Abstract
d-Tagatose has attracted a great deal of attention in recent years due to its health benefits and similar properties to sucrose. d-Tagatose can be used as a low-calorie sweetener, as an intermediate for synthesis of other optically active compounds, and as an additive in detergent, cosmetic, and pharmaceutical formulation. Biotransformation of d-tagatose has been produced using several biocatalyst sources. Among the biocatalysts, l-arabinose isomerase has been mostly applied for d-tagatose production because of the industrial feasibility for the use of d-galactose as a substrate. In this article, the characterization of many l-arabinose isomerases and their d-tagatose production is compared. Protein engineering and immobilization of the enzyme for increasing the conversion rate of d-galactose to d-tagatose are also reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
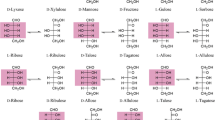
d-Tagatose, a rare natural hexoketose, is an isomer of d-galactose. The cyclic form of d-tagatose consists of α-d-tagato-2,6-pyranose (79%), β-d-tagato-2,6-pyranose (14%), α-d-tagato-2,5-furanose (2%), and β-d-tagato-2,6-furanose (5%; Köpper and Freimund 2003). d-Tagatose occurs naturally in Sterculia setigera gum, and it is also found in small quantities in various foods such as sterilized and powdered cow’s milk, hot cocoa, and a variety of cheeses, yogurts, and other dairy products (Mendoza et al. 2005; Richards and Chandrasekhara 1960; Troyono et al. 1992).
d-Tagatose production can be produced from d-galactose by a chemical method using a calcium catalyst (Beadle et al. 1991), but the chemical process has some disadvantages, such as complex purification steps, chemical waste formation, and by-products formation. To overcome these disadvantages, biological manufactures of d-tagatose using several biocatalyst sources have been studied intensively in recent years. Among the biocatalysts, l-arabinose isomerase (EC 5.3.1.4) catalyzes the conversion of d-galactose to d-tagatose as well as the conversion of l-arabinose to l-ribulose, owing to the similar configurations of the substrates (Cheetham and Wootton 1993; Roh et al. 2000a).
In this article, biological production of d-tagatose using l-arabinose isomerase is mainly described. The reaction conditions, protein engineering, and immobilization on l-arabinose isomerase are introduced in the viewpoint of effective d-tagatose production. Moreover, high production of d-tagatose using equilibrium-shifted conversion is suggested.
Properties
The melting temperature of d-tagatose is 134°C, and it is stable at pH 2–7. d-Tagatose has high solubility [58% (w/w) at 21°C], which makes it ideal as a flavor enhancer or fiber in soft drinks and yogurts. Its humectant properties are similar to those of sorbitol. d-Tagatose is less hygroscopic than fructose, and it is lower in viscosity [180 cP at 70% (w/w) and 20°C] than sucrose at the same concentration but slightly higher than fructose and sorbitol. As d-tagatose is a reducing sugar, it is involved in browning reactions during heat treatment. It decomposes more readily than sucrose at high temperatures (Kim 2004; Levin 2002).
d-Tagatose is a malabsorbing sugar, as it is poorly absorbed in the small intestine (Buemann et al. 1999a, b, 2000; Lærke and Jensen 1999). The unabsorbed fraction of d-tagatose reaches the large intestine where it is completely fermented by the intestinal microflora. The formed short chain fatty acids are absorbed almost completely and are metabolized. During fermentation, there is a relatively low energy recovery, and a certain amount of energy is lost due to increased biomass excretion of the microflora (Bertelsen et al. 2001).
The sweetness of d-tagatose is 92% that of sucrose when compared in 10% solutions. d-Tagatose has a sucrose-like taste with no cooling effect or aftertaste. d-Tagatose is similar to the polyols in having a low caloric value and tooth-friendly property. However, it has no laxative effect unlike polyols (Levin et al. 1995). Although the taste quality of d-tagatose is similar to sucrose, d-tagatose does not contribute to calorie production (Levin 2002; Zehner and Lee 1988). A growth study on rats reports that d-tagatose contributed no net energy (Bar et al. 1999; Livesey and Brown 1996), and human clinical trials shows that subjects gradually and consistently lose weight at medically desirable rates (Levin 2002). As a result of these properties, d-tagatose is considered to be a potential reduced-energy sweetener.
Applications
d-Tagatose have numerous health benefits, including promotion of weight loss (Buemann et al. 2000), no glycemic effect (Donner et al. 1999; Seri et al. 1993), anti-plaque, non-cariogenic, anti-halitosis, prebiotic, and anti-biofilm properties (Bertelsen et al. 1999; Cisar et al. 1979; Lærke et al. 2000; Wong 2000), organ transplants (Paterna et al. 1998), enhancement of flavor (Rosenplenter and Mende 2004), improvement of pregnancy and fetal development (Levin 2000), treatment of obesity (Moore 2006), and reduction in symptoms associated with type 2 diabetes, hyperglycemia, anemia, and hemophilia (Levin 2002; Seri et al. 1993). The heath benefits and application of d-tagatose are summarized in Table 1.
d-Tagatose can be used as a low-calorie sweetener in nonchronic drugs, tooth paste, and mouth wash and in a wide variety of foods, beverages, health foods, and dietary supplements; it can be applied to products of low-carbohydrate diets, cereals, health bars, chocolate, candy, chewing gum, yogurt, soft drink, bakery, milk-based drink, and confectionery. d-Tagatose is also useful as an intermediate for synthesis of other optically active compounds and as an additive in detergent, cosmetic, and pharmaceutical formulation (Ibrahim and Spradlin 2000).
Biological production of tagatose
Biological manufactures of d-tagatose have been studied using several biocatalyst sources. Arthrobacter globiformis (Izumori et al. 1984), Gluconobacter oxydans (Manzoni and Rollini 2001; Rollini and Manzoni 2005), Mycobacterium smegmatis (Izumori and Tsuzaki 1988), Enterobacter agglomerans (Muniruzzanman et al. 1994), and Klebsiella pneumoniae (Shimonishi et al. 1994) have been reported to convert galactitol into d-tagatose. The responsible enzyme for the biotransformation from galactitol to d-tagatose is a sorbitol dehydrogenase (Rollini and Manzoni 2005). Agrobacterium tumefaciensd-psicose 3-epimerase (Kim et al. 2006a) and Pseudomonas cichoriid-tagatose 3-epimerase convert d-sorbose to d-tagatose (Itoh et al. 1994; Ishida et al. 1997; Yoshida et al. 2007). However, d-sorbose or galactitol is an expensive substrate that seems to have little potential for commercial application.
Various strains of Mucoraceae fungi convert d-psicose to d-tagatose (Yoshihara et al. 2006). As the mass production of d-psicose from d-fructose has became industrial feasible in recent years (Kim et al. 2006a; Takeshita et al. 2000), the production of d-tagatose from d-fructose via d-psicose as an alternative method can be proposed in spite of the requirement for further intensive investigation.
A new process to convert d-galactose to d-tagatose is introduced using lactic acid bacteria (Cheetham and Wootton 1993). Enterobacter agglomerans also produces d-tagatose from d-galactose when grown on an l-arabinose pre-induced medium (Oh et al. 1998). The cloned l-arabinose isomerases of Escherichia coli, Bacillus subtilis, and Salmonella typhimurium mediate the conversion of d-tagatose from d-galactose (Roh et al. 2000a). l-Arabinose isomerase has been of interest and studied intensively in recent years due to its industrial feasibility in d-tagatose production (Kim et al. 2003a).
Characterization of l-arabinose isomerase
For effective d-tagatose productions using the enzymes, the reaction conditions such as pH, temperature, and metal ion should be optimized. The optimal reaction conditions of these bacterial l-arabinose isomerases are compared (Table 2). The optimum temperature for l-arabinose isomerase is in the range of 30–50°C in mesophiles such as Aerobacter aerogenes (Yamanaka and Wood 1966), Alicyclobacillus acidocaldarius (Lee et al. 2005b), Bacillus halodurans (Lee et al. 2005a), Escherichia coli (Kim et al. 2001a, b; Roh et al. 2000a, b; Yoon et al. 2003), Lactobacillus gayonii (Nakamatu and Yamanaka 1969), and M. smegmatis (Izumori et al. 1978); of 60–80°C in thermophiles such as B. stearothermophilus (Rhimi 2007a, b), Geobacillus stearothermophilus (Jung et al. 2005; Kim et al. 2003a; Lee et al. 2005a; Oh et al. 2006a; Ryu et al. 2003), G. thermodenitrificans (Baek et al. 2004; Kim and Oh 2005; Oh et al. 2006b), Thermus sp. (Kim et al. 2003b), and Thermoanaerobacter mathranii (Jørgensen et al. 2004); and of 85-90°C in hyperthermophiles such as Thermotoga neapolitana (Kim et al. 2002; Hong et al. 2007) and T. maritima (Lee et al. 2004).
The optimum pH for l-arabinose isomerase from Alicyclobacillus acidocaldarius is 6.0 (Lee et al. 2005b), while the enzymes from G. thermodenitrificans and Thermus sp. have optimum pH of 8.5 (Kim and Oh 2005; Kim et al. 2003b). The optimum pHs of the others are in the range 6.0–8.0.
The mesophilic and thermophilic l-arabinose isomerases require Mn2+ as a cofactor to enhance the isomerization rate reaction (Patrick and Lee 1968), while the hyperthermophilic l-arabinose isomerases require Co2+ (Kim et al. 2002; Lee et al. 2004). Mn2+ ion has also been shown to enhance the rate of the arabinose–ribulose isomerization reaction (Nakamatu and Yamanaka 1969; Patrick and Lee 1968).
The kinetic parameters of various bacterial l-arabinose isomerases for l-arabinose or d-galactose as a substrate are presented in Table 3. All reported bacterial l-arabinose isomerases have higher specificity for l-arabinose than d-galactose, suggesting that more research to alter substrate specificity into d-galactose should be needed. The ratio of catalytic efficiency (k cat/K m) for d-galactose to l-arabinose decreased when the optimum temperature of l-arabinose isomerase was increased; the ratio of k cat/K m was 128 in the mesophilic enzyme from B. halodurans, 51 in the thermophilic enzyme from G. stearothermophilus, respectively, and 9 in the hyperthermophilic enzyme from T. maritima. These results suggested that substrate specificity at high temperatures altered from l-arabinose into d-galactose.
Tagatose production using l-arabinose isomerase
Protein engineering of l-arabinose isomerase
The three-dimensional structure of E. colil-arabinose isomerase was solved (Manjasetty and Chance 2006). The crystal structure and structural comparison with E. colil-fucose isomerase suggested a possible metal binding site. The crystal structure forms a basis for identifying molecular determinants responsible for isomerization of galactose to tagatose. Based on the solved crystal structure and sequence alignments, the residues for the essential catalytic site and substrate recognition of G. stearothermophilus US 100 l-arabinose isomerase was suggested (Rhimi et al. 2007b).
Molecular evolution strategies for improving d-galactose isomerization are proposed as the increases of substrate specificity, thermostability, and optimum temperature and the decrease in optimum pH (Kim 2004). Directed evolution of the l-arabinose isomerase gene has been suggested as a powerful tool for increasing the reaction rate (Kim et al. 2001a). A mutated l-arabinose isomerase from G. stearothermophilus was obtained by an error-prone polymerase chain reaction, and it exhibits the change of three amino acids (V322M, A393T, and A408V) compared to the wild-type enzyme. The mutated l-arabinose isomerase shows increases in d-galactose isomerization activity, optimum temperature, catalytic efficiency (k cat/K m) for d-galactose, and the production rate of d-tagatose from d-galactose (Kim et al. 2006b). A double-sites mutant enzyme, C450S-N475K of l-arabinose isomerase from G. thermodenitrificans, was obtained by site-directed mutagenesis. The double-sites mutant enzyme converts d-galactose to d-tagatose with a yield of 58%, whereas a wild type gives 46% of d-tagatose conversion after 300 min at 65°C (Oh et al. 2006b).
Commercial application of l-arabinose isomerase requires an acidic pH range to reduce nonspecific side and browning reactions (Kim 2004). The optimum pHs of Q408V and R408V mutants are shifted to pH 7.5 from 8.5, and the galactose isomerization activities of the mutants are 60 and 30% higher than that of wild type, respectively (Oh et al. 2006a). Using site-directed mutagenesis, comparative studies with the wild type and its mutants are performed, and it is found that the Lys-269 of acidophilic l-arabinose isomerases from Alicyclobacillus acidocaldarius has a role to determine pH optimum (Lee et al. 2005b).
Tagatose production using immobilized biocatalyst
To produce d-tagatose effectively, l-arabinose isomerase or cells containing the enzyme should be immobilized, and a bioreactor containing immobilized biocatalyst is used for industrial production of d-tagatose. In terms of the productivity relative to the biomass as specific productivity, the immobilized cells are more efficient than the immobilized enzyme. Using the same mass of cells, d-tagatose production by immobilized cells is higher than that by immobilized enzyme (Jung et al. 2005). The lower tagatose production of the immobilized enzymes obtained from the same cell mass was due to the decreased activity resulting from enzyme purification process, such as cell lysis, precipitation, and dialysis.
The immobilized enzyme reactor can be used at the highly concentrated enzyme, whereas the immobilized cell reactor cannot be used because at high cell concentrations, immobilized cells contain high levels of other cell materials, which have no tagatose-producing activity and cause steric hindrance (Jung et al. 2005). As a result, the immobilized enzyme reactor has a higher tagatose titer, conversion yield, and volumetric productivity than the immobilized cell reactor, immobilized G. stearothermophilusl-arabinose isomerase entrapped by alginate produces d-tagatose at an average d-tagatose productivity of 1,296 g l−1 day−1 as the reported highest productivity (Ryu et al. 2003). The immobilized E coli cells containing G. stearothermophilusl-arabinose isomerase in alginate produces a productivity of 70 g l−1 d−1 in a bioreactor (Jung et al. 2005) while the immobilized E coli cells containing Thermotoga neapolitanal-arabinose isomerase produces a productivity of 98 g l−1 day−1 (Hong et al. 2007).
Comparison of tagatose production
A summary of d-tagatose production from d-galactose by various l-arabinose isomerases and cells containing the enzyme is shown in Table 4. The d-tagatose equilibrium from d-galactose is 28.8% at 30°C in E. colil-arabinose isomerase (Kim et al. 2001b), 58% at 65°C in the enzyme from G. thermodenitrificans (Oh et al. 2006b), and 68% at 85°C in Thermotoga neapolitana (Kim et al. 2002). The content of product of isomerization reaction is determined by the reaction temperature, and it is shifted toward d-tagatose as a product at higher temperatures.
The highest levels of d-tagatose production have been reported 230 g l−1 tagatose from 500 g l−1d-galactose using immobilized G. stearothermophilusl-arabinose isomerase in continuous recycling mode of a packed-bed bioreactor with a tagatose productivity of 230 g l−1 day−1 (Kim et al. 2003a). In continuous mode using the immobilized enzyme, 145 g l−1d-tagatose from 300 g l−1d-galactose was produced in the bioreactor with an average tagatose productivity of 1,296 g l−1 day−1 as the reported highest productivity (Ryu et al. 2003), which was 5.6-fold higher than that in continuous recycling mode. The increase in productivity may be due to the use of 1.5-fold higher amount of enzyme and continuous mode and operation under the optimized conditions such as bead size, length/diameter (L/D) of the reactor, dilution rate, total loaded enzyme amount, and substrate concentration. The highest reported yield of d-tagatose, which is catalyzed by Thermotoga neapolitanal-arabinose isomerase using 1.8 g l−1d-galactose, is 68% at 85°C (Kim et al. 2002).
Equilibrium-shifted production of tagatose
The equilibrium between substrate and product cannot be simply changed by protein engineering of the enzyme, as it is controlled by the reaction temperature (Chang et al. 1999). To obtain a higher conversion yield of product than that defined at the specific temperature, the equilibrium should be shifted toward product. If a material binds more specific to product (d-tagatose) than substrate (d-galactose), the addition of a material (like boric acid) causes an equilibrium shift by complex formation between the product and material. Once formed, the product–material complex does not participate in the equilibrium reaction. When residual substrate is converted to product to reestablish the reaction equilibrium, the result is the shifting of the equilibrium toward product formation. The formation of the product–material complex can be proposed as an alternative to high-temperature biotransformation for feasible industrial production of d-tagatose.
Borate forms complexes with carbohydrates (De Muynck et al. 2006) and interacts with enzyme systems and changes the equilibrium of any reaction involving cis-diol carbohydrates (Smith and Johnson 1976). Some ketoses such as lactulose, maltulose, and celluobiulose are synthesized from aldoses such as lactose, maltose, and cellobiose, respectively, by the high-yielding isomerization in alkaline solutions containing borate (Hicks et al. 1983; Hicks and Parrish 1980). We found the greater complexing capacity of borate to d-tagatose than d-galactose. In the isomerization reaction between d-galactose and d-tagatose catalyzed by l-arabinose isomerase, the favored formation of d-tagatose/borate complexes shifted the equilibrium toward d-tagatose formation. Now, we attempt high production of d-tagatose using the equilibrium-shifted conversion.
Further research
The three-dimensional structure of E. colil-arabinose isomerase was already solved (Manjasetty and Chance 2006), and the residues for the essential catalytic site and substrate recognition was suggested based on the solved crystal structure and sequence alignments (Rhimi et al. 2007b). Mutation studies for the amino acids on or near the active site should be intensively performed to know the molecular determinants of l-arabinose isomerase activity. The molecular determinants will change the substrate specificity, increase conversion rate, and alter the pH range for activity, and it will provide information for the development of industrial valuable l-arabinose isomerases.
d-Tagatose production by enzyme or cell of non-generally recognized as safe (GRAS) host may cause safety problem (Burdock and Carabin 2004). The problem can be solved by transferring the gene of l-arabinose isomerase to GRAS host such as Bacillus megaterium, Corynebacterium ammonagenes, and C. glutamicum.
In the future, l-arabinose isomerase will be able to produce industrially d-tagatose because the biotransformation has some advantages over the chemical process, such as mild reaction conditions, d-tagatose production without by-products, no salt in waste water, and d-galactose recycling during purification.
References
Baek DH, Lee Y, Sin HS, Oh DK (2004) A new thermophile strain of Geobacillus thermodenitrificans having l-arabinose isomerase activity for tagatose production. J Microbiol Biotechnol 14:312–316
Bar A, Lina BA, de Groot DM, de Bie B, Appel MJ (1999) Effect of d-tagatose on liver weight and glycogen content of rats. Regul Toxicol Pharmacol 29:S11–S28
Beadle JR, Saunder JP, Wajada TJ (1991) Process for manufacturing tagatose. US patent 500261
Bertelsen H, Jensen BB, Buemann B (1999) d-Tagatose: a novel low-caloric bulk sweetener with prebiotic properties. World Rev Nutr Diet 85:98–109
Bertelsen H, Andersen H, Tvede M (2001) Fermentation of d-tagatose by human intestinal bacteria and dairy lactic acid bacteria. Microb Ecol Health Dis 13:87–95
Buemann B, Toubro S, Astrup A (1999a) Human gastrointestinal tolerance to d-tagatose. Regul Toxicol Pharmacol 29:S71–S77
Buemann B, Toubro S, Raben A, Astrup A (1999b) Human tolerance to a single, high dose of d-tagatose. Regul Toxicol Pharmacol 29:S66–S70
Buemann B, Toubro S, Raben A, Blundell J, Astrup A (2000) The acute effect of d-tagatose on food intake in human subjects. Br J Nutr 84:227–231
Burdock GA, Carabin IG (2004) Generally recognized as safe (GRAS): history and description. Toxicol Lett 150:3–18
Chang C, Park BC, Lee DS, Suh SW (1999) Crystal structures of thermostable xylose isomerases from Thermus caldophilus and Thermus thermophilus: possible structural determinants of thermostability. J Mol Biol 288:623–634
Cheetham PSJ, Wootton AN (1993) Bioconversion of d-galactose into d-tagatose. Enzyme Microb Technol 15:105–108
Cisar JO, Kolenbrander PE, Mclntire FC (1979) Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun 24:742–752
De Muynck C, Beauprez J, Soetaert W, Vandamme EJ (2006) Boric acid as a mobile phase additive for high performance liquid chromatography separation of ribose, arabinose and ribulose. J Chromatogr 1101:115–121
Donner TW, Wilber JF, Ostrowski D (1999) d-Tagatose, a novel hexose: acute effects on carbohydrate tolerance in subjects with and without type 2 diabetes. Diabetes Obes Metab 1:285–291
Hicks KB, Parrish FW (1980) A new method for the preparation of lactulose from lactose. Carbohydr Res 82:393–397
Hicks KB, Symanski EV, Pfeffer PE (1983) Synthesis and high-performance liquid chromatography of maltulose and cellobiulose. Carbohydr Res 112:37–50
Hong YH, Lee DW, Lee SJ, Choe EA, Kim SB, Lee YH, Cheigh CI, Pyun YR (2007) Production of d-tagatose at high temperatures using immobilized Escherichia coli cells expressing l-arabinose isomerase from Thermotoga neapolitana. Biotechnol Lett 29:569–574
Ibrahim OO, Spradlin JE (2000) Process for manufacturing d-tagatose. US Patent 6057135
Ishida Y, Kamiya T, Itoh H, Kimura Y, Izumori K (1997) Cloning and characterization of the d-tagatose 3-epimerase gene from Pseudomonas cichorii ST-24. J Ferment Bioeng 83:529–534
Itoh H, Okaya H, Khan AR, Tajima S, Hayakawa S, Izumori K (1994) Purification and characterization of d-tagatose 3-epimerase from Pseudomonas sp. ST-24. Biosci Biotechnol Biochem 58:2168–2171
Izumori K, Tsuzaki K (1988) Production of d-tagatose from d-galactitol by Mycobacterium smegmatis. J Ferment Technol 66:225–227
Izumori K, Ueda Y, Yamanaka K (1978) Pentose metabolism in Mycobacterium smegmatis: comparison of l-arabinose isomerases induced by l-arabinose and d-galactose. J Bacteriol 133:413–414
Izumori K, Miyoshi T, Tokuda S, Yamabe K (1984) Production of d-tagatose from ducitol by Arthrobacter globiformis. Appl Environ Microbiol 46:1055–1057
Jørgensen F, Hansen OC, Stougaard P (2004) Enzymatic conversion of d-galactose to d-tagatose: heterologous expression and characterisation of a thermostable l-arabinose isomerase from Thermoanaerobacter mathranii. Appl Microbiol Biotechnol 64:816–822
Jung ES, Kim HJ, Oh DK (2005) Tagatose production by immobilized recombinant Escherichia coli cells containing Geobacillus stearothermophilusl-arabinose isomerase mutant in a packed-bed bioreactor. Biotechnol Prog 21:1335–1340
Kim P (2004) Current studies on biological tagatose production using l-arabinose isomerase: a review and future perspective. Appl Microbiol Biotechnol 65:243–249
Kim HJ, Oh DK (2005) Purification and characterization of an l-arabinose isomerase from an isolated strain of Geobacillus thermodenitrificans producing d-tagatose. J Biotechnol 120:162–173
Kim P, Yoon SH, Seo MJ, Oh DK, Choi JH (2001a) Improvement of tagatose conversion rate by genetic evolution of thermostable galactose isomerase. Biotechnol Appl Biochem 34:99–102
Kim P, Yoon SH, Roh HJ, Choi JH (2001b) High production of d-tagatose, a potential sugar substitute, using immobilized l-arabinose isomerase. Biotechnol Prog 17:208–210
Kim BC, Lee YH, Lee HS, Lee DW, Choe EA, Pyun YR (2002) Cloning, expression and characterization of l-arabinose isomerase from Thermotoga neapolitana: bioconversion of d-galactose to d-tagatose using the enzyme. FEMS Microbiol Lett 212:121–126
Kim HJ, Ryu SA, Kim P, Oh DK (2003a) A feasible enzymatic process for d-tagatose production by an immobilized thermostable l-arabinose isomerase in a packed-bed bioreactor. Biotechnol Prog 19:400–404
Kim JW, Kim YW, Roh HJ, Kim HY, Cha JH, Park KH, Park CS (2003b) Production of tagatose by a recombinant thermostable l-arabinose isomerase from Thermus sp. IM6501. Biotechnol Lett 25:963–967
Kim HJ, Hyun EK, Kim YS, Lee YJ, Oh DK (2006a) Characterization of an Agrobacterium tumefaciensd-psicose-3-epimerase that converts d-fructose to d-psicose. Appl Environ Microbiol 72:981–985
Kim HJ, Kim JH, Oh HJ, Oh DK (2006b) Characterization of a mutated Geobacillus stearothermophilusl-arabinose isomerase that increases the production rate of d-tagatose. J Appl Microbiol 101:213–221
Köpper S, Freimund S (2003) The composition of keto aldoses in aqueous solution as determined by NMR spectroscopy. Helv Chim Acta 86:827–843
Lærke HN, Jensen BB (1999) d-Tagatose has low small intestinal digestibility but high large intestinal fermentability in pigs. J Nutr 129:1002–1009
Lærke HN, Jensen BB, Højsgaard S (2000). In vitro fermentation pattern of d-tagatose is affected by adaptation of the microbiota from the gastrointestinal tract of pigs. J Nutr 130:1772–1779
Lee DW, Jang HJ, Choe EA, Kim BC, Lee SJ, Kim SB, Hong YH, Pyun YR (2004) Characterization of a thermostable l-arabinose (d-galactose) isomerase from the hyperthermophilic eubacterium Thermotoga maritima. Appl Environ Microbiol 70:1397–1404
Lee DW, Choe EA, Kim BC, Eom SH, Hong YH, Lee SJ, Lee HS, Lee DY, Pyun YR (2005a) Distinct metal dependence for catalytic and structural functions in the l-arabinose isomerase from the mesophilic Bacillus halodurans and the thermophilic Geobacillus stearothermophilus. Arch Biochem Biophys 434:333–343
Lee SJ, Lee DW, Choe EA, Hong YH, Kim SB, Kim BC, Pyun YR (2005b) Characterization of a thermoacidophilic l-arabinose isomerase from Alicyclobacillus acidocaldarius: role of Lys-269 in pH optimum. Appl Environ Microbiol 71:7888–7896
Levin GV (2000) Increased fertility and improved fetal development. US patent 6225452
Levin GV (2002) Tagatose, the new GRAS sweetener and health product. J Med Food 5:23–36
Levin GV, Zehner LR, Saunders JP, Beadle JR (1995) Sugar substitutes: their energy values, bulk characteristics, and potential health benefits. Am J Clin Nutr 62:S1161–S1168
Livesey G, Brown JC (1996) d-Tagatose is a bulk sweetener with zero energy determined in rats. J Nutr 126:1601–1609
Manjasetty BA, Chance MR (2006) Crystal Structure of Escherichia colil-arabinose isomerase (ECAI), the putative target of biological tagatose production. J Mol Biol 360:297–309
Manzoni M, Rollini M (2001) Bioconversion of d-galactitol to tagatose by acetic acid bacteria. Process Biochem 36:971–977
Mendoza MR, Olano A, Villamiel M (2005) Chemical indicators of heat treatment in fortified and special milks. J Agric Food Chem 53:2995–2999
Moore MC (2006) Drug evaluation: tagatose in the treatment of type 2 diabetes and obesity. Curr Opin Investig Drugs 7:924–935
Muniruzzanman S, Tokunaga H, Izumori K (1994) Isolation of Enterobacter agglomerans strain 221e from soil, a potent d-tagatose producer from galactitol. J Ferment Bioeng 78:145–148
Nakamatu T, Yamanaka K (1969) Crystallization and properties of l-arabinose isomerase from Lactobacillus gayonii. Biochim Biophys Acta 178:156–165
Oh DK, Roh HJ, Kim SY, Noh BS (1998) Optimization of culture conditions for d-tagatose production from d-galactose by Enterobacter agglomerans. Kor J Appl Microbiol Biotechnol 26:250–256
Oh DK, Kim HJ, Ryu SA, Kim P (2001) Development of an immobilization method of l-arabinose isomerase for industrial production of tagatose. Biotechnol Lett 23:1859–1862
Oh DK, Kim HJ, Oh HJ, Cheon J, Kim P (2006a) Modification of optimal pH in l-arabinose isomerase from Geobacillus stearothermophilus for d-galactose isomerization. J Mol Catal B Enzym 43:108–112
Oh HJ, Kim HJ, Oh DK (2006b) Increase in d-tagatose production rate by site-directed mutagenesis of l-arabinose isomerase from Geobacillus thermodenitrificans. Biotechnol Lett 28:145–149
Paterna JC, Boess F, Stäubli A, Boelsterli UA (1998) Antioxidant and cytoprotective properties of d-tagatose in cultured murine hepatocytes. Toxicol Appl Pharm 148:117–125
Patrick JW, Lee N (1968) Purification and properties of an l-arabinose isomerase from Escherichia coli. J Biol Chem 243:4312–4318
Rhimi M, Messaoud EB, Borgi MA, Khadra KB, Bejar S (2007a) Co-expression of l-arabinose isomerase and d-glucose isomerase in E. coli and development of an efficient process producing simultaneously d-tagatose and d-fructose. Enzyme Microb Technol 40:1531–1537
Rhimi M, Juy M, Aghajari N, Haser R, Bejar S (2007b) Probing the essential catalytic residues and the substrate affinity in the thermoactive Bacillus stearothermophilus US100 l-arabinose isomerase by site-directed mutagenesis. J Bacteriol 189:3556–3563
Richards EL, Chandrasekhara MR (1960) Chemical changes in dried skim milk during storage. J Dairy Res 27:59–66
Roh HJ, Kim P, Park YC, Choi JH (2000a) Bioconversion of d-galactose into d-tagatose by expression of l-arabinose isomerase. Biotechnol Appl Biochem 31:1–4
Roh HJ, Yoon SH, Kim P (2000b) Preparation of l-arabinose isomerase originated from Escherichia coli as a biocatalyst for d-tagatose production. Biotechnol Lett 22:197–199
Rollini M, Manzoni M (2005) Bioconversion of d-galactitol to tagatose and dehydrogenase activity induction in Gluconobacter oxydans. Process Biochem 40:437–444
Rosenplenter K, Mende K (2004) Use of d-tagatose for improving aroma and flavor in foods and beverages. WO patent 2004/073419
Ryu SA, Kim CS, Kim HJ, Baek DH, Oh DK (2003) Continuous d-tagatose production by immobilized thermostable l-arabinose isomerase in a packed-bed bioreactor. Biotechnol Prog 19:1643–1647
Seri K, Sanai K, Negishi S, Akino T (1993) Prophylactic and remedial preparation for diseases attendant on hyperglycemia, and wholesome food. European Patent 560284
Shimonishi T, Okumura Y, Izumori K (1994) Production of d-tagatose from galactitol by Klebsiella pneumoniae strain 40b. J Ferment Bioeng 78:145–148
Smith KW, Johnson SL (1976) Borate inhibition of yeast alcohol dehydrogenase. Biochemistry 15:560–565
Takeshita K, Suga A, Takada G, Izumori K (2000) Mass production of d-psicose from d-fructose by a continuous bioreactor system using immobilized d-tagatose 3-epimerase. J Biosci Bioeng 90:453–455
Troyono E, Martinez-Castro I, Olano A (1992) Kinetics of galactose and tagatose formation during heat-treatment of milk. Food Chem 45:41–43
Wong D (2000) Sweetener determined safe in drugs, mouthwashes, and toothpastes. Dent Today 19:34–35
Yamanaka K, Wood WA (1966) l-Arabinose isomerase. Methods Enzymol 9:596–602
Yoon SH, Kim P, Oh DK (2003) Properties of l-arabinose isomerase from Escherichia coli as biocatalysis for tagatose production. World J Microbiol Biotechnol 19:47–51
Yoshida H, Yamada M, Nishitani T, Takada G, Izumori K, Kamitori S. (2007) Purification, crystallization and preliminary X-ray diffraction studies of d-tagatose 3-epimerase from Pseudomonas cichorii. Acta Crystallog Sect F Struct Biol Cryst Commun 63:123–125
Yoshihara K, Shinohara Y, Hirotsu T, Izumori K (2006) Bioconversion of d-psicose to d-tagatose and d-talitol by Mucoraceae Fungi. J Biosci Bioeng 100:219–222
Zehner LR, Lee R (1988) d-Tagatose as a low-calorie carbohydrate sugar and bulking agent. European patent 257626
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, DK. Tagatose: properties, applications, and biotechnological processes. Appl Microbiol Biotechnol 76, 1–8 (2007). https://doi.org/10.1007/s00253-007-0981-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-0981-1