.org/10.1111/j.1365-2672.2008.03998.x
Giardina P, Sannia G (2015) Laccases: old enzymes with a promising future. Cell Mol Life Sci 72(5):855–856. https://doi.org/10.1007/s00018-014-1821-y
Giardina P, Autore F, Faraco V, Festa G, Palmieri G, Piscitelli A, Sannia G (2007) Structural characterization of heterodimeric laccases from Pleurotus ostreatus. Appl Microbiol Biotechnol 75(6):1293–1300. https://doi.org/10.1007/s00253-007-0954-4
Goudopoulou A, Krimitzas A, Typas MA (2010) Differential gene expression of ligninolytic enzymes in Pleurotus ostreatus grown on olive oil mill wastewater. Appl Microbiol Biotechnol 88(2):541–551. https://doi.org/10.1007/s00253-010-2750-9
Grabherr M, Haas B, Yassour M, Levin J, Thompson D, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, Palma F, Birren B, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652. https://doi.org/10.1038/nbt.1883
Guo C, Zhao L, Wang F, Lu J, Ding Z, Shi G (2017) beta-Carotene from yeasts enhances laccase production of Pleurotus eryngii var. ferulae in co-culture. Front Microbiol 8:1101. https://doi.org/10.3389/fmicb.2017.01101
Hailei W, Guangli Y, Ping L, Yanchang G, Jun L, Guosheng L, Jianming Y (2009) Overproduction of Trametes versicolor laccase by making glucose starvation using yeast. Enzym Microb Tech 45(2):146–149. https://doi.org/10.1016/j.enzmictec.2009.04.003
Hoegger PJ, Kilaru S, James TY, Thacker JR, Kües U (2006) Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J 273(10):2308–2326. https://doi.org/10.1111/j.1742-4658.2006.05247.x
Hou H, Zhou J, Wang J, Du C, Yan B (2004) Enhancement of laccase production by Pleurotus ostreatus and its use for the decolorization of anthraquinone dye. Process Biochem 39(11):1415–1419. https://doi.org/10.1016/s0032-9592(03)00267-x
Hu J, Zhang Y, Xu Y, Sun Q, Liu J, Fang W, Xiao Y, Kües U, Fang Z (2019) Gongronella sp. w5 elevates Coprinopsis cinerea laccase production by carbon source syntrophism and secondary metabolite induction. Appl Microbiol Biotechnol 103(1):411–425. https://doi.org/10.1007/s00253-018-9469-4
Hyun M, Kim J, Dumur C, Schroeder FC, You YJ (2016) BLIMP-1/BLMP-1 and metastasis-associated protein regulate stress resistant development in Caenorhabditis elegans. Genetics 203(4):1721–1732. https://doi.org/10.1534/genetics.116.190793
Janusz G, Kucharzyk KH, Pawlik A, Staszczak M, Paszczynski AJ (2013) Fungal laccase, manganese peroxidase and lignin peroxidase: gene expression and regulation. Enzym Microb Technol 52(1):1–12. https://doi.org/10.1016/j.enzmictec.2012.10.003
Kang G, Li G, Ma H, Wang C, Guo T (2013) Proteomic analysis on the leaves of TaBTF3 gene virus-induced silenced wheat plants may reveal its regulatory mechanism. J Proteome 83:130–143. https://doi.org/10.1016/j.jprot.2013.03.020
Kües U, Rühl M (2011) Multiple multi-copper oxidase gene families in basidiomycetes – What for? Current Genomics 12(2):72–94. https://doi.org/10.2174/138920211795564377
Kuramoto N, Goto E, Masamune Y, Gion K, Yoneda Y (2002) Existence of xenobiotic response element binding in Dictyostelium. Biochim Biophys Acta 1578(1-3):1–11. https://doi.org/10.1016/S0167-4781(02)00449-9
Li B, Dewey CN (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinforma 12(1):323–323. https://doi.org/10.1186/1471-2105-12-323
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Luo F, Zhong Z, Liu L, Igarashi Y, Xie D, Li N (2017) Metabolomic differential analysis of interspecific interactions among white rot fungi Trametes versicolor, Dichomitus squalens and Pleurotus ostreatus. Sci Rep 7(1):5265. https://doi.org/10.1038/s41598-017-05669-3
Lv Y, Chen Y, Sun S, Hu Y (2014) Interaction among multiple microorganisms and effects of nitrogen and carbon supplementations on lignin degradation. Bioresour Technol 155:144–151. https://doi.org/10.1016/j.biortech.2013.12.012
Morin E, Kohler A, Baker AR, Foulongne-Oriol M, Lombard V, Nagy LG, Ohm RA, Patyshakuliyeva A, Brun A, Aerts AL, Bailey AM, Billette C, Coutinho PM, Deakin G, Doddapaneni H, Floudas D, Grimwood J, Hilden K, Kües U, Labutti KM, Lapidus A, Lindquist EA, Lucas SM, Murat C, Riley RW, Salamov AA, Schmutz J, Subramanian V, Wösten HA, Xu J, Eastwood DC, Foster GD, Sonnenberg AS, Cullen D, de Vries RP, Lundell T, Hibbett DS, Henrissat B, Burton KS, Kerrigan RW, Challen MP, Grigoriev IV, Martin F (2012) Genome sequence of the button mushroom Agaricus bisporus reveals mechanisms governing adaptation to a humic-rich ecological niche. Proc Natl Acad Sci U S A 109(43):17501–17506. https://doi.org/10.1073/pnas.1206847109
Niu W, Lu ZJ, Zhong M, Sarov M, Murray JI, Brdlik CM, Janette J, Chen C, Alves P, Preston E, Slightham C, Jiang L, Hyman AA, Kim SK, Waterston RH, Gerstein M, Snyder M, Reinke V (2011) Diverse transcription factor binding features revealed by genome-wide ChIP-seq in C. elegans. Genome Res 21(2):245–254. https://doi.org/10.1101/gr.114587.110
Piscitelli A, Giardina P, Lettera V, Pezzella C, Sannia G, Faraco V (2011) Induction and transcriptional regulation of laccases in fungi. Current Genomics 12(2):104–112. https://doi.org/10.2174/138920211795564331
Pollegioni L, Tonin F, Rosini E (2015) Lignin-degrading enzymes. FEBS J 282(7):1190–1213. https://doi.org/10.1111/febs.13224
Qi-He C, Krugener S, Hirth T, Rupp S, Zibek S (2011) Co-cultured production of lignin-modifying enzymes with white-rot fungi. Appl Biochem Biotechnol 165(2):700–718. https://doi.org/10.1007/s12010-011-9289-9
Ramírez DFG, Vázquez RR, Pérez PA, Bustillos LGT, López RA, Horcasitas MCM, García FJE (2018) Crude enzymatic extract from Trametes maxima and Paecilomyces carneus mixed culture entrapped on alginate for phenanthrene removal in water. Environ Eng Sci 35(10):1126–1135. https://doi.org/10.1089/ees.2018.0064
Rivas-San Vicente M, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62(10):3321–3338. https://doi.org/10.1093/jxb/err031
Rivera-Hoyos CM, Morales-Alvarez ED, Poveda-Cuevas SA, Reyes-Guzman EA, Poutou-Pinales RA, Reyes-Montano EA, Pedroza-Rodriguez AM, Rodriguez-Vazquez R, Cardozo-Bernal AM (2015) Computational analysis and low-scale constitutive expression of laccases synthetic genes GlLCC1 from Ganoderma lucidum and POXA 1B from Pleurotus ostreatus in Pichia pastoris. PLoS One 10(1):e0116524. https://doi.org/10.1371/journal.pone.0116524
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1):139–140. https://doi.org/10.1093/bioinformatics/btp616
Schroeckh V, Scherlach K, Nützmann H, Shelest E, Schmidt-Heck W, Schuemann J, Martin K, Hertweck C, Brakhage AA (2009) Intimate bacterial–fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc Natl Acad Sci USA 106(34):14558–14563. https://doi.org/10.1073/pnas.0901870106
Sharma A, Jain KK, Jain A, Kidwai M, Kuhad RC (2018) Bifunctional in vivo role of laccase exploited in multiple biotechnological applications. Appl Microbiol Biotechnol 102(24):10327–10343. https://doi.org/10.1007/s00253-018-9404-8
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28(5):511–515. https://doi.org/10.1038/nbt.1621
Wang H, Yu G, Li P, Gu Y, Li J, Liu G, Yao J (2009) Overproduction of Trametes versicolor laccase by making glucose starvation using yeast. Enzym Microb Technol 45(2):146–149. https://doi.org/10.1016/j.enzmictec.2009.04.003
Wang HJ, Liang R, Fu LM, Han RM, Zhang JP, Skibsted LH (2014) Nutritional aspects of beta-carotene and resveratrol antioxidant synergism in giant unilamellar vesicles. Food Funct 5(7):1573–1578. https://doi.org/10.1039/c4fo00225c
Wang H, Peng L, Ding Z, Wu J, Shi G (2015) Stimulated laccase production of Pleurotus ferulae JM301 fungus by Rhodotorula mucilaginosa yeast in co-culture. Process Biochem 50(6):901–905. https://doi.org/10.1016/j.procbio.2015.03.004
Wang L, Gao W, Wu X, Zhao M, Qu J, Huang C, Zhang J (2018) Genome-wide characterization and expression analyses of Pleurotus ostreatus MYB transcription factors during developmental stages and under heat stress based on de novo sequenced genome. Int J Mol Sci 19(7):2052. https://doi.org/10.3390/ijms19072052
Wei F, Hong Y, Liu J, Yuan J, Fang W, Peng H, Xiao Y (2010) Gongronella sp. induces overproduction of laccase in Panus rudis. J Basic Microbiol 50(1):98–103. https://doi.org/10.1002/jobm.200900155
Xiang Y, Karaveg K, Moremen KW (2016) Substrate recognition and catalysis by GH47 alpha-mannosidases involved in Asn-linked glycan maturation in the mammalian secretory pathway. Proc Natl Acad Sci U S A 113(49):E7890–E7899. https://doi.org/10.1073/pnas.1611213113
Xiao YZ, Hong YZ, Li JF, Hang J, Tong PG, Fang W, Zhou CZ (2006) Cloning of novel laccase isozyme genes from Trametes sp. AH28-2 and analyses of their differential expression. Appl Microbiol Biotechnol 71(4):493–501. https://doi.org/10.1007/s00253-005-0188-2
Yang J, Fong HT, Xie Z, Tan JW, Inoue T (2015) Direct and positive regulation of Caenorhabditis elegans bed-3 by PRDM1/BLIMP1 ortholog BLMP-1. Biochim Biophys Acta 1849(9):1229–1236. https://doi.org/10.1016/j.bbagrm.2015.07.012
Yang L, Zheng C, Chen Y, Ying H (2018) FLO genes family and transcription factor MIG1 regulate Saccharomyces cerevisiae biofilm formation during immobilized fermentation. Front Microbiol 9:1860. https://doi.org/10.3389/fmicb.2018.01860
Zengler K, Zaramela LS (2018) The social network of microorganisms - how auxotrophies shape complex communities. Nat Rev Microbiol 16(6):383–390. https://doi.org/10.1038/s41579-018-0004-5
Zhong Z, Li L, Chang P, Xie H, Zhang H, Igarashi Y, Li N, Luo F (2017) Differential gene expression profiling analysis in Pleurotus ostreatus during interspecific antagonistic interactions with Dichomitus squalens and Trametes versicolor. Fungal Biol 121(12):1025–1036. https://doi.org/10.1016/j.funbio.2017.08.008
Zhong Z, Li L, He B, Igarashi Y, Luo F (2019) Transcriptome analysis of differential gene expression in Dichomitus squalens during interspecific mycelial interactions and the potential link with laccase induction. J Microbiol 57(2):127–137. https://doi.org/10.1007/s12275-019-8398-y
Zhou L, Ouyang L, Lin S, Chen S, Liu Y, Zhou W, Wang X (2018) Protective role of beta-carotene against oxidative stress and neuroinflammation in a rat model of spinal cord injury. Int Immunopharmacol 61:92–99. https://doi.org/10.1016/j.intimp.2018.05.022
Acknowledgments
Funding for this study was provided by the National Natural Science Foundation of China (31571822) and the Science and Technology Project of Jiangsu Province (social development category, BE2017683). This study was also sponsored by the National First-Class Discipline Programme of Light Industry Technology and Engineering (LITE2018-22).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
This research did not involve any human participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 765 kb)
Rights and permissions
About this article
Cite this article
Zhang, Q., Zhao, L., Li, Y. et al. Comparative transcriptomics and transcriptional regulation analysis of enhanced laccase production induced by co-culture of Pleurotus eryngii var. ferulae with Rhodotorula mucilaginosa. Appl Microbiol Biotechnol 104, 241–255 (2020). https://doi.org/10.1007/s00253-019-10228-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10228-z