Abstract
Phycocyanin (PC) is a light-harvesting protein isolated from Spirulina and has health benefits for a range of diseases including pulmonary fibrosis (PF). In this study, a bleomycin-induced pulmonary fibrosis model was used to determine whether PC attenuates PF and modulates the intestinal microbiota. The results showed that PC intervention attenuated the pulmonary fibrosis, demonstrated by hematoxylin-eosin staining (HE), Masson’s trichrome staining, and lung dry-wet weight ratio, and PC significantly inhibited the production of interleukin-1 beta (IL-1β), tumor necrosis factor-α (TNF-α), and lipopolysaccharide (LPS). Additionally, intestinal microbiota analysis revealed that PC intervention significantly increased the bacterial diversity and richness. Correlation analysis indicated that 9 families and 17 genes were significantly associated with at least 1 physiological index. And PC intervention significantly decreased the bacteria which is related to inflammation and dramatically increased the SCFAs-producing bacteria and probiotics. These data indicated that PC can decrease the pro-inflammatory cytokines and regulate the intestinal microbiota in BLM-induced PF mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a disease characterized by macrophages, lymphocytes, and other inflammatory cells in the interstitial infiltration, fibroblast proliferation, and fibrous connective tissue deposition, which is the ultimate outcome of a series of chronic lung diseases and it causes great harm to human health with median survival of 2–3 years (Shappell et al. 1998). Pulmonary fibrosis is a complex pathophysiological process. In recent years, the incidence of pulmonary fibrosis has been increasing and there is still no effective treatment in clinic (Noble et al. 2012). Therefore, the current treatment of this devastating disease is basically unsuccessful (Raghu et al. 2013; Wilson et al. 2014), resulting in an average survival time of only 3–5 years after diagnosis (Yao et al. 2016). The search for effective treatment of pulmonary fibrosis has become an urgent need for current medicine (Lissi et al. 2000).
Phycocyanin (PC) is a light-harvesting protein isolated from Spirulina platensis and participates in algal photosynthesis. The role of PC in promoting health has been widely accepted in the past two decades (Wu et al. 2016). Many researches have shown that PC has significant antioxidant, anti-inflammatory, liver-protecting, anti-arthritic, neuroprotective, and anti-tumor effects (Cherng et al. 2007; Li et al. 2005; Romay et al. 2003; Sathyasaikumar et al. 2007). Previous study showed that PC could attenuate acute lung injury in rats by reducing oxidative stress damages and inhibiting NF-κB-mediated cytotoxicity. And the effect of PC in attenuation lung injury was also detected in lipopolysaccharide (LPS)-induced rats (Sun et al. 2011).
In recent years, with the deepening of research on lung diseases (Ndj and Marsland 2017; Samuelson et al. 2015), more and more studies have proved that there are important crosstalk between intestinal microbiota and lung, which has an important role in maintaining host health. Numerous studies have shown that many lung diseases are often accompanied by changes in intestinal microbiota, which in turn can affect lung immunity (He et al. 2017).
In this study, we use bleomycin (BLM) to induce pulmonary fibrosis mice model. And the effects of PC on pulmonary fibrosis were evaluated by pathological section staining of the lung, the levels of pro-inflammatory and intestinal microbiota in mice. We are the first to report the improvement and regulation of PC in pro-inflammatory cytokines and intestinal microbiota in PF mice.
Materials and methods
Materials
PC was purchased from King Dnarmse Spirulina Company (Fuqing, China). The mouse feed was purchased from the Jinan Pengyue Animal Breeding Center (Jinan, China). Table S1 shows the daily dietary components of mice. IL-1β and TNF-α enzyme-linked immunosorbent assay (ELISA) kits were purchased from proteintech™. HE and MASSON stain kits were purchased from Solarbio (Beijing, China).
Animals and sample collection
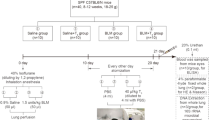
In this study, 72 6-week-old SPF male C57BL/6 mice were used. The mice were acclimated for 1 week under conditions controlled by temperature (23 ± 2 °C) and humidity (50–60%), maintaining a 12 h/12 h light/dark cycles and allowing mice to freely harvest energy. Then we use SPSS software to divide the mice into four groups (n = 18 for each group): control group (NC), bleomycin group (BL), bleomycin + 50 mg/kg phycocyanin group (PC), and bleomycin + pirfenidone group (PFD). And mice were placed in an IVC closed cage in a sober condition and 0.25% BLM was atomized with a nebulizer and sprayed into the cage through a spray tube for 30 min each time for six times. The control mice were treated with the same method; only BLM was changed to deionized water. Then, mice in PC group were intragastrically administered with 50 mg/kg PC per day, mice in PFD group were intragastrically administered with 50 mg/kg PFD per day, and the other two groups were given with the same volume of deionized water per day. At 7 and 28 days, nine mice were euthanized. Mice serum, lung, and gut tissue were excised and stored in liquid nitrogen until use.
At the beginning and end of the experiment, each mouse was placed in a separate sterile cage and 150 mg of fresh stool samples were collected in sterile cryopreserved tubes and immediately stored in liquid nitrogen until use.
Pathological changes in lung tissue of mice
Pathological examination in lung tissue was performed using an optical microscope. In our study, the left lung of the mice were perfused and fixed with 4% formaldehyde and routinely processed to embed the samples in paraffin. Lung tissues were sectioned and subjected to HE and MASSON stain.
Sections were observed using BX-51M fluorescence microscope (Olympus, JP). Lung injury scores 0, 1, 2, 3, and 4 indicate no injury, mild injury, moderate injury, severe injury, and very severe histological changes respectively. And fibrosis was scored using Ashcroft score (Hubner et al. 2008).
Determination serum and lung tissues IL-1β, TNF-α, and lipopolysaccharide levels
To detect IL-1β, TNF-α, and LPS levels in serum and lung tissue, the IL-1β, TNF-α, and LPS ELISA kits were used. Absorbance values were measured at 450 nm using an enzyme labeling instrument according to the instructions.
Determination of lung dry-wet weight ratio
Seven days after, the bleomycin was atomized. The right lung of the mouse was taken and the right lower lobe was blotted to its “wet” weight. It was then placed in an oven at 60 °C and after 72 h weighed to obtain a “dry” weight.
Extraction of genomic DNA, amplicon generations, and Illumina sequencing
The QIAamp DNA Stool Mini Kit (Qiagen, Germany) was used to extract the total genomic DNA in the feces samples. The 16S rRNA gene V3-V4 hypervariable region was selected and the barcode-specific primers (338F and 806R) were used for amplification. Gel electrophoresis was used to detect the presence of the amplicons and AxyPrep DNA Purification Kit (Axygen Biosciences, Union City, CA, USA) was used to purificate the amplications, and then the PCR product was quantified using a QuantiFluor-ST fluorometer (Promega, USA) and analyzed using the MiSeq platform (Illumina, Novogene, Beijing, China) according to standard protocols for paired end sequencing (2 × 300).
Data accessibility
The raw data were uploaded to the National Center for Biotechnology Information, and SRA database under the accession number PRJNA546446.
Data analysis
The paired-end-sequenced fragments were spliced with FLASH and 0.97 was set as the operational classification units (OTUs) and species classification threshold. The representative sequence of each OTU was annotated by the RDP classifier, and then the corresponding species information and species-based abundance distribution were obtained. The sequence was analyzed using QIIME software. The alpha (α) diversity was analyzed by mother. The difference between different groups was analyzed based on the principal coordinate analysis (PCoA) of UniFrac. And the correlation between different physiological indexes and the intestinal microbiota was analyzed by SPSS.
Statistical analysis
Before the difference analysis, we performed the homogeneity test of variance. All data are expressed as mean ± SDs. Comparisons between groups were tested by one-way ANOVA (Tukey’s test); p < 0.05 was considered significantly different. Correlations were calculated by Spearman’s correlation analyses with 95% confidence intervals.
Results
Pathological changes of lung tissue in mice after PC intervention
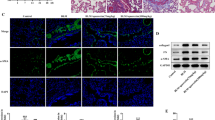
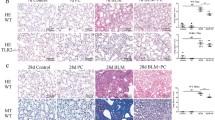
HE staining and Masson staining was used to analyze lung injury and fibrosis. Compared with the NC group, obvious inflammatory cell infiltration in the lung tissue, blood cells, and tissue edema in the alveoli and a small amount of blue collagen fibers appeared in mice 7 days after the BLM was atomized (Fig. 1a). Alveolar structural disorder, abnormal thickening of the alveolar wall, massive deposition of matrix, amount of blue collagen fibers, and fibroblasts appeared in mice 28 days after the BLM was atomized (Fig. 1b). Obviously, PC and PFD treatment groups significantly reduced lung injury and fibrosis induced by BLM at 7 and 28 days. The PC group and the PFD group have no significant differences.
The effects of PC on the pulmonary fibrosis tissue morphology on day 7 and 28 after nebulization. a Observation of lung morphology by hematoxylin-eosin (HE) staining and Masson’s trichrome staining on the 7th day after nebulization. b Pulmonary morphology observed with HE and MASSON staining on the 28th day after nebulization. (HE and Masson stained sections were imaged at × 200 magnification). NC blank control group, PC phycocyanin treatment group, PFD Pirfenidone treatment group, BL bleomycin model group
The effect of PC on lung dry-wet weight ratio
The lung dry-wet weight ratio (D/W) was used to assess the extent of pulmonary edema. Compared with the NC group, the lung dry-wet weight ratio was significantly increased in the BL group (p < 0.05), and the PC and PFD treatments significantly decreased the ratio (Fig. 2). The results indicate that PC might attenuate the pulmonary fibrosis by decreasing the lung edema.
The effect of PC on BLM-induced cytokines and LPS release
The levels of IL-1β, TNF-α, and LPS in lung serum and gut of mice were measured by ELISA kit after 7 days of BLM nebulization (Fig. 3). The ELISA results showed that compared to the control group, the levels of IL-1β, TNF-α, and LPS in lung tissue, serum, and gut tissue of the BLM group were significantly increased on day 7 after BLM atomization, and the inflammatory cytokines were significantly decreased after PC and PFD treatment (p < 0.05).
The effect of PC on BLM-induced cytokines release. a The levels of IL-1β in lung, serum, and gut of mice. b The levels of TNF-α in lung, serum and gut of mice. c The levels of LPS in lung, serum, and gut of mice. Data were represented as mean ± SD (n = 5). One-way ANOVA (Tukey’s test) was used to test the difference in different groups, p < 0.05(*) was considered significantly different compared with the BL group (p < 0.05)
The effects of PC on modulation of intestinal microbiota structure
After high-throughput sequencing, we obtained 32,179 effective sequences. The sample rarefaction curve tended to be stable, indicating that sequencing data was sufficient (Fig. 4a). The richness and diversity of bacterial in the intestinal microbiota were determined. Compared to NC group, the index of Shannon and Simpson were lower in the BL group (Fig. 4b, c). And the index of Chao1 and ACE was also lower in the BL group (Fig. 4d, e). The diversity and richness in intestinal microbiota was significantly increased after PC and PFD intervention (p < 0.05). The overall structure of intestinal microbiota was analyzed using weighted UniFrac PCoA (Fig. 4f). The result indicated that the structure of the overall intestinal microbiota was significantly changed after BLM intervention, and the overall structure of the intestinal microbiota was shifted toward the NC group after PC and PFD treatment.
Analysis of α diversity and overall structure of mouse intestinal microbiota. a Rarefaction curve of intestinal microbiota in each group of mice. b Shannon index of intestinal microbiota in each group of mice. c Simpson index of intestinal microbiota in each group of mice. d Chao1 value of intestinal microbiota in each group of mice. e ACE value of intestinal microbiota in each group of mice. f Principal coordinates analysis (PCoA) of mice based on weighted UniFrac distances. Data were represented as mean ± SD (n = 5). One-way ANOVA (Tukey’s test) was used to test the difference in different groups, p < 0.05(*) was considered significantly different compared with the BL group (p < 0.05)
At the phylum level, the most abundant phyla in intestinal microbiota were Firmicutes, Bacteroidetes, Proteobacteria, Deferribacteres, Actinobacteria, and Tenericutes, and the dominant bacteria were Firmicutes and Bacteroidetes, accounting for more than 90% in all bacteria (Fig. 5a). Compared to PC group, the abundance of Firmicutes and Proteobacteria was increased and the abundance of Bacteroidetes was decreased after BLM treatment; PC and PFD treatment inverse the effects (Fig. 5a and Table S2).
At the family level, high abundance of the intestinal microbiota in all groups was shown in Fig. 5b. Compared to NC group, the relative abundance of Muribaculaceae, Lachnospiraceae, Lactobacillaceae, Ruminococcaceae, Rikenellaceae, Akkermansiaceae, and Bacteroidaceae was significantly reduced and the relative abundance of Erysipelotrichaceae, Helicobacteraceae, and Staphylococcaceae was significantly increased after BLM treatment. The treatment of PC and PFD significantly inversed the effects (Fig. 5b and Table S3).
The correlation coefficients of the differential abundance of the bacterial families and the ratio of lung dry-wet weight or expression of pro-inflammatory cytokines in lung and serum were calculated by spearman’s correlation analyses in mice (n = 5). The results (Fig. 6) showed that the abundance of the bacterial families Muribaculaceae, Lachnospiraceae, Lactobacillaceae, Ruminococcaceae, Rikenellaceae, and Akkermansiaceae were negatively correlated with the ratio of lung dry-wet weight and expression of pro-inflammatory cytokines, while the community abundance of Erysipelotrichaceae, Helicobacteraceae, and Staphylococcaceae were positively correlated with the ratio of lung dry-wet weight and expression of pro-inflammatory cytokines. In addition, high abundance of the intestinal microbiota in all groups of 22 genus were identified after PC intervention. Spearman’s correlation analyses of the differential abundance of the 22 genus and the ratio of lung dry-wet weight or expression of pro-inflammatory cytokines in lung and serum were calculated in mice (n = 5). The results (Fig. 7) showed that 17 genus were significantly correlated with at least 1 of the physiological index. These results indicated that intestinal microbiota might be associated with inflammation in the mice and anti-inflammation effects of PC were associated with the specific intestinal microbiota in a certain degree. The effect of intestinal microbiota changes on pulmonary fibrosis caused by BLM was shown in Fig. 8. The result showed that changes in intestinal microbiota might influence the lung through the blood.
Correlation analysis between the bacteria of family and mouse physiological index (dry-wet weight ratio (g/g), IL-1β level, and TNF-α level). A positive correlation was expressed as red and a negative correlation was expressed as blue. The degree of correlation between the family abundant and the physiological index of the hos was expressed by intensity of the colors. *p < 0.05; **p < 0.01
Effects of PC on the genus level of intestinal microbiota. a The abundance of 22 OTUs was shown as Heatmap. b Correlation analysis of genus abundance and mouse phenotypes (dry-wet weight ratio (g/g), IL-1β level, and TNF-α level). A positive correlation was expressed as red and a negative correlation was expressed as blue. The degree of correlation between the genus abundant and the physiological index of the hos was expressed by intensity of the colors. *p < 0.05; **p < 0.01
Discussion
The study showed that PC attenuated BLM-induced pulmonary inflammatory and fibrosis demonstrated by HE and MASSON. And the PC intervention significantly decreased the levels of lung, serum, and gut pro-inflammatory cytokines and LPS. Additionally, BLM treatment significantly increased the abundance of some harmful bacteria, while the abundance of some beneficial bacteria was significantly reduced, and PC treatment can effectively reverse this phenomenon.
As one of the chemotherapeutic drugs, BLM is widely used for the induction of mouse IPF model. The drug causes a time-dependent increase in tissue infiltration of pro-inflammatory cells and cytokines (Aono et al. 2012). In this study, HE and MASSON stain were used to analyze lung injury and fibrosis. Under the influence of BLM, alveolar structure and interstitial lung presented different degrees of injury and fibrosis (Fig. 1a, b). PC and PFD intervention significantly attenuated the injury and fibrosis, which indicated that PC can reduce the degree of pulmonary fibrosis to a certain extent. Pro-inflammatory cytokines directly damage lung tissue cells, resulting in extensive damage and permeability of pulmonary vascular endothelial cells and alveolar epithelial cells, pulmonary edema, and microthrombus formation, which finally lead to the progress of pulmonary fibrosis. Pro-inflammatory cytokines are also associated with a variety of pathways, such as NF-κB. Effective inhibition of the production of pro-inflammatory cytokines can reduce the progression of pulmonary fibrosis. Numerous studies have shown that the production of pulmonary fibrosis is associated with increased inflammation, mainly as an increase in IL-1β and TNF-α (Meziani et al. 2018; Shih et al. 2009). In this study, PC intervention significantly reduced the levels of IL-1β, TNF-α, and LPS in BLM-treated mice. The protective effect of PC on pulmonary fibrosis might be highly related to the reduction of inflammation and LPS. Moreover, PC reversed the increasing of lung dry-wet weight ratio in BLM-treated mice, that may reduce the lung injury. Studies have shown that PC significantly inhibits TNF-α, IL-1β, IL-6 release, and neutrophil infiltration in experimental hyperalgesia inflammation sites. PC can reverse the increase of TNF-α, IL-1β, and IL-6 levels after microglia LPS induced (Chen et al. 2012). In addition, PC administration also exerts significant protective effects on inflammatory bowel disease, including a reduction in weight loss and inflammatory lesions in the colon, and inhibition of macrophage activation by inhibition of nuclear translocation (Budden et al. 2017). These results indicate that PC can alleviate a variety of diseases by inhibiting the inflammatory response, including BLM-induced pulmonary fibrosis.
Not only the lung functions were changed but also the intestinal microbiota were dysbiosis after BLM treatment. The intestinal microbiota structure analysis showed that the diversity and richness of intestinal microbiota increased after PC and PFD treatment compared to BL group, which indicated that PC and PFD treatment can significantly improve the reduction of intestinal microbiota in mice caused by BLM. Additionally, the structure of the intestinal microbiota in BLM-treated mice was significantly changed compared to the NC group mice, and the overall structure of the intestinal microbiota was shifted toward the NC group after PC and PFD treatment.
On the basis of the analysis of the correlation between intestinal microbiota and pulmonary fibrosis physiological indicators, we determined the possible regulation of PC on intestinal microbiota.
First, numerous studies have shown that many bacteria in the intestinal microbiota were related to inflammation (Dziarski et al. 2016; Lavelle et al. 2015; Loy et al. 2017). For example, the genes of Helicobacter, which is represented by Helicobacter pylori, can induce a variety of cytokines and chemokines, such as TNF-α, IL-6, IL-8, and histamine (Kountouras et al. 2015). Helicobacter pylori harms the intestinal wall by producing ammonia and some biochemicals. Ammonia, which used to regulate pH, is toxic to epithelial cells, and biochemicals such as protease, vacuolating cytotoxin A (VacA), and some phospholipases, which are produced by Helicobacter pylori, can destroys epithelial cells, disrupts tight junctions, and leads to apoptosis (Smoot 1997). Compared to BL group, PC intervention significantly decreased the relative abundance of Helicobacter. And other bacterial associated with inflammation include Erysipelactoclostridium and Parasultterella, which followed the same trend with Helicobacter in our study.
Second, a large number of studies have shown that many bacteria in the intestine were associated with the production of short-chain fatty acids (SCFAs), such as Muribacculum, Ruminiclostridium, Lachnoclostridium, and Butyricicoccus (Chen et al. 2011; Meehan and Beiko 2014). SCFAs are produced by fermentation of the intestinal microbiota, which can directly supply energy to the intestinal epithelium, improve intestinal digestion, nutrient absorption, and intestinal immunity (Vital et al. 2017). SCFAs could regulate at least two signaling molecular systems, which are histone deacetylase (HDACs) and G protein coupled receptors (GPCRs). SCFAs are natural inhibitors of HDACs and GPCRs (Tan et al. 2014). HDACs are involved in traumatic injury, and GPCRs are involved in lung diseases (Edwards 2010). Compared to BL group, PC intervention significantly increased the relative abundance of Muribacculum, Ruminiclostridium, Lachnoclostridium, and Butyricicoccus, which increased the synthesis of SCFAs and enhance its inhibitory effects of HDACs and GPCRs.
In addition, studies have shown that many of the microbiota in the gut are probiotics, such as Lactobacillus (Daughtry et al. 2018). Probiotics have an important regulatory effect on human health; it can increase the immunity of human mucous intestinal tract by increasing the amount of LgA(+)(Ashraf and Shah 2014). And probiotics help to treat the disease by regulating the balance of the intestinal microbiota in the body, when the level of opportunistic pathogens in the intestinal microbiota is abnormally elevated (Ford et al. 2014). Additionally, Lactobacillus salivarius interacts with many pathogens by producing salivary hormone B (a bacteriocin) (ten Brink et al. 1994). Compared to BL group, the relative abundance of Lactobacillus was significantly increased after PC intervention, which may increase the immunity and balance of the intestinal microbiota.
In conclusion, pulmonary fibrosis mouse model induced by BLM was used in this study, which has been shown that PC intervention (i) significantly attenuated pulmonary fibrosis scores demonstrated by HE and MASSON staining; (ii) significantly reduced pro-inflammatory cytokine and LPS levels demonstrated by reducing IL-6, TNF-α, and LPS levels in lung, serum, and gut; and (iii) significantly shifted the overall structure of the intestinal microbiota toward the NC group. And the changes of IL-6, TNF-, and LPS levels in lung, serum, and gut demonstrated that there was a significant crosstalk between the intestines and the lungs. However, the crosstalk mechanism between PC attenuate pulmonary fibrosis and intestinal microbiota needs further exploration.
References
Aono Y, Ledford JG, Mukherjee S, Ogawa H, Nishioka Y, Sone S, Beers MF, Noble PW, Wright JR (2012) Surfactant protein-D regulates effector cell function and fibrotic lung remodeling in response to bleomycin injury. Am J Respir Crit Care Med 185(5):525–536. https://doi.org/10.1164/rccm.201103-0561OC
Ashraf R, Shah NP (2014) Immune system stimulation by probiotic microorganisms. Crit Rev Food Sci Nutr 54(7):938–956. https://doi.org/10.1080/10408398.2011.619671
Budden KF, Gellatly SL, Wood DLA, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM (2017) Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol 15(1):55–63. https://doi.org/10.1038/nrmicro.2016.142
Chen X, Zuo Q, Hai Y, Sun XJ (2011) Lactulose: an indirect antioxidant ameliorating inflammatory bowel disease by increasing hydrogen production. Med Hypotheses 76(3):325–327. https://doi.org/10.1016/j.mehy.2010.09.026
Chen JC, Liu KS, Yang TJ, Hwang JH, Chan YC, Lee IT (2012) Spirulina and C-phycocyanin reduce cytotoxicity and inflammation-related genes expression of microglial cells. Nutr Neurosci 15(6):252–256. https://doi.org/10.1179/1476830512Y.0000000020
Cherng SC, Cheng SN, Tarn A, Chou TC (2007) Anti-inflammatory activity of c-phycocyanin in lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci 81(19–20):1431–1435. https://doi.org/10.1016/j.lfs.2007.09.009
Daughtry KV, Johanningsmeier SD, Sanozky-Dawes R, Klaenhammer TR, Barrangou R (2018) Phenotypic and genotypic diversity of Lactobacillus buchneri strains isolated from spoiled, fermented cucumber. Int J Food Microbiol 280:46–56. https://doi.org/10.1016/j.ijfoodmicro.2018.04.044
Dziarski R, Park SY, Kashyap DR, Dowd SE, Gupta D (2016) Pglyrp-regulated gut microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii enhance and Alistipes finegoldii attenuates colitis in mice. PLoS One 11(1):e0146162. https://doi.org/10.1371/journal.pone.0146162
Edwards A (2010) The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clin Exp Allergy 38(1):4–18. https://doi.org/10.1111/j.1365-2222.2007.02886.x
Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Moayyedi P (2014) Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol 109(10):1547–1561. https://doi.org/10.1038/ajg.2014.202
He Y, Wen Q, Yao F, Xu D, Huang Y, Wang J (2017) Gut-lung axis: the microbial contributions and clinical implications. Crit Rev Microbiol 43(1):81–95. https://doi.org/10.1080/1040841X.2016.1176988
Hubner RH, Gitter W, El Mokhtari NE, Mathiak M, Both M, Bolte H, Freitag-Wolf S, Bewig B (2008) Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 44(4):507–517. https://doi.org/10.2144/000112729
Kountouras J, Zavos C, Polyzos SA, Deretzi G (2015) The gut-brain axis: interactions between Helicobacter pylori and enteric and central nervous systems. Ann Gastroenterol 28(4):506–506
Lavelle A, Lennon G, O'Sullivan O, Docherty N, Balfe A, Maguire A, Mulcahy HE, Doherty G, O'Donoghue D, Hyland J, Ross RP, Coffey JC, Sheahan K, Cotter PD, Shanahan F, Winter DC, O'Connell PR (2015) Spatial variation of the colonic microbiota in patients with ulcerative colitis and control volunteers. Gut 64(10):1553–1561. https://doi.org/10.1136/gutjnl-2014-307873
Li B, Zhang X, Gao M, Chu X (2005) Effects of CD59 on antitumoral activities of phycocyanin from Spirulina platensis. Biomed Pharmacother 59(10):551–560. https://doi.org/10.1016/j.biopha.2005.06.012
Lissi EA, Pizarro M, Aspee A, Romay C (2000) Kinetics of phycocyanine Bilin groups destruction by peroxyl radicals. Free Radic Biol Med 28(7):1051–1055. https://doi.org/10.1016/s0891-5849(00)00193-3
Loy A, Pfann C, Steinberger M, Hanson B, Herp S, Brugiroux S, Gomes Neto JC, Boekschoten MV, Schwab C, Urich T, Ramer-Tait AE, Rattei T, Stecher B, Berry D (2017) Lifestyle and horizontal gene yransfer-mediated rvolution of mucispirillum schaedleri, a core member of the murine gut microbiota. mSystems 2(1):e00171–e00116. https://doi.org/10.1128/mSystems.00171-16
Meehan CJ, Beiko RG (2014) A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol Evol 6(3):703–713. https://doi.org/10.1093/gbe/evu050
Meziani L, Mondini M, Petit B, Boissonnas A, Thomas de Montpreville V, Mercier O, Vozenin MC, Deutsch E (2018) CSF1R inhibition prevents radiation pulmonary fibrosis by depletion of interstitial macrophages. Eur Respir J 51(3):1702120. https://doi.org/10.1183/13993003.02120-2017
Ndj U, Marsland BJ (2017) Mechanistic insight into the function of the microbiome in lung diseases. Eur Respir J 50(3):1602467. https://doi.org/10.1183/13993003.02467-2016
Noble PW, Barkauskas CE, Jiang D (2012) Pulmonary fibrosis: patterns and perpetrators. J Clin Invest 122(8):2756–2762. https://doi.org/10.1172/JCI60323
Raghu G, Behr J, Brown KK, Egan JJ, Kawut SM, Flaherty KR, Martinez FJ, Nathan SD, Wells AU, Collard HR, Costabel U, Richeldi L, de Andrade J, Khalil N, Morrison LD, Lederer DJ, Shao L, Li X, Pedersen PS, Montgomery AB, Chien JW, O'Riordan TG, Investigators* A-I (2013) Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med 158(9):641–649. https://doi.org/10.7326/0003-4819-158-9-201305070-00003
Romay C, Gonzalez R, Ledon N, Remirez D, Rimbau V (2003) C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr Protein Pept Sci 4(3):207–216. https://doi.org/10.2174/1389203033487216
Samuelson DR, Welsh DA, Shellito JE (2015) Regulation of lung immunity and host defense by the intestinal microbiota. Front Microbiol 6:1085. https://doi.org/10.3389/fmicb.2015.01085
Sathyasaikumar KV, Swapna I, Reddy PV, Murthy Ch R, Roy KR, Dutta Gupta A, Senthilkumaran B, Reddanna P (2007) Co-administration of C-phycocyanin ameliorates thioacetamide-induced hepatic encephalopathy in Wistar rats. J Neurol Sci 252(1):67–75. https://doi.org/10.1016/j.jns.2006.10.014
Shappell SB, Gurpinar T, Lechago J, Suki WN, Truong LD (1998) Chronic obstructive uropathy in severe combined immunodeficient (SCID) mice: lymphocyte infiltration is not required for progressive tubulointerstitial injury. J Am Soc Nephrol 9(6):1008–1017. https://doi.org/10.1089/end.1998.12.291
Shih CM, Cheng SN, Wong CS, Kuo YL, Chou TC (2009) Antiinflammatory and antihyperalgesic activity of C-phycocyanin. Anesth Analg 108(4):1303–1310. https://doi.org/10.1213/ane.0b013e318193e919
Smoot DT (1997) How does Helicobacter pylori cause mucosal damage? Direct mechanisms. Gastroenterology 113(6 Suppl):S31–S34. https://doi.org/10.1016/S0016-5085(97)80008-X
Sun Y, Zhang J, Yan Y, Chi M, Chen W, Sun P, Qin S (2011) The protective effect of C-phycocyanin on paraquat-induced acute lung injury in rats. Environ Toxicol Pharmacol 32(2):168–174. https://doi.org/10.1016/j.etap.2011.04.008
Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L (2014) The role of short-chain fatty acids in health and disease. Adv Immunol 121:91–119. https://doi.org/10.1016/B978-0-12-800100-4.00003-9
Ten Brink B, Minekus M, van der Vossen JM, Leer RJ, Huis in't Veld JH (1994) Antimicrobial activity of lactobacilli: preliminary characterization and optimization of production of acidocin B, a novel bacteriocin produced by Lactobacillus acidophilus M46. J Appl Bacteriol 77(2):140–148. https://doi.org/10.1111/j.1365-2672.1994.tb03057.x
Vital M, Karch A, Pieper DH (2017) Colonic butyrate-producing communities in humans: an overview using omics data. Msystems 2(6):e00130–e00117. https://doi.org/10.1128/mSystems.00130-17
Wilson EC, Shulgina L, Cahn AP, Chilvers ER, Parfrey H, Clark AB, Twentyman OP, Wilson AM (2014) Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: an economic evaluation alongside a randomised controlled trial. Pharmacoeconomics 32(1):87–99. https://doi.org/10.1007/s40273-013-0112-z
Wu Q, Liu L, Miron A, Klimova B, Wan D, Kuca K (2016) The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch Toxicol 90(8):1817–1184. https://doi.org/10.1007/s00204-016-1744-5
Yao Y, Wang Y, Zhang Z, He L, Zhu J, Zhang M, He X, Cheng Z, Ao Q, Cao Y, Yang P, Su Y, Zhao J, Zhang S, Yu Q, Ning Q, Xiang X, Xiong W, Wang CY, Xu Y (2016) Chop deficiency protects mice against bleomycin-induced pulmonary fibrosis by attenuating M2 macrophage production. Mol Ther 24(5):915–925. https://doi.org/10.1038/mt.2016.36
Acknowledgements
This work was supported by The National Key Research and Development Program of China (2018YFD0901102) and The Key Research and Development Program of Yantai (2019XDHZ101).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The study design was approved by the Animal Care and Maintenance Committee of Shandong International Biotechnology Park (SCXK 20140007) (Yantai, China), and complied with NIH guidelines for the care and use of laboratory animals. All authors confirm that ethical principles have been followed in the experiments.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 396 kb)
Rights and permissions
About this article
Cite this article
Xie, Y., Li, W., Lu, C. et al. The effects of phycocyanin on bleomycin-induced pulmonary fibrosis and the intestinal microbiota in C57BL/6 mice. Appl Microbiol Biotechnol 103, 8559–8569 (2019). https://doi.org/10.1007/s00253-019-10018-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10018-7