Abstract
Laccases are multicopper enzymes present in plants, fungi, bacteria, and insects, which catalyze oxidation reactions together with four electron reduction of oxygen to water. Plant, bacterial, and insect laccases have a polymerizing role in nature, implicated in biosynthesis of lignin, melanin formation, and cuticle hardening, respectively. On the other hand, fungal laccases carry out both polymerizing (melanin synthesis and fruit body formation) as well as depolymerizing roles (lignin degradation). This bifunctionality of fungal laccases can be attributed to the presence of multiple isoforms within the same as well as different genus and species. Interestingly, by manipulating culture conditions, these isoforms with their different induction patterns and unique biochemical characteristics can be expressed or over-expressed for a targeted biotechnological application. Consequently, laccases can be considered as one of the most important biocatalyst which can be exploited for divergent industrial applications viz. paper pulp bleaching, fiber modification, dye decolorization, bioremediation as well as organic synthesis. The present review spotlights the role of fungal laccases in various antagonistic applications, i.e., polymerizing and depolymerizing, and co-relating this dual role with potential industrial significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
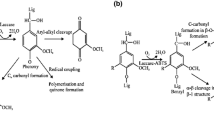
Laccases are polyphenol multicopper oxidases which catalyze oxidation of various phenols and anilines with the concomitant reduction of molecular oxygen to water (Thurston 1994; Solomon et al. 1996). The enzyme is ubiquitous in nature being found in plants (Berthret et al. 2012), fungi (Baldrian 2006), bacteria (Claus 2003), insects (Dittmer and Kanost 2010) as well as lichens (Lisov et al. 2007) and sponges (Li et al. 2015). The genes that encode for laccases belong to members of a multi-gene family with different isozymes expressed in different space and time (Gianfreda et al. 1999; Kumar et al. 2017). The presence of multiple laccase isoforms explains the diverse and multiple functions of this enzyme within same as well as diverse species (Sharma and Kuhad 2008). In plants, laccases are involved in lignin and polyflavanoid synthesis (Ranocha et al. 2002; Liang et al. 2006), while, in fungi, they have a role in delignification (Eggert et al. 1996), fruit body formation (Zhang et al. 2015, pigmentation (Eisenman et al. 2007), and pathogenesis (Zhu and Williamson 2004). In insects, the function of laccases is sclerotization of cuticle (Gorman et al. 2012) and in bacteria they regulate copper homeostasis, morphogenesis, melanization (Claus 2003), and pathogenesis (Singh et al. 2016). While in lichens, laccases are considered to carry out metabolism of lichen acids and other phenols (Lisov et al. 2007). In sponges, laccase is involved in the antibacterial defense of the sponge organism (Li et al. 2015). Therefore, it can be seen that in vivo function of laccases is synthetic in all the organisms except in fungi (Fig. 1), where they carry out both synthetic (fruit body formation, pigment synthesis) and degradative roles (lignin degradation). In all laccase-mediated catalysis, reaction begins with single electron oxidation of substrate to corresponding radicals, which can then subsequently either repolymerize or lead to depolymerization of the substrate (Madhavi and Lele 2009; Jones and Solomon 2015) depending on the reaction conditions, metabolism and half-life of the radicals as well as enzymes redox potential (Jeon and Chang 2013). The polymerizing function of the enzyme occurs by oxidative coupling of the substrate producing dimers and polymers. On the other hand, the depolymerizing role of fungal laccase owe to their higher redox potential (Fig. 2), enabling them to oxidize lignin with the help of small molecular weight mediators (Jeon et al. 2012). It is suggested that parallel polymerization and depolymerization reactions compete during treatment with laccase as phenolic groups in lignin serves as sites for lignin polymerization, which in turn obstruct ligninolysis (Srebotnik and Hammel 2000). Therefore, in order to exploit the depolymerizing role of fungal laccases, various natural/ synthetic mediators are added to the reaction mixture, as these mediators act as diffusible electron carriers which enhance substrate conversion (Mate and Alcalde 2016). Synthetic laccase mediators [2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)], 1-hydroxybenzotriazole, and violuric acid (VA) in conjunction with laccase, called as laccase mediator system (LMS) have been demonstrated to effectively enhance the degradative role of the enzyme (Baldrian 2006). Furthermore, instead of using the abovementioned synthetic mediators, compounds involved in the natural degradation of lignin by fungi can also be used for depolymerizing application of laccase as these natural mediators have an added advantages of being cost effective and eco-friendly (Camarero et al. 2005). These include p-coumaric acid, vanillin, acetovanillone, methyl vanillate, phenol, aniline, 4-hydroxybenzoic acid, and 4-hydroxybenzyl alcohol syringaldehyde and acetosyringone (Johannes and Majcherczyk 2000; Camarero et al. 2005).
Mechanism of action of laccase isozymes (*Lac B has higher redox potential than Lac A). Many organics can be converted to their corresponding radicals by laccase-mediated catalysis. These radicals per se can then undergo synthetic or degradative processes. For instance, a Lac B with higher redox potential can cause oxidative bond cleavage of substrate, while Lac A with lower redox potential will cause condensation of targets. b However, in presence of redox mediators, Lac A can also carry out depolymerization of end products. c The inducible nature of laccase isoforms can result in overexpression of a particular isoform, and thus increased catalytic activity
Nonetheless, the in vivo bifunctionality of laccases is also incurred to the presence of numerous laccase isozymes with different induction patterns as well as unique biochemical properties (Fig. 2). Most white rot fungi produce more than one laccase isozyme that differ in the degree of glycosylation, amino acid sequence, molecular weight, pI, and substrate specificity (Mansur et al. 2003; Kumar et al. 2017). As a result, diverse functions of laccase isozymes is dependent on cell type and intra- or extra-cellular conditions in which it gets expressed. Isoforms expressed in the lag or logarithmic phase of fungal fermentation are mainly drawn in degradation of substrate, while the ones detected in the stationary phase are related to pigmentation and morphogenesis (Lettera et al. 2010). The effect of metal ions, phenolic compounds, nutrient nitrogen and carbon are also critical in the expression of a particular laccase isoform (Piscitelli et al. 2011). Some of the isoforms are constitutively expressed, while others are inducible (Soden and Dobson 2001). In this regard, positive induction by nitrogen sources has been reported for two isozymes of laccase (lac2 and lac4) from Pleurotus sajor-caju, while the expression of other two (lac1 and lac3) are not affected (Soden and Dobson 2001). While, Galhaup and co-workers (Galhaup et al. 2002) observed that Trametes pubescens secrete eight laccase isoforms, with LAP2 being majorly induced in the presence of copper ions in the media. On the other hand, D’souza et al. (2004) found out that two laccase isoforms (lcc1 and lcc2) from Pleurotus pulmonarius are produced in non-induced cultures after the depletion of carbon and nitrogen sources, while the other two (lcc3 and lcc4) were detected in cultures induced by various phenolic and aromatic compounds related to lignin and its derivatives, with maximum stimulation with ferulic acid and vanillin. In another study, different electrophoretic profiles of laccase isoforms were observed from Ganoderma lucidum in the presence of phenolic and metallic inducers (Kuhar and Papinutti 2014). Similarly, three laccase isoforms were characterized from Steccherinum ochraceum with unusual and different biochemical properties (Chernykh et al. 2008). Furthermore, the three laccase isozymes (LacI, LacII, and LacIII) were purified and characterized from white rot fungus, Trametes sp. HS-03 with different PIs and thermostabilities. While, LacI and LacII showed similar thermo stability, LacIII showed better thermostability (Guo et al. 2012). Likewise, three laccase isozymes (Lac-2, Lac-3, and Lac-4) with different pH stabilities and thermostabilities were purified from the white rot fungus Ganoderma sp. En3 (He et al. 2014). Among the three isolated isonzymes, Lac-2 showed stronger pH stability and thermostability than the other two isoforms. Furthermore, it was found that Lac-2 had a stronger ability to tolerate metal ions and organic solvents compared with the other two isoenzymes. The authors also deciphered a positive synergistic effect of the three isozymes on decolorization of azo dyes, with Lac-3 alone having a negligible effect on dye decolorization but when combined with the other two isoenzymes Lac-2 or Lac-4, enhanced their decolorization potential against the tested dyes (He et al. 2014). Very interestingly, Kumar et al. (2017) observed differential expression of laccase isozymes from Ganoderma strains, G. lucidum MDU-7, and Ganoderma sp. kk-02 in the presence of diverse aromatic compounds and metal salts. While, O-toluidine induced isozyme production from G. lucidum MDU-7, it did not have any effect on isozyme pattern of Ganoderma sp. kk-02. On the other hand, copper and tannic acid induced laccase production from both the strains. Thus, Kumar and co-workers (Kumar et al. 2017) concluded that the species-specific action of different aromatic compounds on the production of laccase isozymes might be due to differences in ecological habitat, which eventually helps in adaptation of the fungus. However, actual mechanism and purposes of temporal and substrate specific laccase isozyme secretion is unknown and therefore needs to be scientifically elaborated and ecologically related. In contrast, structural studies of two laccase isoforms from Pycnoporous sanguineus were performed by Orlikowska et al. (2018) and significant differences were found in their substrate binding pockets, thermal and pH stabilities as well as tolerance against inhibitors. Therefore, it can be concluded that by manipulating culture conditions or adding a specific inducer, a particular laccase isozyme targeted for an application can be expressed or over-expressed (Fig. 2). The approach can also be exploited for increasing total laccase activity in the culture medium, bypassing need of recombinant gene expression. Likewise, two laccase isozymes (Lacc1 and Lacc2) from Agaricus bisporus were purified and characterized. While, Lacc1 was found to be thermostable (retaining 80% activity at 60 °C after 90 min), Lacc2 was alkali stable (retaining 93% activity at pH 9.0). Further, the activity of both the isozymes was differently affected by metal ions and the decolorizing activity was also found to be different with Lacc2 more superior in terms of decolorization of Acid blue dye solution (Othman et al. 2018). From the application point of view, co-relating the in vivo duplicate role of enzyme (polymerizing and depolymerizing roles) to the presence of inducible isoforms, the enzyme can be used for divergent biotechnological processes viz. pulp bleaching, fiber modification, dye decolorization, and organic synthesis (Fig. 3). For instance, the lignin polymerization function of laccases can be used for in vitro polymerization of lignocellulosic materials and also for grafting of phenolic compounds onto pulp fibers producing boards with improved properties (Schubert et al. 2015). In contrast, the depolymerizing action of the enzyme can be used for the delignification of wood pulp for paper making (Sharma et al. 2005) with the mediation of synthetic mediators, thereby sinking the consumption of toxic chemicals used for the same purpose. The degradative role of the enzyme can also be exploited for oxidative bond cleavage of toxic xenobiotic compounds (Yang et al. 2017) and synthetic dyes (Vantamuri and Kaliwal 2016) structurally related to lignin and its derivatives. On the other hand, the polymerizing action of the enzyme can be used for polymerization of pollutants which can then be subsequently removed by filtration/sedimentation (Steevensz et al. 2012). In organic synthesis, the polymerization of same or different substrates by laccase result in the formation of homo and heterodimers, respectively, for the production of new antibiotic derivatives and complex products with enhanced physiological properties (Wellington et al. 2013). The present review discusses the synthetic and degradative in vitro applications of laccase viz. paper pulp bleaching and pulp fiber modification in the pulp and paper industry, bioremediation, dye decolorization, and organic synthesis.
Applications of laccase (polymerizing and depolymerizing role)
Organic synthesis (polymerizing role)
Oxidation reactions are an essential part of organic synthesis but the conventional oxidation technologies use environmentally toxic chemicals such as chromium compounds, permanganate, manganese dioxide, and periodate and produce non-specific and undesirable side-reactions (Kidwai et al. 2012b). The growing public concern over the use of such hazardous chemicals has provoked search for new oxidation technologies based on biological systems such as enzymatic oxidation (Sanchez and Demain 2011). In this regard, laccases are of great interest as an enzyme which oxidizes a wide variety of phenolic and aromatic compounds using oxygen from the environment and producing water as the sole by-product, thereby ideal for future sustainable green chemistry (Kidwai et al. 2012b). Since fungal laccases have higher redox potential than laccases from other species, they are more fitted to perform the polymerizing role in organic synthesis (Christopher et al. 2014). For example, laccases secreted by the white rot fungi Trametes versicolor and Neurospora crassa have a high redox potential of 0.78–0.80 V, whereas the redox potential of laccases from the plant, Rhus vernicifera is only 0.42 V (Mikolasch and Schauer 2009). Table 1 lists some fungal laccases used by many research groups for organic synthesis. Furthermore, the application of laccases in the production of pharmaceutically important moieties, synthesis of compounds with increased antioxidant potential, and various valuable polymers is discussed below:
Production of compounds with improved antioxidant potential
Reactive oxygen species (ROS) are produced in the human body as by-products of normal metabolism and are also reduced by the human defense system comprising of glutathione and other thiols. However, in case of oxidative stress, there is an imbalance between ROS formation and cellular antioxidant capacity, leading to the development of various neuro-generative disorders. In such cases, supplementation with external antioxidant agents is needed (Chen et al. 2012). Unfortunately, the clinically effective antioxidant drugs are scarce. As a result, novel compounds are being constantly synthesized chemically and evaluated for their increased antioxidant potential (Gupta et al. 2012; Apotrosoaei et al. 2014). Phenyl propanoid acids and flavonoids (polyphenols) are strong antioxidants that protects from oxidative stress caused by surplus ROS. However, the position and stereo-electronic properties of the substituents on the aromatic rings of polyphenols as well as the presence of catechol and pyrogallol pharmacophores tune the antioxidant effect of these compounds (Botta et al. 2017). Therefore, the oxidative effect of laccases on phenols can further enhance their antioxidant potential. In this regard, laccase-catalyzed polymerization of quercetin as well as of kampeferol was performed separately to produce aggregates with higher antioxidant properties than the monomeric quercetin and kampeferol (Desentis-Mendoza et al. 2006). While, silybin dimers were successfully prepared by laccase-catalyzed polymerization of silybin derivatives (Gažák et al. 2008). Silybin dimers are commonly used in the treatment of liver dysfunction, as a hepatoprotectant and also as an antioxidant (Agarwal et al. 2006). In another reaction, conjugate of catechin (poly(allylamine)) was synthesized by the polymerization of catechins present in green tea and was found to have improved properties compared to the unconjugated catechin in terms of antioxidant potential (Gogoi et al. 2010). Nemadziva and co-workers (Nemadziva et al. 2018) synthesized a β-β caffeic acid dimer, phellinsin A, using laccase-mediated catalysis and found that the dimer had almost 1.8-fold higher antioxidant property compared to caffeic acid. Fascinatingly, three dimers were synthesized from 2,6-dimethoxyphenol using Botryosphaeria rhodina laccase under different reaction conditions (pH and reaction times) and it was found that dimer II, 3,3′,5,5′-tetramethoxy-biphenyl-4,4′-diol (TMBP), being synthesized at pH 6.5 in 120 h had high antioxidant activity, similar to the commercial standard, butyl hydroxytoluene (Schirmann et al. 2018). The authors also indicated that TMBP can be used as an alternative antioxidant to stabilize biodiesel (Schirmann et al. 2018).
Production of pharmaceutically and industrially important compounds
Selective oxidations catalyzed by laccase have been applied for the manufacture of pharmaceutically vital compounds (Mogharabi and Faramarzi 2014). For instance, laccase-catalyzed oxidative domino reaction of cylcohexane-1,3-dione with catechol produced 3,4-dihydro-7,8-dihydroxy-2-H-debenzofuran-1-ones with yields ranging from 70 to 97% (Hajdok et al. 2007). Because of their biological chemistry, these dibenzofuranones are of great interest to medicinal chemistry. In another study, dimers were produced by laccase-catalyzed oxidation of catechol. Further, these dimers reacted with chalcones producing oxaflavins, which owe their importance as probable redox co-enzymes (Kidwai et al. 2009). While, benzofurans were synthesized using laccase-catalyzed oxidation of catechol to corresponding dimers (Witayakran and Ragauskas 2009). Benzofurans are known to have good antimicrobial and anti-inflammatory activities but are produced conventionally by lanthanide metal catalysts. In another work, a new series of quinoxaline derivatives have been effectively produced by laccase-mediated oxidative coupling of dihyroxy benzene and diamines (Kidwai et al. 2012b). Quinoxaline derivatives form basic skeleton of many antibiotics (levomycin and actinomycin), synthesized conventionally by electrochemical methods. In another study, aminonapthoquinones were synthesized by laccase-catalyzed nuclear monoanimation of 1,4-hydroquinone with primary aromatic amines (Wellington and Kolesnikova 2012). The authors also demonstrated the cytostatic effects of the produced aminonapthoquinones against cancer cell lines. In yet another reaction, benzoquinones were synthesized by laccase-catalyzed domino reaction between cyclic 1.3-dicarbonyls and hydroquinones (Hajdok et al. 2012). Likewise, laccase-catalyzed coupling of 1,2- ethanedithiol with substituted hydroquinones for the eco-friendly one-pot synthesis of 2,3-ethylenedithio-1,4-quinones was performed (Cannatelli and Ragauskas 2015). Interestingly, an efficient synthesis of benzofuro(2,3-c) pyrazoles by polymerization of catechols with pyrazolin-5-ones in the presence of laccase was also carried out (Kidwai et al. 2013a). The fusion of benzofurans with pyrazoles can lead to superpotent pharmaceutical compounds (Kidwai et al. 2013a). The same research group (Kidwai et al. 2013b) fused meldrum’s acid with catechols and flavanoids in the presence of laccase and produced some new superior compounds, thereby making a significant input in the field of biocatalysis (Kidwai et al. 2013b). Meldrum’s acid is known to have a depressant effect on the central nervous system and possess low toxicity like barbiturates, whereas, catechols and flavanoids are good antimicrobial agents. Furthermore, laccase was employed in enzymatic derivatization of amino acids, such as L-tryptophan, L-phenyalanine, or L-lysine by Mogharabi and Faramarzi (2014). On the other hand, laccase in association with the water-soluble palladium complex catalyzed aerobic oxidation of alcohols (Mekmouche et al. 2015). Recently, T. versicolor laccase was used for aerobic oxidative coupling of 4-substituted urazoles with sodium arylsulphinates for the synthesis of arylsulfonyl triazolidinediones (Rahimi et al. 2018).
Synthesis of valuable polymers and dyes
Polyanniline has good electrical and optical properties as well as remarkable environmental stability and therefore used as an active constituent of organic light weight batteries, optical display, micro-electronics, anti-corrosive protection, and in bioanalysis. Similarly, polycatechol is also a valuable polymer, used as a chromatographic resin and also for the production of thin films in biosensors (Kunamneni et al. 2008). Existing processes for synthesis of these polymers use horseradish peroxidase (HRP) per se, which shows low activity and stability at pH below pH 4.5 and also gets inactivated in the presence of high concentrations of H2O2 (Karamyshev et al. 2003). To address this issue, laccases have been used for the synthesis of polyanniline (de Salas et al. 2016) and polycatechol (Aktaş et al. 2003).
Mild conditions of synthesis thereof, as well as a lack of any toxic by-products, constitute advantages of the application of laccase-mediated biocatalysis in the synthesis of dyes (Polak et al. 2016). Laccase-based polymerization of different phenols in different combinations (gallic acid and syringic acid; catechin and catechol; ferulic acid and syringic acid) yielded diverse hair dyes (Jeon et al. 2010). Each of the formulated dye showed resistance to conventional shampooing, thereby showing the potential of laccase-mediated catalysis in the development of non-toxic hair dyes. The conventional hair dyeing methodology uses H2O2 as an oxidizing agent and phenylenediamine as a dye precursor. While H2O2 can cause damage to the hair, phenylendiamine is a potential carcinogen. In another work, laccase-catalyzed dimerization of various ortho and meta, para-disubstituted aromatic amines into phenazine and phenoxazinone chromophores was performed (Sousa et al. 2014). Very interestingly, laccases from Myceliphthora thermophila were evolved by directed evolution and further expressed in Saccharomyces cerevisiae to be finally used for the synthesis C-H heteropolymeric dyes at alkaline pH (Vicente et al. 2016). The study thus provided useful laccase mutant for organic synthesis at basic pH. In another very interesting report, the toxicity and dyeing properties of several orange-red biodyes, which were obtained after laccase-catalyzed biotransformation of aromatic precursors was studied by Polak et al. (2016). The authors found out that the dyes were non-toxic on bioluminescent marine bacterium (Vibrio fischeri) as a test organism as well as on cultures of normal human colon epithelial cells (Polak et al. 2016).
Bioremediation (polymerizing and depolymerizing role)
Bioremediation is one such important application of laccase where the enzyme is involved in removal of toxic environmental pollutants either by an oxidative bond cleavage or by oxidative coupling mechanism depending on the reaction conditions. For instance, Murugesan and co-workers (Murugesan et al. 2010) studied transformation as well as detoxification of triclosan (TCS) by laccase in the presence and absence of redox mediators. It was observed that TCS was removed by 56.5% in the absence of redox mediator within 24 h, along with formation of new products (dimers and trimers of TCS) with molecular weights greater than that of the parent compound. However, in the presence of mediators (HBT and syringaldehyde), 90% of TCS was removed and compounds with molecular weight lower than TCS were detected by GC-MS. Hence, involvement of two mechanisms in laccase-mediated reactions was postulated: (i) polymerization or oxidative coupling in the absence of redox mediators, and (ii) bond cleavage as well as dechlorination in the presence of redox mediators (Murugesan et al. 2010). The results also suggest that laccase with redox mediators can be used for detoxification or elimination of pollutants by oxidative bond cleavage while, in the absence of mediators, enzyme polymerizes small organics with pollutants forming adducts that can be subsequently removed by filtration.
Laccase-catalyzed bioremediation by oxidative coupling (polymerizing role)
Oxidative coupling of small organic compounds with pollutants in the presence of laccase lead to their removal by consequent filtration or sedimentation. This is specifically valuable as well as practical in the treatment of water as the pollutant-containing co-polymeric compounds will become insoluble in water (Kulys et al. 2003), thereby making their consequent removal by filtration or sedimentation easier (Lante et al. 2000). In this regard, Han-Ko and Chen (2008) elucidated mechanisms for enhanced removal of common phenolic compounds present in various industrial effluents by laccase polymerization. The authors found out that guaiacol, catechol, and m-cresol got polymerized by laccase to products of average molecular weight of 9600, 8350, and 5400 Da, respectively, and thus can be easily removed by ultra and microfiltration membrane systems. Similarly, transformation of chlorinated hydroxybiphenyls by laccase from Pycnoporus cinnabarinus was investigated by Schultz et al. (2001). It was found out that the compounds used were transformed to sparingly water-soluble colored precipitates, identified as oligomerization products of the chlorinated hydroxybiphenyls by gas chromatography-mass spectrometry (Schultz et al. 2001). In another study, cell cultures of a laccase-producing fungus, T. versicolor, were compared with the immobilized cultures on nylon mesh in a 2 L bioreactor for transformation and adsorption of pentachlorophenol (PCP) and 2,4- dichlorophenol (2,4-DCP) by Sedarati et al. (2003). The authors observed that the immobilized cultures performed better with 85% of 2,4-DCP and 70% of PCP transformed; 5% of 2,4-DCP and 28% of PCP adsorbed by the biomass; and 10% of 2,4-DCP and 2% of PCP retained in the medium at the termination of the fermentation after 1020 h. While, Gullato and co-workers (Gullotto et al. 2008) exploited combination of two enzymes, namely toluene o-xylene monooxygenase from Pseudomonas sp. OX1 and laccase from P. ostreatus for the polymerization of mono and poly-aromatic hydrocarbons into polymers with reduced toxicity. Bhattacharya and Banerjee (2008) observed faster biodegradation of 2,4-DCP with Pleurotus sp. laccase compared with whole-cell biodegradation and obtained 98% degradation of the xenobiotic compound in 9 h. Furthermore, Bhattacharya et al. (2009) used RSM with a developed genetic algorithm for reducing contact time of laccase-mediated biodegradation of 2,4-DCP and successfully achieved 99% biodegradation in 8 h.Similarly, laccase-mediated coupling of aromatic amines, produced after decolorization of textile dyes, was performed by Franciscon et al. (2010). In another report, Steevensz and co-authors (Steevensz et al. 2012) studied laccase initiated oxidative coupling of various aromatic phenols and anilines present in water. Likewise, laccase-mediated oxidative coupling of phenol and its derivatives with 4-aminoantipyrene was investigated by Kidwai et al. (2012a). The authors obtained different yields of antipyrilquinoneimine dye as the colored product using laccase isolated from three different organisms (P. cinnarabanius, Ganoderma sp., and fungal isolate RCK-3), with better catalytic efficiency of P. cinnabarinus laccase w.r.t this particular reaction compared to the laccase from Ganoderma sp., and isolate RCK-3. The strategy can prove to be useful in estimating phenols in aqueous solutions (Kidwai et al. 2012a).Very interestingly, Sumathi et al. (2016) isolated the laccase-producing fungal strain Cochliobolus sp. from plastic-dumped soils and found it to be effective in the degradation of low molecular weight polyvinyl chloride. Recently, Huber et al. (2018) performed laccase-catalyzed elimination of morphine from an aqueous system and obtained complete elimination of 60 g/L within 6 h.
Laccase mediator system catalyzed bioremediation by oxidative bond cleavage (depolymerizing role)
It has been widely reported that laccases alone can carry out degradation of only low-redox-potential phenolic compounds, and not the oxidation of the most recalcitrant nonphenolic contaminants (Xu et al. 1996). However, in the presence of mediators, small molecules that act as electron shuttles between enzymes and target molecules, rate of reaction, and the range of substrates oxidized can be enhanced (Vallecillos et al. 2017). Several polluting substances like textile dyes, pesticides, PAHs, and cholorophenols can be degraded effectively by the laccase mediator system (Camarero et al. 2005). Recently, laccase was also found competent in degradation of lipids as well as oxidation of olefin units present in plastic (Zhang et al. 2002). Therefore, the enzyme in combination with other existing techniques can also be implicated in plastic degradation (Durán et al. 2002). Since, substrate specificity of laccases vary from one to another laccase secreted from different sources and thus can be applied for degrading a variety of environmental contaminants in the presence of synthetic/natural mediators (Table 2).
Pulp and paper industry (polymerizing and depolymerizing role)
The in vivo degradative role of fungal laccases in delignification can be exploited for removal of lignin from paper pulp in the presence of redox mediators, while the in vivo polymerizing role of the enzyme can be used for grafting functional groups on pulp fibers, producing fiber boards with improved properties. Andreu and Vidal (2011) compared the effect of laccase treatment on kenaf pulp in conjunction with a number of diverse natural mediators (vanillin, syringaldehyde, acetosyringone, p-coumaric acid, acetovanillone) and a synthetic mediator (HBT). Interestingly, both the treatments showed opposite results with increase in kappa no. (delignification) and decrease in pulp brightness after enzymatic treatment in the presence of natural mediators. On the other hand, laccase treatment in conjunction with HBT (synthetic mediator) decreased the kappa no. and increased brightness of the pulp. The effect was explained by the authors as partial condensation reactions by laccase on kenaf pulp, leading to grafting of moieties on the fiber, thereby concluding that the effect of the laccase natural mediator system on substrate depends on the delicate equilibrium between reactions of oxidative degradation and polymerization (grafting) (Andreu and Vidal 2011).
Laccase mediator system catalyzed delignification of paper pulp (depolymerizing role)
Conventionally, pulp bleaching was carried out using chlorine, which the released bulk of halogenated organic compounds, measured as adsorbable organic halogens (AOX), in the effluents (Sharma et al. 2014). However, growing public concern and strict legislative laws over the harmful effects of AOX generated in the pulp bleaching processes lead paper mills to replace chlorine from their bleaching sequence with chlorine dioxide in an elemental chlorine-free bleaching (Bajpai et al. 2007). However, many new technologies, such as enzyme-mediated pulp treatment (bio-bleaching) and ozone-based bleaching, have also been studied and used to further reduce the environmental impact of pulp bleaching using chlorine dioxide (Singh et al. 2014). In this regard, application of laccases in bio-bleaching has been thoroughly studied by many researchers (Table 3). Along with reducing the consumption of chemicals for pulp bleaching sequence, the enzyme also helps in improving properties of the resultant paper (Sharma et al. 2014). Nevertheless, it was observed that laccase-mediated delignification of pulp without the addition of synthetic mediators was unsuccessful. The large size as well as lower redox potential limits action of the enzyme on the fiber surface. While, low molecular weight mediators act as a shuttle in transferring electrons from laccase to lignin (Oudia et al. 2007; Morozova et al. 2007). It was also seen by some researchers that introduction of a xylanase stage prior to treatment of pulp with laccase results in a noteworthy reduction of bleaching chemicals (Kapoor et al. 2007; Bajpai et al. 2007; Valls and Roncero 2009). This effect was explained by the action of xylanase on xylan, which lies between lignin and cellulose, thereby increasing the exposure of lignin in the pulp to laccase mediator system (Kapoor et al. 2007). The increased bleach response by introducing a xylanase stage before laccase treatment of the pulp can also be explained by increased accessibility of laccase to hexauronic acid after xylanase treatment and thereby facilitating their removal and thus reducing the kappa number of the pulp (Valls et al. 2010a). The fact was supported by a further study (Valls et al. 2010b) which reported more efficient removal of hexauronic acids by XLMS sequence than by laccase alone. Similarly, Aracri and Vidal (Aracri and Vidal 2011) also concluded that if xylanase treatment was applied before LMS treatment, better quality pulps were retained at the end of the bleaching sequence in terms of lower kappa number and higher cellulose content. Recently, Sharma and co-workers (Sharma et al. 2014) observed that sequential xylanase and laccase treatment of pulp at pilot scale (50 Kg pulp) saved 35% chlorine dioxide in the bleaching sequence to obtain the same targeted brightness as in the control pulp, resulting in 34% decreased AOX levels in bleach effluents along with improvement in properties of the formed paper (reduction in post color number by 50% and increase in tear index by 15.71%).
Laccase-catalyzed fiber modification (polymerizing role)
Laccase with its property to oxidize lignin can be applied in the manufacture of composites like liner and fiber boards and thus replacing toxic chemicals (urea, formaldehyde, isocyanate, and petrochemical resins) used for the purpose (Euring et al. 2011). By this way, laccase from Trametes villosa was used for grafting a variety of amino acids onto high-lignin softwood kraft pulp and it was observed that the strength properties of the paper formed from pulp treated with laccase-histidine were increased significantly (Witayakran and Ragauskas 2009). While, LMS was used to activate lignin on wood fiber surfaces by Euring et al. (2011). Two different mediators (vanillic acid (VAN) and 4-hydroxybenzoic acid (HBA)) were tested in the study, of which HBA performed better. 13C-NMR revealed more structural changes in the wood fibers using LMS with HBA than LMS with VAN. Similarly, ESR spectroscopy also indicated a higher amount of phenoxy radicals on the fiber surface after treatment with LMS containing HBA as a mediator. But VAN also performed well, which showed a high potential to produce eco-friendly MDF (medium-density fiberboards) by using LMSs in the future (Euring et al. 2011). On the other hand, laccase-catalyzed grafting of protein-flavanoid conjugates was performed onto flax fiber, resulting in better color and increased antioxidant activity of the final product (Kim and Cavaco-Paulo 2012). In another study, Li et al. (2013) observed increased carboxyl group and surface lignin content of pulps treated with laccase and ferulic acid compared to the untreated pulps. Interestingly, commercial laccase from Myceliophtora thermophila was used along with latex to catalyze the surface modification of thermo-mechanical pulp (TMP) in process water obtained from the production of low-density wood fiber, which is known to contain natural phenolic extractives (Schubert et al. 2015). The researchers observed changed surface chemistry of the fiber due to grafting of phenolic compounds present in process water onto it by laccase, thereby enhancing mechanical strength properties of the resultant boards (Schubert et al. 2015). Table 4 lists laccases from different sources used for fiber modification.
LMS-catalyzed dye decolorization (depolymerizing role)
The prevalent use of synthetic dyes in a number of industries has resulted in the production of more than 100,000 hazardous dyes of different types such as heterocyclic, anthraquinone, phthalocyanine, triphenylmethane, and azo-based chemical structures (Cristóvão et al. 2008; Ayed et al. 2011). However, there is an urgent need to treat the strong and intense color of dyes discharged in the water from these industries (Cristóvão et al. 2008). This is because even lower concentration of dyes (less than 1 ppm) is distressing for the receiving water bodies as it severely affects the penetration of light and the gas solubility. The dyes are also found to be toxic for aquatic life, microorganisms as well as food chain organisms (Husain 2006). Therefore, treatment technologies need to be investigated for degradation of dyes in water bodies. Many physicochemical techniques are being used for dye removal (Saratale et al. 2011). However, these methods are economically unfeasible, generate large amounts of sludge, causing secondary pollution problem, and are also unable to remove recalcitrant azo dyes (Anjaneyulu et al. 2005). The enzymatic degradation of synthetic dyes provides an environment-friendly and cost effective process for dye removal of waste waters (Forootanfar et al. 2010; Telke et al. 2011). In this regard, laccases have gained much attention as they oxidize a broad range of aromatic compounds including synthetic dyes (Kuhad et al. 2004). During recent decades, many studies have been done on LMS-catalyzed degradation of synthetic dyes (Table 5).
Concluding remarks
The bifunctional roles of laccases owing to the presence of multi-gene families of the enzyme make them useful in diverse biotechnological applications of industrial significance. In addition, with their lesser reaction requirements as well as broad substrate specificity, laccases can be seen as model green catalysts for various industrial processes. Successful application of lignin-degrading ability of the enzyme in pulp bleaching in paper mills will reduce the use of hazardous chemicals to a sizeable extent. Further, the dye decolorizing ability of laccase can be exploited for developing a process for decolorization of textile effluents. On the other hand, the polymerizing ability of the enzyme used for coupling of various phenolic moieties on fiber-boards will help enhance their properties in an eco-friendly manner. While, application of enzyme in organic synthesis for the development of pharmaceutically important compounds as well as valuable polymers and dyes represent a milestone along the path of future sustainable chemistry. Bifunctionally, the enzyme can also be used in bioremediation, wherein it can remove recalcitrant toxic compounds from the environment either by oxidative bond cleavage (depolymerizing role) or by oxidative polymerization (polymerizing role). Nevertheless, the existence of multiple inducible isoforms of the enzyme gives added advantage of selectively expressing a particular isozyme for a desired application using definite inducers. Furthermore, altering reaction conditions (use of natural/synthetic laccase mediators) can also help laccase-based catalysis in either the polymerization/depolymerization direction. Thus, the in-depth knowledge of bifunctionality of this wonderful enzyme and future research on laccase-catalyzed biochemical reactions can definitely pave the way for designing novel biocatalysts with customized features.
References
Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB (2006) Anticancer potential of silymarin: from bench to bed side. Anticancer Res 26:4457–4498
Aktaş N, Şahiner N, Kantoğlu Ö, Salih B, Tanyolaç A (2003) Biosynthesis and characterization of laccase catalyzed poly (catechol). J Polym Environ 11:123–128
Andreu G, Vidal T (2011) Effects of laccase-natural mediator systems on kenaf pulp. Bioresour Technol 102:5932–5937. https://doi.org/10.1016/j.biortech.2011.03.008
Anjaneyulu Y, Sreedhara Chary N, Samuel Suman Raj D (2005) Decolourization of industrial effluents—available methods and emerging technologies—a review. Rev Environ Sci Biotechnol 4:245–273. https://doi.org/10.1007/s11157-005-1246-z
Apotrosoaei M, Vasincu I, Constantin S, Buron F, Routier S, Profire L (2014) Synthesis, characterization and antioxidant activity of some new thiazolidin-4-one derivatives. Rev Med Chir Soc Med Nat Iasi 118:213–218
Aracri E, Vidal T (2011) Xylanase- and laccase-aided hexenuronic acids and lignin removal from specialty sisal fibres. Carbohydr Polym 83:1355–1362
Asgher M, Bhatti HN, Ashraf M, Legge RL (2008) Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation 19:771–783
Ashrafi SD, Rezaei S, Forootanfar H, Mahvi AH, Faramarzi MA (2013) The enzymatic decolorization and detoxification of synthetic dyes by the laccase from a soil-isolated ascomycete, Paraconiothyrium variabile. Int Biodeterior Biodegrad 85:173–181
Ayed L, Mahdhi A, Cheref A, Bakhrouf A (2011) Decolorization and degradation of azo dye methyl red by an isolated Sphingomonas paucimobilis: biotoxicity and metabolites characterization. Desalination 274:272–277
Babot ED, Rico A, Rencoret J, Kalum L, Lund H, Romero J, del Río JC, Martínez ÁT, Gutiérrez A (2011) Towards industrially-feasible delignification and pitch removal by treating paper pulp with Myceliophthora thermophila laccase and a phenolic mediator. Bioresour Technol 102:6717–6722
Bajpai P, Anand A, Sharma N, Mishra SP, Bajpai PK, Lachenal D (2007) Enzymes improve ECF bleaching of pulp. BioResources 1:34–44
Baldrian P (2004) Purification and characterization of laccase from the white-rot fungus Daedalea quercina and decolorization of synthetic dyes by the enzyme. Appl Microbiol Biotechnol 63:560–563
Baldrian P (2006) Fungal laccases - occurrence and properties. FEMS Microbiol Rev 30:215–242
Bayramoğlu G, Yilmaz M, Yakup Arica M (2010) Reversible immobilization of laccase to poly(4-vinylpyridine) grafted and cu(II) chelated magnetic beads: biodegradation of reactive dyes. Bioresour Technol 101:6615–6621
Berthret S, Thevenin J, Baratiny D, Demont-Caulet N, Debeaujon I, Bidzinski P, Leple J-C, Huis R, Hawkins S, Gomez L-D, Lapierre C, Jouanin L (2012) Role of plant laccases in lignin polymerization. Adv Bot Res 61:145–172
Bhattacharya SS, Banerjee R (2008) Laccase mediated biodegradation of 2,4-dichlorophenol using response surface methodology. Chemosphere 73:81–85
Bhattacharya SS, Karmakar S, Banerjee R (2009) Optimization of laccase mediated biodegradation of 2,4-dichlorophenol using genetic algorithm. Water Res 43:3503–3510
Botta L, Brunori F, Tulimieri A, Piccinino D, Meschini R, Saladino R (2017) Laccase-mediated enhancement of the antioxidant activity of Propolis and Poplar bud exudates. ACS Omega 2:2515–2523
Camarero S, Ibarra D, Martínez MJ, Martínez ÁT (2005) Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl Environ Microbiol 71:1775–1784
Cannatelli MD, Ragauskas AJ (2015) Laccase-catalyzed synthesis of 2,3-ethylenedithio-1,4-quinones. J Mol Catal B Enzym 119:85–89
Casas N, Parella T, Vicent T, Caminal G, Sarrà M (2009) Metabolites from the biodegradation of triphenylmethane dyes by Trametes versicolor or laccase. Chemosphere 75:1344–1349
Ceylan H, Kubilay S, Aktas N, Sahiner N (2008) An approach for prediction of optimum reaction conditions for laccase-catalyzed bio-transformation of 1-naphthol by response surface methodology (RSM). Bioresour Technol 99:2025–2031
Champagne P-P, Ramsay JA (2010) Dye decolorization and detoxification by laccase immobilized on porous glass beads. Bioresour Technol 101:2230–2235
Chen X, Guo C, Kong J (2012) Oxidative stress in neurodegenerative diseases. Neural Regen Res 7:376–385
Chernykh A, Myasoedova N, Kolomytseva M, Ferraroni M, Briganti F, Scozzafava A, Golovleva L (2008) Laccase isoforms with unusual properties from the basidiomycete Steccherinum ochraceum strain 1833. J Appl Microbiol 105:2065–2075
Christopher LP, Yao B, Ji Y (2014) Lignin biodegradation with laccase-mediator systems. Front Energy Res 2:12
Claus H (2003) Laccases and their occurrence in prokaryotes. Arch Microbiol 179:145–150
Couto SR, Sanroman MA, Hofer D, Gübitz GM (2004) Production of laccase by Trametes hirsuta grown in an immersion bioreactor and its application in the decolorization of dyes from a leather factory. Eng Life Sci 4:233–238
Cristóvão RO, Tavares APM, Ribeiro AS, Loureiro JM, Boaventura RAR, Macedo EA (2008) Kinetic modelling and simulation of laccase catalyzed degradation of reactive textile dyes. Bioresour Technol 99:4768–4774
Da Re V, Papinutti L, Forchiassin F, Levin L (2010) Biobleaching of loblolly pine kraft pulp with Trametes trogii culture fluids followed by a peroxide stage. Application of Doehlert experimental design to evaluate process parameters. Enzym Microb Technol 46:281–286
de Carvalho Peixoto-Nogueira S, Betini JHA, Michelin M, de Carvalho CC, Lucca AL, Vici AC, Jorge JA, de Lourdes Teixeira de Moraes M, Polizeli M (2015) Laccase production by Aspergillus niveus on SSF using wheat bran as alternative carbon source and its synergistic effect on pulp biobleaching using a mix of laccase/xylanase from the same microorganism. J Biochem Technol 6:929–937
de Salas F, Pardo I, Salavagione HJ, Aza P, Amougi E, Vind J, Martínez AT, Camarero S (2016) Advanced synthesis of conductive polyaniline using laccase as biocatalyst. PLoS One 11:e0164958
de Souza CGM, Tychanowicz GK, de Souza DF, Peralta RM (2004) Production of laccase isoforms by Pleurotus pulmonarius in response to presence of phenolic and aromatic compounds. J Basic Microbiol 44:129–136
Desentis-Mendoza RM, Hernandez-Sanchez H, Moreno A, Rojas del c E, Chel-Guerrero L, Tamariz J, Jaramillo-Flores ME (2006) Enzymatic polymerization of phenolic compounds using laccase and tyrosinase from Ustilago maydis. Biomacromolecules 7:1845–1854
Dhillon GS, Kaur S, Brar SK (2012) In-vitro decolorization of recalcitrant dyes through an ecofriendly approach using laccase from Trametes versicolor grown on brewer’s spent grain. Int Biodeterior Biodegrad 72:67–75
Dittmer NT, Kanost SR (2010) Insect multicopper oxidases: diversity, properties, and physiological roles. Insect Biochem Mol Biol 40:179–188
Durán N, Rosa MA, D’Annibale A, Gianfreda L (2002) Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: a review. Enzym Microb Technol 31:907–931
Eggert C, Temp U, Eriksson KE (1996) The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol 62:1151–1158
Eisenman HC, Mues M, Weber SE, Frases S, Chaskes S, Gerfen G, Casadevall A (2007) Cryptococcus neoformans laccase catalyses melanin synthesis from both D- and L-DOPA. Microbiology 153:3954–3962
Erkurt EA, Ünyayar A, Kumbur H (2007) Decolorization of synthetic dyes by white rot fungi, involving laccase enzyme in the process. Process Biochem 42:1429–1435
Eugenio ME, Santos SM, Carbajo JM, Martín JA, Martín-Sampedro R, González AE, Villar JC (2010) Kraft pulp biobleaching using an extracellular enzymatic fluid produced by Pycnoporus sanguineus. Bioresour Technol 101:1866–1870
Euring M, Rühl M, Ritter N, Kües U, Kharazipour A (2011) Laccase mediator systems for eco-friendly production of medium-density fiberboard (MDF) on a pilot scale: physicochemical analysis of the reaction mechanism. Biotechnol J 6:1253–1261
Fillat U, Blanca Roncero M (2009) Effect of process parameters in laccase-mediator system delignification of flax pulp: part I. pulp properties. Chem Eng J 152:322–329
Fillat U, Roncero MB (2010) Optimization of laccase-mediator system in producing biobleached flax pulp. Bioresour Technol 101:181–187
Fillat U, Pepió M, Vidal T, Roncero MB (2010) Flax fibers as a raw material: how to bleach efficiently a non-woody plant to obtain high-quality pulp. Biomass Bioenergy 34:1896–1905
Forootanfar H, Faramarzi M, Shahverdi AR, Tabatabaei Yazdi M (2010) Purification and biochemical characterization of extracellular leccase from the ascomycete Paraconiothyrium variable. Bioresour Technol 102:1808–1814
Forootanfar H, Rezaei S, Zeinvand-Lorestani H, Tahmasbi H, Mogharabi M, Ameri A, Faramarzi MA (2016) Studies on the laccase-mediated decolorization, kinetic, and microtoxicity of some synthetic azo dyes. J Environ Health Sci Eng 14:7
Franciscon E, Piubeli F, Fantinatti-Garboggini F, Ragagnin de Menezes C, Serrano Silva I, Cavaco-Paulo A, Grossman MJ, Durrant LR (2010) Polymerization study of the aromatic amines generated by the biodegradation of azo dyes using the laccase enzyme. Enzym Microb Technol 46:360–365
Galhaup C, Goller S, Peterbauer CK, Strauss J, Haltrich D (2002) Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions. Microbiology 148:2159–2169
Gažák R, Sedmera P, Marzorati M, Riva S, Křen V (2008) Laccase-mediated dimerization of the flavonolignan silybin. J Mol Catal B Enzym 50:87–92
Gianfreda L, Xu F, Bollag J-M (1999) Laccases: a useful group of oxidoreductive enzymes. Bioremediation J 3:1–26
Gogoi P, Hazarika S, Dutta NN, Rao PG (2010) Kinetics and mechanism on laccase catalyzed synthesis of poly(allylamine)–catechin conjugate. Chem Eng J 163:86–92
Gorman MJ, Sullivan LI, Nguyen TDT, Dai H, Arakane Y, Dittmer NT, Syed LU, Li J, Hua DH, Kanost MR (2012) Kinetic properties of alternatively spliced isoforms of laccase-2 from Tribolium castaneum and Anopheles gambiae. Insect Biochem Mol Biol 42:193–202
Grassi E, Scodeller P, Filiel N, Carballo R, Levin L (2011) Potential of Trametes trogii culture fluids and its purified laccase for the decolorization of different types of recalcitrant dyes without the addition of redox mediators. Int Biodeterior Biodegrad 65:635–643
Gullotto A, Branciamore S, Duchi I, Caño MFP, Randazzo D, Tilli S, Giardina P, Sannia G, Scozzafava A, Briganti F (2008) Combined action of a bacterial monooxygenase and a fungal laccase for the biodegradation of mono- and poly-aromatic hydrocarbons. Bioresour Technol 99:8353–8359
Guo W, Yao Z, Zhou C, Li D, Chen H, Shao Q, Li Z, Feng H (2012) Purification and characterization of three laccase isozymes from the white rot fungus Trametes sp. HP-03. Afr J Biotechnol 11:7916–7922
Gupta AK, Kalpana S, Malik JK (2012) Synthesis and in vitro antioxidant activity of new 3-substituted-2-oxindole derivatives. Indian J Pharm Sci 74:481–486
Hajdok S, Leutbecher H, Greiner G, Conrad J, Beifuss U (2007) Laccase initiated oxidative domino reactions for the efficient synthesis of 3,4-dihydro-7,8-dihydroxy-2H-dibenzofuran-1-ones. Tetrahedron Lett 48:5073–5076
Hajdok S, Conrad J, Beifuss U (2012) Laccase-catalyzed domino reactions between hydroquinones and cyclic 1,3-dicarbonyls for the regioselective synthesis of substituted p-benzoquinones. J Org Chem 77:445–459
He F, Qin X, Zhang H, Yang Y, Zhang X, Yang Y (2014) Characterization of laccase isozymes from the white rot fungus Ganoderma sp. EN3 and synergistic action of isozymes and dye decolorization. Chem Tech Biotechnol 90:2265–2279
Hu X, Wang P, Hwang H (2009) Oxidation of anthracene by immobilized laccase from Trametes versicolor. Bioresour Technol 100:4963–4968
Huber D, Bleymaier K, Pellis A, Vielnascher R, Daxbacher A, Greimel KJ, Guebitz GM (2018) Laccase catalyzed elimination of morphine from aqueous systems. New Biotechnol 42:19–25
Husain Q (2006) Potential applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: a review. Crit Rev Biotechnol 26:201–221
Ibarra D, Romero J, Martínez MJ, Martínez AT, Camarero S (2006) Exploring the enzymatic parameters for optimal delignification of eucalypt pulp by laccase-mediator. Enzym Microb Technol 39:1319–1327
Jeon J-R, Chang Y-S (2013) Laccase-mediated oxidation of small organics: bifunctional roles for versatile applications. Trends Biotechnol 31:335–341
Jeon J, Kim E, Murugesan K, Park H, Kim Y, Kwon J, Kim W, Lee J, Chang Y (2010) Laccase-catalysed polymeric dye synthesis from plant-derived phenols for potential application in hair dyeing: enzymatic colourations driven by homo- or hetero-polymer synthesis. Microb Biotechnol 3:324–335
Jeon J-R, Baldrian P, Murugesan K, Chang Y-S (2012) Laccase-catalysed oxidations of naturally occurring phenols: from in vivo biosynthetic pathways to green synthetic applications. Microb Biotechnol 5:318–332
Jin X, Yu X, Zhu G, Zheng Z, Feng F, Zhang Z (2016) Conditions optimizing and application of laccase-mediator system (LMS) for the laccase-catalyzed pesticide degradation. Sci Rep 6:35787
Johannes C, Majcherczyk A (2000) Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl Environ Microbiol 66:524–528
Jones SM, Solomon EL (2015) Electron transfer and reaction mechanism of laccase. Cell Mol Life Sci 72:869–883
Kandelbauer A, Maute O, Kessler RW, Erlacher A, Gübitz GM (2004) Study of dye decolorization in an immobilized laccase enzyme-reactor using online spectroscopy. Biotechnol Bioeng 87:552–563
Kapoor M, Kapoor RK, Kuhad RC (2007) Differential and synergistic effects of xylanase and laccase mediator system (LMS) in bleaching of soda and waste pulps. J Appl Microbiol 103:305–317
Karamyshev AV, Shleev VS, Koroleva OV, Yaropolov AI, Sakharov IV (2003) Laccase-catalyzed synthesis of conducting polyaniline. Enzym Microb Technol 33:556–564
Keum YS, Li QX (2004) Fungal laccase-catalyzed degradation of hydroxy polychlorinated biphenyls. Chemosphere 56:23–30
Kidwai M, Poddar R, Diwaniyan S, Kuhad RC (2009) Laccase from basidiomycetous fungus catalyzes the synthesis of substituted 5-Deaza-10-oxaflavins via a domino reaction. Adv Synth Catal 351:589–595
Kidwai M, Jain A, Sharma A, Chander Kuhad R (2012a) Ecofriendly approach for detection of phenols in water using laccase from different fungi. Water Sci Technol J Int Assoc Water Pollut Res 66:385–393
Kidwai M, Jain A, Sharma A, Kuhad RC (2012b) First time reported enzymatic synthesis of new series of quinoxalines—a green approach. J Mol Catal B Enzym 74:236–240
Kidwai M, Jain A, Sharma A, Kuhad RC (2013a) Laccase—a natural source for the synthesis of benzofuro[2,3- c ]pyrazolin-5-ones. Catal Sci Technol 3:230–223
Kidwai M, Jain A, Sharma A, Kuhad RC (2013b) Laccase-catalysed reaction between Meldrum’s acid and catechols/hydroquinones—an investigation. Comptes Rendus Chim 16:728–735
Kim S, Cavaco-Paulo A (2012) Laccase-catalysed protein-flavonoid conjugates for flax fibre modification. Appl Microbiol Biotechnol 93:585–600
Ko C-H, Chen S-S (2008) Enhanced removal of three phenols by laccase polymerization with MF/UF membranes. Bioresour Technol 99:2293–2298
Kuhad RC, Sood N, Tripathi KK, Singh A, Ward OP (2004) Developments in microbial methods for the treatment of dye effluents. Adv Appl Microbiol 56:185–213
Kuhar F, Papinutti L (2014) Optimization of laccase production by two strains of Ganoderma lucidum using phenolic and metallic inducers. Rev Argent Microbiol 46:144–149
Kulys J, Vidziunaite R, Schneider P (2003) Laccase-catalyzed oxidation of naphthol in the presence of soluble polymers. Enzym Microb Technol 32:455–463
Kumar A, Singh D, Sharma KK, Arora S, Singh AK, Gill SS, Singhal B (2017) Gel-based purification and biochemical study of lacacse isozymes from Ganoderma sp. and its role in enhanced cotton callogenesis. Front Microbiol 8:674
Kumar VV, Sathyaselvabala V, Premkumar MP, Vidyadevi T, Sivanesan S (2012) Biochemical characterization of three phase partitioned laccase and its application in decolorization and degradation of synthetic dyes. J Mol Catal B Enzym 74:63–72
Kunamneni A, Camarero S, García-Burgos C, Plou FJ, Ballesteros A, Alcalde M (2008) Engineering and applications of fungal laccases for organic synthesis. Microb Cell Factories 7:32
Lante A, Crapisi A, Krastanov A, Spettoli P (2000) Biodegradation of phenols by laccase immobilised in a membrane reactor. Process Biochem 36:51–58
Lettera V, Piscitelli A, Leo G, Birolo L, Pezzella C, Sannia G (2010) Identification of a new member of Pleurotus ostreatus laccase family from mature fruiting body. Fungal Biol 114:724–730
Levin L, Papinutti L, Forchiassin F (2004) Evaluation of Argentinean white rot fungi for their ability to produce lignin-modifying enzymes and decolorize industrial dyes. Bioresour Technol 94:169–176
Levin L, Forchiassin F, Viale A (2005) Ligninolytic enzyme production and dye decolorization by Trametes trogii: application of the Plackett–Burman experimental design to evaluate nutritional requirements. Process Biochem 40:1381–1387
Li H, Fu S, Peng L (2013) Fiber modification of unbleached kraft pulp with laccase in the presence of ferulic acid. BioResources 8:5794–5806
Li Q, Wang X, Korzhev M, Schröder HC, Link T, Tahir MN, Diehl-Seifert B, Müller WEG (2015) Potential biological role of laccase from the sponge Suberites domuncula as an antibacterial defense component. Biochim Biophys Acta 1850:118–128
Liang M, Davis E, Gardner D, Cai X, Wu Y (2006) Involvement of AtLAC15 in lignin synthesis in seeds and in root elongation of Arabidopsis. Planta 224:1185–1196
Lisov AV, Zavarzina AG, Zavarzin AA, Leontievsky AA (2007) Laccases produced by lichens of the order Peltigerales. FEMS Microbiol Lett 275:46–52
Lloret L, Eibes G, Feijoo G, Moreira MT, Lema JM (2012) Degradation of estrogens by laccase from Myceliophthora thermophila in fed-batch and enzymatic membrane reactors. J Hazard Mater 213–214:175–183
Macellaro G, Pezzella C, Cicatiello P, Sannia G, Piscitelli A (2014) Fungal laccases degradation of endocrine disrupting compounds. In: BioMed Res. Int. https://www.hindawi.com/journals/bmri/2014/614038/. Accessed 18 Nov 2017
Madhavi V, Lele SS (2009) Laccase: properties and applications. BioResources 4:1694–1717
Mansur M, Arias ME, Copa-Patiño JL, Flärdh M, González AE (2003) The white-rot fungus Pleurotus ostreatus secretes laccase isozymes with different substrate specificities. Mycologia 95:1013–1020
Mate DM, Alcalde M (2016) Laccase: a multi-purpose biocatalyst at the forefront of biotechnology. Microb Biotechnol 10:1457–1467
Máximo C, Costa-Ferreira M (2004) Decolourisation of reactive textile dyes by Irpex lacteus and lignin modifying enzymes. Process Biochem 39:1475–1479
Mazmanci M, Ünyayar A (2005) Decolorization of Reactive Black 5 by Funalia trogii immobilized on Luffa cylindrica sponge. Process Biochem 40:337–342
Mekmouche Y, Schneider L, Rousselot-Pailley P, Faure B, Jalila Simaan A, Bochot C, Réglier M, Tron T (2015) Laccases as palladium oxidases. Chem Sci 6:1247–1251
Menale C, Nicolucci C, Catapane M, Rossi S, Bencivenga U, Mita DG, Diano N (2012) Optimization of operational conditions for biodegradation of chlorophenols by laccase-polyacrilonitrile beads system. J Mol Catal B Enzym 78:38–44
Michniewicz A, Ledakowicz S, Ullrich R, Hofrichter M (2008) Kinetics of the enzymatic decolorization of textile dyes by laccase from Cerrena unicolor. Dyes Pigments 77:295–302
Mikolasch A, Schauer F (2009) Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl Microbiol Biotechnol 82:605–624
Mirzadeh S-S, Khezri S-M, Rezaei S, Forootanfar H, Mahvi AH, Faramarzi MA (2014) Decolorization of two synthetic dyes using the purified laccase of Paraconiothyrium variabile immobilized on porous silica beads. J Environ Health Sci Eng 12:6
Mogharabi M, Faramarzi MA (2014) Laccase and laccase-mediated systems in the synthesis of organic compounds. Adv Synth Catal 356:897–927
Moldes D, Vidal T (2008) Laccase-HBT bleaching of eucalyptus kraft pulp: influence of the operating conditions. Bioresour Technol 99:8565–8570
Morozova OV, Shumakovich GP, Shleev SV, Yaropolov YI (2007) Laccase-mediator systems and their applications: a review. Appl Biochem Microbiol 43:523–535
Murugesan K, Kim Y-M, Jeon J-R, Chang Y-S (2009) Effect of metal ions on reactive dye decolorization by laccase from Ganoderma lucidum. J Hazard Mater 168:523–529
Murugesan K, Chang Y-Y, Kim Y-M, Jeon J-R, Kim E-J, Chang Y-S (2010) Enhanced transformation of triclosan by laccase in the presence of redox mediators. Water Res 44:298–308
Nemadziva B, Le Roes-Hill M, Koorbanally N, Kudanga T (2018) Small laccase-catalyzed synthesis of a caffeic acid dimer with high antioxidant capacity. Process Biochem 69:99–105
Okazaki S, Michizoe J, Goto M, Furusaki S, Wariishi H, Tanaka H (2002) Oxidation of bisphenol A catalyzed by laccase hosted in reversed micelles in organic media. Enzym Microb Technol 31:227–232
Orlikowska M, de Jesus Rostro-Alanis M, Bujacz A, Hernández-Luna C, Rubio R, Parra R, Bujacz G (2018) Structural studies of two thermostable laccases from the white-rot fungus Pycnoporus sanguineus. Int J Biol Macromol 107:1629–1640
Osma JF, Toca-Herrera JL, Rodríguez-Couto S (2010) Biodegradation of a simulated textile effluent by immobilised-coated laccase in laboratory-scale reactors. Appl Catal A Gen 373:147–153
Othman AM, Elsayed MA, Elshafei AM, Hassan MA (2018) Purification and biochemical characterization of two isolated laccase isoforms from Agaricus bisporus CU13 and their potency in dye decolorization. Int J Biol Macromol 113:1142–1147
Oudia A, Mészáros E, Simões R, Queiroz J, Jakab E (2007) Pyrolysis-GC/MS and TG/MS study of mediated laccase biodelignification of Eucalyptus globulus kraft pulp. J Anal Appl Pyrolysis 78:233–242
Piscitelli A, Giardina P, Lettera V, Pezzella C, Sannia G, Faraco V (2011) Induction and transcriptional regulation of laccases in fungi. Curr Genomics 12:104–112
Polak J, Jarosz-Wilkolazka A, Szuster-Ciesielska A, Wlizlo K, Kopycinska M, Sojka-Ledakowicz J, Lichawska-Olczyk J (2016) Toxicity and dyeing properties of dyes obtained through laccase-mediated synthesis. J Clean Prod 112:4265–4272
Punnapayak H, Prasongsuk S, Messner K, Danmek K, Lotrakul P (2009) Polycyclic aromatic hydrocarbons (PAHs) degradation by laccase from a tropical white rot fungus Ganoderma lucidum. Afr J Biotechnol 8:5897–5900
Rahimi A, Habibi D, Rostami A, Ali Zolfigol M, Mallakpour S (2018) Laccase-catalyzed, aerobic oxidative coupling of 4-substituted urazoles with sodium arylsulfinates: green and mild procedure for the synthesis of arylsulfonyl triazolidinediones. Tetrahedron Lett 59:383–387
Ranocha P, Chabannes M, Chamayou S, Danoun S, Jauneau A, Boudet A-M, Goffner D (2002) Laccase down-regulation causes alterations in phenolic metabolism and cell wall structure in poplar. Plant Physiol 129:145–155
Sadhasivam S, Savitha S, Swaminathan K (2010) Deployment of Trichoderma harzianum WL1 laccase in pulp bleaching and paper industry effluent treatment. J Clean Prod 18:799–806
Sanchez S, Demain AL (2011) Enzymes and bioconversions of industrial, pharmaceutical, and biotechnological significance. Org Process Res Dev 15:224–230
Santo M, Weitsman R, Sivan A (2013) The role of the copper-binding enzyme—laccase—in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int Biodeterior Biodegrad 84:204–210
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42:138–157
Schirmann JG, Dekker RFH, Borsato D, Barbosa-Dekker AM (2018) Selective control for the laccase-catalyzed synthesis of dimers from 2,6-dimethoxyphenol: optimization of 3,3′,5,5′-tetramethoxy-biphenyl-4,4′-diol synthesis using factorial design, and evaluation of its antioxidant action in biodiesel. Appl Catal A Gen 555:88–97
Schubert M, Ruedin P, Civardi C, Richter M, Hach A, Christen H (2015) Laccase-catalyzed surface modification of thermo-mechanical pulp (TMP) for the production of wood fiber insulation boards using industrial process water. PloS One 10:e0128623
Schultz A, Jonas U, Hammer E, Schauer F (2001) Dehalogenation of chlorinated hydroxybiphenyls by fungal laccase. Appl Environ Microbiol 67:4377–4381
Sedarati MR, Keshavarz T, Leontievsky AA, Evans CS (2003) Transformation of high concentrations of chlorophenols by the white-rot basidiomycete Trametes versicolor immobilized on nylon mesh. Electron J Biotechnol 6:104–114
Sharma KK, Kuhad RC (2008) Laccase: enzyme revisited and function redefined. Indian J Microbiol 48:309–316
Sharma KK, Kapoor M, Kuhad RC (2005) In vivo enzymatic digestion, in vitro xylanase digestion, metabolic analogues, surfactants and polyethylene glycol ameliorate laccase production from Ganoderma sp. kk-02. Lett Appl Microbiol 41:24–31
Sharma A, Thakur VV, Shrivastava A, Jain RK, Mathur RM, Gupta R, Kuhad RC (2014) Xylanase and laccase based enzymatic kraft pulp bleaching reduces adsorbable organic halogen (AOX) in bleach effluents: a pilot scale study. Bioresour Technol 169:96–102
Sigoillot C, Camarero S, Vidal T, Record E, Asther M, Pérez-Boada M, Martínez MJ, Sigoillot J-C, Asther M, Colom JF, Martínez AT (2005) Comparison of different fungal enzymes for bleaching high-quality paper pulps. J Biotechnol 115:333–343
Singh G, Kaur K, Puri S, Sharma P (2014) Critical factors affecting laccase-mediated biobleaching of pulp in paper industry. Appl Microbiol Biotechnol 99:154–164
Singh D, Rawat S, Waseem M, Gupta S, Lynn A, Nitin M, Ramchiary N, Sharma KK (2016) Molecular modeling and simulation studies of recombinant laccase from Yersinia enterocolitica suggests significant role in the biotransformation of non-steroidal anti-inflammatory drugs. Biochem Biophys Res Commun 469:306–312
Soares GM, de Amorim MP, Costa-Ferreira M (2001) Use of laccase together with redox mediators to decolourize Remazol Brilliant Blue R. J Biotechnol 89:123–129
Soden DM, Dobson AD (2001) Differential regulation of laccase gene expression in Pleurotus sajor-caju. Microbiology 147:1755–1763
Solomon EI, Sundaram UM, Machonkin TE (1996) Multicopper oxidases and oxygenases. Chem Rev 96:2563–2605
Sousa AC, Oliveira MC, Martins LO, Robalo MP (2014) Towards the rational biosynthesis of substituted phenazines and phenoxazinones by laccases. Green Chem 16:4127–4136
Srebotnik E, Hammel KE (2000) Degradation of nonphenolic lignin by the laccase/1-hydroxybenzotriazole system. J Biotechnol 81:179–188
Steevensz A, Al-Ansari MM, Taylor KE, Bewtra JK, Biswas N (2012) Oxidative coupling of various aromatic phenols and anilines in water using a laccase from Trametes villosa and insights into the ‘PEG effect. J Chem Technol Biotechnol 87:21–32
Streltsov AV, Morozova OV, Arkharova NA, Klechkovskaya VV, Staroverova IN, Shumakovich GP, Yaropolov AI (2009) Synthesis and characterization of conducting polyaniline prepared by laccase-catalyzed method in sodium dodecylbenzenesulfonate micellar solutions. J Appl Polym Sci 114:928–934
Sumathi T, Viswanath B, Sri Lakshmi A, SaiGopal DVR (2016) Production of laccase by Cochliobolus sp. isolated from plastic dumped soils and their ability to degrade low molecular weight PVC. Biochem Res Int 2016:1–10
Svobodová K, Majcherczyk A, Novotný C, Kües U (2008) Implication of mycelium-associated laccase from Irpex lacteus in the decolorization of synthetic dyes. Bioresour Technol 99:463–471
Tavčar M, Svobodová K, Kuplenk J, Novotnỳ C, Pavko A (2006) Biodegradation of Azo Dye RO16 in different reactors by immobilized Irpex lacteus. Acta Chim Slov 53:338–343
Telke AA, Ghodake GS, Kalyani DC, Dhanve RS, Govindwar SP (2011) Biochemical characteristics of a textile dye degrading extracellular laccase from a Bacillus sp. ADR. Bioresour Technol 102:1752–1756. https://doi.org/10.1016/j.biortech.2010.08.086
Thurston CF (1994) The structure and function of fungal laccases. Microbiology 140:19–26
Tilli S, Ciullini I, Scozzafava A, Briganti F (2011) Differential decolorization of textile dyes in mixtures and the joint effect of laccase and cellobiose dehydrogenase activities present in extracellular extracts from Funalia trogii. Enzym Microb Technol 49:465–471
Tychanowicz GK, Zilly A, de Souza CGM, Peralta RM (2004) Decolourisation of industrial dyes by solid-state cultures of Pleurotus pulmonarius. Process Biochem 39:855–859
Uhnáková B, Petříčková A, Biedermann D, Homolka L, Vejvoda V, Bednář P, Papoušková B, Šulc M, Martínková L (2009) Biodegradation of brominated aromatics by cultures and laccase of Trametes versicolor. Chemosphere 76:826–832
Vallecillos L, Sadef Y, Borrull F, Pocurull E, Bester K (2017) Degradation of synthetic fragrances by laccase-mediated system. J Hazard Mater 334:233–243
Valls C, Roncero MB (2009) Using both xylanase and laccase enzymes for pulp bleaching. Bioresour Technol 100:2032–2039
Valls C, Vidal T, Roncero MB (2010a) Boosting the effect of a laccase-mediator system by using a xylanase stage in pulp bleaching. J Hazard Mater 177:586–592
Valls C, Vidal T, Roncero MB (2010b) The role of xylanases and laccases on hexenuronic acid and lignin removal. Process Biochem 45:425–430
Valls C, Quintana E, Roncero MB (2012) Assessing the environmental impact of biobleaching: effects of the operational conditions. Bioresour Technol 104:557–564
Vantamuri AB, Kaliwal BB (2016) Purification and characterization of laccase from Marasmius species BBKAV79 and effective decolorization of selected textile dyes. 3 Biotech 6:189
Vats A, Mishra S (2018) Identification and evaluation of bioremediation potential of laccase isoforms produced by Cyathus bulleri on wheat bran. J Hazard Mater 344:466–479
Vicente AI, Viña-Gonzalez J, Santos-Moriano P, Marquez-Alvarez C, Ballesteros AO, Alcalde M (2016) Evolved alkaline fungal laccase secreted by Saccharomyces cerevisiae as useful tool for the synthesis of C–N heteropolymeric dye. J Mol Catal B Enzym 134:323–330
Wellington KW, Kolesnikova NI (2012) A laccase-catalysed one-pot synthesis of aminonaphthoquinones and their anticancer activity. Bioorg Med Chem 20:4472–4481
Wellington KW, Qwebani-Ogunleye T, Kolesnikova NI, Brady D, de Koning CB (2013) One-pot laccase-catalysed synthesis of 5,6-dihydroxylated benzo[b]furans and catechol derivatives, and their anticancer activity. Arch Pharm (Weinheim) 346:266–277
Witayakran S, Ragauskas AJ (2009) Cocatalytic enzyme system for the Michael addition reaction of in-situ-generated ortho-quinones. Eur J Org Chem 2009:358–363
Wong K-S, Huang Q, Au C-H, Wang J, Kwan H-S (2012) Biodegradation of dyes and polyaromatic hydrocarbons by two allelic forms of Lentinula edodes laccase expressed from Pichia pastoris. Bioresour Technol 104:157–164
Xu F, Shin W, Brown SH, Wahleithner JA, Sundaram UM, Solomon EI (1996) A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim Biophys Acta 1292:303–311
Yang J, Yang X, Lin Y, Ng TB, Lin J, Ye X (2015) Laccase-catalyzed decolorization of malachite green: performance optimization and degradation mechanism. PLoS One 10:e0127714
Yang J, Li W, Ng TB, Deng X, Lin J, Ye X (2017) Laccases: production, expression regulation, and applications in pharmaceutical biodegradation. Front Microbiol 8:832
Zeng X, Cai Y, Liao X, Zeng X, Luo S, Zhang D (2012) Anthraquinone dye assisted the decolorization of azo dyes by a novel Trametes trogii laccase. Process Biochem 47:160–163
Zeng S, Qin X, Xia L (2017) Degradation of the herbicide isoproturon by laccase-mediator systems. Biochem Eng J 119:92–100
Zhang X, Eigendorf G, Stebbing DW, Mansfield SD, Saddler JN (2002) Degradation of trilinolein by laccase enzymes. Arch Biochem Biophys 405:44–54
Zhang J, Chen H, Chen M, Ren A, Huang J, Wang H, Zhao M, Feng Z (2015). Cloning and functional analysis of a laccase gene during fruiting body formation in Hypsizygus marmoreus. 179: 54–63
Zhang D, Liu M, Liu Y, Li H (2016) Characteristics of lignocellulosic fibers from hardwood pulp by laccase-catalyzed TEMPO oxidation. Fibers Polym 17:1330–1335
Zhu X, Williamson PR (2004) Role of laccase in the biology and virulence of Cryptococcus neoformans. FEMS Yeast Res 5:1–10
Zhuo R, Ma L, Fan F, Gong Y, Wan X, Jiang M, Zhang X, Yang Y (2011) Decolorization of different dyes by a newly isolated white-rot fungi strain Ganoderma sp.En3 and cloning and functional analysis of its laccase gene. J Hazard Mater 192:855–873
Zhuo R, Yu H, Yuan P, Fan J, Chen L, Li Y, Ma F, Zhang X (2018) Heterologous expression and characterization of three laccases obtained from Pleurotus ostreatus HAUCC 162 for removal of environmental pollutants. J Hazard Mater 344:499–510
Zille A, Gornacka B, Rehorek A, Cavaco-Paulo A (2005) Degradation of azo dyes by Trametes villosa laccase over long periods of oxidative conditions. Appl Environ Microbiol 71:6711–6718
Acknowledgements
The authors AS and AJS acknowledge financial assistance received from the Council of Scientific and Industrial Research (CSIR) as Senior Research Fellowship. KKJ acknowledge fellowship received from SERB (FILE NO.PDF/2016/001068) as National Post-Doctoral Fellow.
Funding source
This study was funded as Senior Research Fellowship for AS from the Council of Scientific and Industrial Research (CSIR) as Senior Research Fellowship. KKJ acknowledge fellowship received from SERB (FILE NO.PDF/2016/001068) as National Post-Doctoral Fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
No ethical approval is required as no animals or humans have been used in the study.
Rights and permissions
About this article
Cite this article
Sharma, A., Jain, K.K., Jain, A. et al. Bifunctional in vivo role of laccase exploited in multiple biotechnological applications. Appl Microbiol Biotechnol 102, 10327–10343 (2018). https://doi.org/10.1007/s00253-018-9404-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9404-8