Abstract
Fusarium graminearum is a prominent fungal pathogen that causes economically important losses by infesting a wide variety of cereal crops. F. graminearum produces both asexual and sexual spores which disseminate and inoculate hosts. Therefore, to better understand the disease cycle and to develop strategies to improve disease management, it is important to further clarify molecular mechanisms of F. graminearum conidiogenesis. In this study, we functionally characterized the FgMed1, a gene encoding an ortholog of a conserved MedA transcription factor known to be a key conidiogenesis regulator in Aspergillus nidulans. The gene deletion mutants ΔFgMed1 produced significantly less conidia, and these were generated from abnormal conidiophores devoid of phialides. Additionally, we observed defective sexual development along with reduced virulence and deoxynivalenol (DON) production in ΔFgMed1. The GFP-tagged FgMed1 protein localized to the nuclei of conidiophores and phialides during early conidiogenesis. Significantly, RNA-Seq analyses showed that a number of the conidiation- and toxin-related genes are differentially expressed in the ΔFgMed1 mutant in early conidiogenesis. These data strongly suggest that FgMed1 involved in regulation of genes associated with early conidiogenesis, DON production, and virulence in F. graminearum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fusarium head blight (FHB), also known as scab, caused by Fusarium graminearum (teleomorph Gibberella zeae) is a devastating disease affecting wheat and other small grains worldwide (McMullen et al. 1997). Under favorable conditions, this fungal disease can lead to severe yield losses and poor quality. Infested grains pose a serious safety threat to human and animal health due to harmful mycotoxins such as deoxynivalenol (DON), nivalenol (NIV), and zearalenone (Desjardins 2003; Pestka and Smolinski 2005). Despite the devastating issues caused by FHB, establishing effective disease management strategies has been very difficult. Fundamental biology understanding of pathogen-host association along with clearer characterization of the disease cycle is essential for spearheading new sustainable FHB management strategies. In many plant pathogenic fungi, conidia play a crucial role for disease dissemination as infectious propagules (Park and Yu 2012). The genetic and molecular mechanisms related to conidiation have been studied extensively in model fungi, such as Neurospora crassa and Aspergillus nidulans. In the past decades, a number of conidiation-related transcription factors (TFs) have been identified in plant pathogenic fungi, e.g., Magnaporthe oryzae and Fusarium species, associated with the regulation of cellular signal pathway genes (Odenbach et al. 2007; Ruiz-Roldan et al. 2015; Son et al. 2011; Zheng et al. 2012a, b).
The homeodomain transcription factors are evolutionarily conserved and regulate diverse developmental stages in fungi. Two homeobox genes in Saccharomyces cerevisiae, MATa2 and MATa1, are known to be essential for mating (Ho et al. 2002). The first cloned homeobox gene PAH1 in Podospora anserina was determined as a repressor of microconidiation (Arnaise et al. 2001). In Fusarium species, the conserved homeobox transcription factor Htf1 regulates phialide development and conidiogenesis via distinct signaling pathways (Zheng et al. 2012a, b). And in the rice blast fungus M. oryzae, homeodomain transcription factors are required for conidiation and appressorium development (Kim et al. 2009). Another family of transcription factors, the APSES TFs, is unique to fungi and regulates developmental processes in ascomycetes. StuA was the first APSES family TF to be characterized and was shown to be involved in conidiophore morphogenesis, notably in the formation of metulae and phialides in A. nidulans (Dutton et al. 1997). In F. graminearum, a ΔFgStuA mutant, showed significantly impaired spore production, lacked conidiophores and phialides, and did not develop perithecia and sexual ascospores (Lysoe et al. 2011). Lastly, Myb DNA-binding domain transcription factors are involved in various cellular processes in eukaryotes, including cell proliferation, apoptosis, differentiation, metabolism, and stress responses. In F. graminearum, 20 Myb-like proteins, e.g., Myt1, Myt2, and Myt3, were identified and most of them are required for normal sexual development (Kim et al. 2014; Lin et al. 2011, 2012; Son et al. 2011). In Ashbya gossypii, the deletion of AgBAS1 gene that encodes Myb-like protein showed delayed spore germination (Mateos et al. 2006). The Myb-like transcription factor FlbD in A. nidulans controls asexual and sexual differentiation, as determined by the mutants exhibiting severe defects in conidiation and perithecia development (Arratia-Quijada et al. 2012).
Conidiation gene signaling in the model organism A. nidulans has been extensively studied, and the core regulatory pathway includes TFs BrlA-AbaA-WetA (Martinelli 1979). The TF MedA was first identified and characterized in A. nidulans as a temporal modifier upstream of these core regulatory pathway TFs (Chung et al. 2011). Deletion of MedA resulted in phialide production delay, reduced number of conidia as well as production of medusoid-like conidiophores (Clutterbuck 1969). In A. fumigatus, MedA controls adherence, host cell interactions, and virulence. However, conidiophore morphology is markedly different in A. nidulans and A. fumigatus ΔmedA mutants (Gravelat et al. 2010). The study of MedA in Ustilago maydis found that the gene was required for in vitro mating, pheromone response, and full virulence (Chacko and Gold 2012). In M. oryzae, the insertional mutants of MedA homolog Acr1 produced head-to-tail (acropetal) arrays of elongated conidia. Acr1 was regarded as a stage-specific negative regulator of conidiation that is required for establishing a sympodial pattern of spore formation (Nishimura et al. 2000). F. oxysporum Ren1, a Acr1 homolog, is required for proper differentiation of conidiogenesis for microconidia and macroconidia (Ohara et al. 2004).
With conidia production being a critical element in FHB disease cycle, there is a need to characterize the role of MedA homolog in conidia development and virulence in F. graminearum. Here, we identified the MedA ortholog FgMed1 and showed that null mutants are impaired in sexual and asexual development. The mutants also exhibited defects in DON production and virulence. Further intracellular localization study showed that FgMed1 protein localizes to the nuclei of conidiophores and terminal phialides. Transcriptome analyses further demonstrated that a number of the conidiation and toxin-related proteins are differentially expressed in the ΔFgMed1 mutant, suggesting that FgMed1 is a transcriptional regulator that regulates the differentiation of sexual and asexual reproduction and virulence in F. graminearum.
Materials and methods
Strains and culture conditions
F. graminearum strain PH-1 originally from Fungal Genetics Stock Center in the USA (accession number NRRL 31084) was used as the wild-type strain in this study. FgMed1 gene deletion strains (ΔFgMed1-1, ΔFgMed1-2) and the FgMed1 complemented (FgMed1-Com) strain were generated in this study following our standard protocols (Zheng et al. 2015). All strains were routinely maintained on complete medium (CM) agar plate (Fan et al. 2017). TB3 medium (3 g yeast extract, 3 g acid hydrolysis casein, 20 g sucrose, and 15 g agar powder in 1 L water) was used for regeneration of protoplasts. Carboxymethyl cellulose medium (CMC) and carrot agar (200 g carrots and 20 g agar in 1 L medium) were used for induction of asexual and sexual sporulation, respectively (Cappellini and Peterson 1965). All strains were stored as conidial suspensions in 20% glycerol at − 70 °C.
Nucleic acid manipulations, construction of FgMed1 deletion mutants and Southern blot
The procedure for isolation of genomic DNA was described earlier (Fan et al. 2017). Total RNA samples were isolated from frozen mycelia with Eastep™ Total RNA extraction Kit (Promega (Beijing) Biotech Co., Ltd, China, LS1030) according to the manufacturer’s instruction. PCR primers (Supplementary Table S1) used in this study were synthesized at Beijing Liuhe Huada Genomics Technology Co., LTD, Beijing, China. The gene replacement of FgMed1 in F. graminearum strain PH-1 was generated according to the split-marker recombination approach (Catlett et al. 2003; Son et al. 2014a, b). F. graminearum protoplast preparation and fungal transformation were performed following the protocol previously described (Yang et al. 2015). The transformants were selected on hygromycin-resistant CM culture plates, verified by PCR with genes specific primers, and further characterized by RT-PCR. In total, 5 colonies were selected, and 2 of these were selected for subsequent phenotypic analysis. Southern blot was performed as described (Matar et al. 2017). The genomic DNA of F. graminearum was digested by XhoI and separated by agarose gel. The probe for hybridization was amplified from genomic DNA of PH-1 by PCR using primers Med1AF and Med1AR (Supplementary Table S1). Then, the probe was labeled with the DIG-High Prime DNA Labeling and Detection Starter Kit (Roche, Mannheim, Germany).
Vegetative growth and osmotic sensitivity test
For vegetative growth assay, mycelial pellets with equal colony diameter (5 mm) were collected with a cork borer, placed at the center of SYM medium, and were incubated at 28 °C in inverted manner. For the osmotic sensitivity test, mycelial pellets with equal colony diameter were collected with a cork borer, placed on SYM plates amended with 1 M sorbitol, 0.5 M NaCl, 0.05 mM H2O2, 0.2 mg/ml CFW, 0.05% SDS, or 0.2 mg/ml Congo red, and incubated in the dark at 28 °C to monitor fungal growth. Colony diameter was measured and photographed after 4 days of incubation. All tests were performed in triplicates.
Asexual and sexual production
For asexual production assay, three agar blocks carrying fresh mycelium were inoculated into a 30 ml CMC broth at 28 °C on a rotary shaker for 2–4 days. Quantification of conidia was performed with a hemocytometer and also photographed at different time stages with the Olympus BX51 Research Microscope (Olympus Co. Tokyo, Japan). Nuclei in conidia and phialides were visualized with 4′,6-diamidino-2-phenylindole (DAPI) (10 mg/ml, Sigma-Aldrich Co., St. Louis, USA) staining. For spore germination test, freshly harvested macroconidia were suspended in CM Petri dish with gentle agitation, and observed at 4 h, 12 h, 24 h, and 48 h time points. All experiments were replicated three times. For ascospore production, mycelia agar blocks were cultivated on carrot agar plates for 1 week and pressed down with a spreader after applying 2.5% sterilized Tween 60 per 20 ml Petri dish to induce sexual reproduction (Pasquali and Kistler 2006). Perithecium formation and ascospore production were examined under a dissecting microscope after incubation for 2 weeks at 28 °C under UV light.
Virulence and DON production assays
Virulence assays on flowering wheat heads were performed as previously described (Liu et al. 2013; Zheng et al. 2015). We inoculated agar blocks (5 mm in diameter) with mycelia on the flowering wheat heads, incubated in 100% humidity, and observed symptoms 14 days after inoculation. The disease was calculated by the average disease index (diseased spikelets per head). Disease index was measured by the number of symptomatic spikelet 15 days after inoculation. Mean and standard error were calculated with results from three independent experiments. At least three wheat heads were examined in each repeat. For the DON production test, autoclaved rice grains were inoculated with mycelia of the wild-type and mutant strains, cultured at 28 °C for 3 weeks. DON analysis was performed as previously described (Bluhm et al. 2007).
Construction of FgMed1-GFP fusion vector and cellular localization of FgMed1
The FgMed1 protein was tagged with eGFP at the N-terminus by fusing the respective DNA sequences under control of its native promoter. The FgMed1 gene plus 1722-bp promoter region was amplified using genomic DNA extracted from wild-type PH-1 as a template using appropriate primers (Supplementary Table S1) (Zheng et al. 2015). Then, a SOE-PCR were applied to obtain the native promoter-FgMed1-GFP, cloned into a T vector (pMDTM 18/19-T Vector Cloning Kit, Takara), which was verified by sequencing. The constructed vector was co-transformed into protoplasts of ΔFgMed1 mutant along with pKNTG vector (Khang et al. 2005) harboring the neomycin-resistance marker. Transformants were screened by PCR with primer pairs of pGFPF/FgMed1CR and by phenotypic restoration to ΔFgMed1. GFP fluorescence was followed using a Leica TCS SP5 inverted confocal laser scanning microscope (Leica, Germany).
RNA-sequencing and bioinformatics analysis
Three biological replicates of the wild-type PH-1 and ΔFgMed1 mutants were grown in CMC for 24 h on a rotary shaker (150 rpm). Mycelia and conidia were harvested with Miracloth and were washed with distilled water. Total RNA was extracted with EastepTM Total RNA extraction Kit (Promega (Beijing) Biotech Co., Ltd, China, LS1030) according to the manufacturer’s instruction. RNA-sequencing libraries were created using Illumina TruseqTM RNA sample prep Kit (Illumina, Inc., San Diego, USA). Sequencing was performed on an Illumina HiSeq4000 instrument (Illumina, Inc., San Diego, USA) using the reagents provided in the Illumina Hiseq4000 Truseq SBS Kit (Illumina, Inc., San Diego, USA). The gene differential expression was analyzed by Cuffdiff (http://cole-trapnell-lab.github.io/cufflinks/cuffdiff/index.html). The gene enrichment was carried out with Gene Ontology (http://www.geneontology.org/). The relative transcript abundance was measured in FPKM (expected number of Fragments Per Kilobase of transcript sequence per Millions base pairs sequenced). The log2 ratios of the FPKM values were used to identify differentially expressed genes. All raw data files are available from the NCBI database (accession number PRJNA516068).
Quantitative real-time PCR (qRT-PCR) assays
For the qRT-PCR assays, fresh mycelia of ΔFgMed1 and wild-type strain PH-1 samples were collected at the time points of 12 h, 24 h, 36 h, and 48 h under CMC medium at 28 °C on a rotary shaker. For the expression level of core conidiation regulators, the samples of wild-type PH-1 and ΔFgMed1 mutant were collected at 24 h after grown in CMC on a rotary shaker at 28 °C. RNA was extracted using RNAiso Reagent (TaKaRa Biotechnology Co., Dalian, China) according to the manufacturer’s instructions. qRT-PCR was carried out using primer pairs listed in Supplementary Table S1. The β-tublin gene FGSG_09530 was used as an endogenous control. Relative abundance of transcripts was calculated by the 2−ΔΔCt method. Quantitative RT-PCR was conducted at least twice with three independent biological replicates.
Results
Sequence analysis of the MedA ortholog in F. graminearum
FgMed1 (FGSG_02471.3), a homolog of A. nidulans MedA, was originally identified by a BLASTp searching for A. nidulans MedA. We identified a single homolog of FgMed1 in the F. graminearum genome, which is a 728-amino-acid hypothetical protein (41% identity) (Supplementary Fig. S1A). In addition, the FgMed1 transcript contains a ~ 1273 bp 5′untranslated region (5′UTR), and its open reading fragment contain two introns. A previous study in A. fumigatus confirmed that nuclear localization of MedA was mediated via three nuclear localization signal (NLS) sites NLS2, NLS3 within the conserved C-terminal domain. In our study, we performed sequence alignment analysis of FgMed1 orthologues against other fungi (Al Abdallah et al. 2012), and learned that NLS sites are highly conserved (Supplementary Fig. S1B).
FgMed1 deletion and complementation
To analyze the function of FgMed1, we constructed a null mutant (ΔFgMed11) from wild-type strain PH-1 by homologous recombination strategy as described previously (Fan et al. 2017). Transformants were selected on hygromycin-amended medium, and five individual targeted deletion mutants were created. Two mutants, designated ΔFgMed1-1 and ΔFgMed1-2, were chosen for further confirmed by RT-PCR and Southern blot analysis (Supplementary Fig. S2A). In order to confirm that phenotypic defects in mutants were caused by FgMed1 gene deletion, we complemented the mutant with a wild-type FgMed1 gene with its native promoter, and the FgMed1 was fused to a GFP for gene localization observation. Subsequently, we obtained the complemented strain FgMed1-Com and confirmed the recovery of FgMed1 transcription (Supplementary Fig. S2B).
FgMed1 is involved in vegetative growth
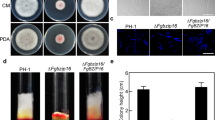
F. graminearum vegetative growth was evaluated by measuring colony diameter on CM agar after incubating for 4 days at 28 °C. The results showed ΔFgMed1 mutants grew approximately 17% slower than the wild-type and FgMed1-Com strains but with normal mycelial morphology. The two mutants showed a diameter growth of 6.46 ± 0.07 and 6.38 ± 0.10 cm on CM after 4 days at room 28 °C whereas wild-type and FgMed1-Com measured 7.78 ± 0.10 and 7.67 ± 0.11 respectively (Fig. 1a, Table 1). These results suggest that FgMed1 plays a role in vegetative growth.
Mycelial growth and macroconidial defect in ΔFgMed1 mutant. a Mycelial growth of F. graminearum strains on complete media (CM). b Self-fertility of F. graminearum strains. Six-day-old carrot agar culture was mock-fertilized to induce sexual reproduction and incubated for an additional 2 weeks. cFgMed1 regulates the development of macroconidia production. ΔFgMed1 produced the macroconidia without terminal phialides. d Morphology of macroconidia produced by PH-1 and ΔFgMed1 mutants. ΔFgMed1 produced both normal and abnormal macroconidia
To determine whether the slower growth was caused by the stress responses or cell wall integrity, F. graminearum strains were exposed to several stress-induced reagents as 1 M sorbitol, 0.5 M NaCl, 0.05 mM H2O2, and several cell membrane damage reagents, e.g., 0.2 mg/ml CFW, 0.05% SDS, and 0.2 mg/ml Congo red. However, there were no significant differences in mycelial growth among all the tested strains under the exposure of the stress-induced reagents and cell membrane damage reagents (data not shown). These results suggested that FgMed1 is involved in vegetative growth but does not play a role in maintaining cell membrane integrity of F. graminearum.
FgMed1 is essential for sexual and asexual reproduction
The MedA was known to regulate conidial development in several other fungal species (Al Abdallah et al. 2012; Gravelat et al. 2010; Ohara et al. 2004), and we performed asexual and sexual reproduction tests in F. graminearum to study the function of FgMed1. For the sexual reproduction test, mutants failed to generate perithecia and ascospores after a cross (Fig. 1b), which indicated that FgMed1 is essential for sexual reproduction in F. graminearum. Asexual conidia production was also dramatically affected by the gene mutation. Cultures of the ΔFgMed1 mutants produced few macroconidia on CMC medium when compared with wild-type and FgMed1-Com strains. After 6 days of incubation, only 300 ± 200 and 400 ± 100 macroconidia/ml were obtained from ΔFgMed-1 and ΔFgMed1-2 mutants, in contrast to 144 ± 11.79 × 106 and 139 ± 7.37 × 106 macroconidia/ml in PH-1 and FgMed1-Com strains, respectively (Table 1).
Normally, the wild-type produces macroconidia on solitary terminal phialides or on multiple terminal phialides borne on conidiophores. However, the mutant only produced macroconidia directly from the abnormal conidiophores without terminal phialides (Fig. 1c, d), which may be directly responsible for the drastic reduction of conidia in the ΔFgMed1 mutant. Moreover, ΔFgMed1 produced both normal and abnormal macroconidia. The tips of the abnormal conidia in the ΔFgMed1 mutant appeared bulbous without a foot cell and an apical cell (Fig. 1c). These results indicated that FgMed1 plays an important role in the development of both sexual reproduction as well as conidiation.
To further study the function of FgMed1 in the development of conidiation, we monitored conidia germination in ΔFgMed1 mutants. The spores collected from PH-1 and ΔFgMed1 were incubated in liquid CM with gentle agitation. The aberrant macroconidia in the mutants was able to germinate but we saw a significant lag time when compared to the wild-type macroconidia. After 4 h, over 56% macroconidia of PH-1 showed at least one germ tube. However, only about 6~7% mutant macroconidia germinated. After 12 h, approximately all PH-1 macroconidia germinated but the germination rate of ΔFgMed1 mutants only reached about 25~30%. After 48 h, ΔFgMed1 macroconidia did reach a germination rate of 82~88% (Table 1).
FgMed1 affected the pathogenicity and DON production
Subsequently, we questioned whether FgMed1 plays a role on pathogenicity, since fungal reproduction and virulence have shown association in other plant pathogenic fungi. We inoculated wheat heads with mycelial plugs of fungal strains (Zheng et al. 2015), and the assays showed that the ΔFgMed1-1 and ΔFgMed1-2 mutants produced moderate scab symptom with a disease index of 6.28 ± 0.86 and 6.76 ± 0.91, respectively (Supplementary Fig. S3). The average disease index of PH-1 and FgMed1-Com was 11.35 ± 0.81 and 12.46 ± 1.00, respectively. We then asked whether this outcome is correlated with mycotoxin deoxynivalenol (DON) production, which is reported as a virulence factors in F. graminearum (Proctor et al. 1995). DON production, assayed in 3-week-old rice grain cultures, showed that ΔFgMed1 mutants have a very low level of DON than the wild-type, suggesting that FgMed1 plays an important role in DON biosynthesis (Table 1).
Intracellular localization of the GFP-tagged FgMed1
The sequence alignment analysis of FgMed1 ortholog against those of other fungi found that nuclear localization signal (NLS) sites are highly conserved in the conserved C-terminal domain of MedA homologs (Supplementary Fig. 1B). We examined the cellular localization of FgMed1 fused to enhanced green fluorescent protein (eGFP) to understand the temporal and spatial pattern of FgMed1. We examined the eGFP expression at different time points (12 h, 24 h, 36 h, and 48 h) after inoculating the mycelia of FgMed1-eGFP strain into CMC medium. The eGFP signals were not detectable until 12 h post inoculation (hpi). The signals were extremely strong at 24 hpi (Fig. 2), but no signal could be detected beyond 36 hpi. Monitoring of the eGFP fusion protein and 4,6-diamidino-2-phenylindole (DAPI)-stained nuclei by fluorescent microscopy confirmed the nuclear localization of FgMed1-eGFP in conidiophores and terminal phialides. These results demonstrated that FgMed1 is important for F. graminearum conidiogenesis.
Moreover, we tested the expression patterns of FgMed1 in the wild-type strain PH-1 at 12-h, 24-h, 36-h, and 48-h time points. The real-time PCR results showed that the FgMed1 expression level increased from 12 to 24 hpi and then decreased from 24 to 48 hpi (Fig. 3). The highest FgMed1 expression was detected at 24 hpi during the sporulation stage. This result corresponded with our eGFP localization study. Therefore, we concluded that the transcription factor FgMed1 is activated and localized to the nucleus, and is predicted to participate in regulating conidiogenesis.
Expression levels of FgMed1 at different time points (12 h, 24 h, 36 h, and 48 h) after inoculation in CMC medium. qRT-PCR was used to quantify transcript level of FgMed1 relative to that of the reference gene β-tubulin using the 2-ΔΔCt method. Error bars represent the standard deviation. Double asterisks indicated significant difference (P = 0.01)
RNA-sequencing based transcriptome analysis
To further gain insight into the FgMed1 function on downstream genes, we conducted a RNA-seq study of wild-type PH-1 and ΔFgMed1 deletion mutant strains at 24 h after the induction of conidiogenesis. Differentially expressed genes (DEGs) were identified by 1.2-fold (log2_fold change) in FKPM values (FPKM values of zero were converted to 1 for the calculation of fold change). A total of 1158 up-regulated (> 1.2-fold) genes and 581 down-regulated (< 1.2-fold) genes were identified in ΔFgMed1 when compared to those DEGs in PH-1 (Supplementary Fig. S4). When the DEGs were mapped to the chromosomes, they were distributed evenly except in chromosome 4 (Supplementary Fig. S5). Using the WEGO (Web Gene Ontology Annotation Plot) to plot GO annotation (Ye et al. 2018), the 1158 up-regulated genes and 581 down-regulated were grouped into three functional categories, 552 and 289 to molecular function, 391 and 200 to biological process, and 152 and 110 to cellular component, respectively (Fig. 4a, b) (Supplementary Table S2). For the down-regulated genes, most genes were associated with the organic substance metabolic process, primary metabolic process, hydrolase activity, organic cyclic compound binding, and heterocyclic compound binding. For the up-regulated genes, most genes were classified into oxidoreductase activity, organic substance metabolic process, and heterocyclic compound binding.
Gene ontology (GO) of FgMed1 targeted DEGs by WEGO 2.0 (Ye et al. 2018). a The WEGO histogram of FgMed1 targeted DEGs. The x-axis displays the GO terms. The right y-axis shows the gene numbers, while the left y-axis shows the percentages (the y-axis was log scaled). b Log of P values of GO terms indicated the significant differences between the down and up-regulated genes
Functional categorization of the targeted DEGs was further conducted manually by the gene annotation into XI groups through the NCBI blast and the published literature (Table 2 and Table 3). Not surprisingly, twenty genes were identified to spore-related protein group (including asexual or sexual development) according to the reported literature (Table 2). Among these were GzBrlA FgAbaA and FgwetA, the orthologs of A. nidulans brlA, abaA, and wetA, respectively, which make up the well-characterized conidiation regulatory pathway. Furthermore, the velvet protein FgVeA was also identified, which is known to control asexual and sexual development as well as secondary metabolisms in several fungal species (Jiang et al. 2011). Additional genes, such as FgFGP1, MAT1-2-1, FgPBS2, and GzRFX1, that are involved in asexual or sexual development were also identified. A number of genes involved in mycotoxin and aurofusarin biosynthesis were identified, including a transcription factor gene DAL81 (FGSG_01134), ZEB2 (FGSG_02398) and aurofusarin biosynthesis genes. Some DEGs were classified to other groups, including integral membrane protein, vegetative incompatibility protein HET-E-1, chitin synthase related protein, multiple antibiotic resistance protein, F-box Protein, and osmotic related protein (Table 3). These results indicated that FgMed1 is a transcription factor with a broad regulatory role in diverse cellular processes, including control of asexual, sexual development, virulence, and toxin production as well as secondary metabolisms in F. graminearum.
The genetic relationship of Med1 and the conidiation regulators genes brlA, abaA, and wetA
RNA-seq results showed that the expression of the central conidiation regulator genes brlA, abaA, and wetA was all down-regulated in the ΔFgMed1 mutant. To confirm the reduction in expression of brlA, abaA, and wetA in the ΔFgMed1 mutant, qRT-PCR were conducted using the RNA extracted at 24 h after the induction of conidiogenesis. The results showed a relative down-regulation in expression of brlA, abaA, and wetA genes in the ΔFgMed1 mutant when compared to the wild-type strain (Fig. 5), suggesting that FgMed1 is an upstream activator of the central conidiation regulators brlA, abaA, and wetA.
Real-time PCR analyses of the central conidiation regulators: brlA, abaA, and wetA. The relative quantity of the transcripts of each gene was compared between the ΔFgMed1 mutant and wild-type strain. The experiment was repeated three times with similar results and the representative data from one experiment was presented. The stable expressed β-tublin gene (FGSG_09530) was used to normalize all candidate genes. Error bars represent the standard deviation. Double asterisks indicated significant difference (P = 0.01)
Discussion
In this study, we characterized FgMed1 in F. graminearum, the homolog of A. nidulans MedA, by generating deletion mutants and analyzing the phenotypic defects. We found that FgMed1 was specifically involved in conidiogenesis and conidium formation in F. graminearum. The FgMed1 deletion mutants failed to produce perithecia and ascospores after a cross, which indicated that FgMed1 is essential for sexual reproduction in F. graminearum. Moreover, the FgMed1 deletion mutants produce macroconidia directly from the abnormal conidiophores in the absence of terminal phialides. Subsequently, we found that FgMed1 affected the pathogenicity and DON production. The intracellular localization of the GFP-tagged FgMed1 confirmed the nuclear localization of FgMed1-GFP in the cell of conidiophores and terminal phialides in F. graminearum. Furthermore, the RNA-seq analysis showed an overall gene expression profile and identified some potential targeted genes that FgMed1 regulated in F. graminearum.
Thus, we conclude that the transcription factor FgMed1 is involved in regulation of diverse cellular processes, including control of asexual sporulation, sexual development, and virulence as well as toxin production in F. graminearum.
The TF MedA has been characterized as a key developmental regulator and has been studied extensively in the model organism A. nidulans. Orthologs of MedA in other filamentous fungi have also been studied, including in A. fumigatus, F. oxysporum, M. oryzae, N. crassa, and U. maydis. The studies showed that MedA orthologs play a conserved role in conidiation, albeit some different phenotypic consequences in each species. The deletion of MedA led to medusoid-like conidiophores in A. nidulans, but was normal in A. fumigatus (Chung et al. 2011). In U. maydis, MedA is needed for successful sexual mating (Chacko and Gold 2012). In M. oryzae and F. oxysporum, the MedA mutation showed deficiencies in conidiophore architecture (Lau and Hamer 1998; Ohara et al. 2004), which are very similar to the FgMed1 mutant producing macroconidia directly from the abnormal conidiophores without terminal phialides. Moreover, N. crassa acon-3, an ortholog of MedA, is required for early conidiophore development and female fertility, and can complement the conidiation defects in A. nidulans ΔmedA (Chung et al. 2011). Our results, along with published reports, showed that MedA orthologs play a conserved role in conidiation in fungi.
One of our key aims in this study is to investigate the molecular mechanisms underlying conidiogenesis in F. graminearum. Therefore, the RNA-seq experiments were conducted by comparing wild-type and ΔFgMed1 strains during conidiation. 1158 up-regulated and 581 down-regulated genes in ΔFgMed1 were identified when compared to PH-1. Significantly, the key genetic components of brlA, abaA, and wetA involved in conidiation were identified in the model organism A. nidulans, these key cores regulatory proteins were studied extensively, and MedA was identified as a temporal modifier of expression of these genes. However, in A. fumigatus, it was determined that the expression of the core conidiation pathway genes was independent of MedA (Gravelat et al. 2010). In our RNA-seq study, we found the expression of FgAbaA, FgWetA, and FgBrlA was all significantly reduced in the ΔFgMed1, which was also confirmed by qPCR. These indicate that the conidiation core regulatory pathway FgBrlA-Aba-WetA regulated by FgMed1 is conserved in F. graminearum. The other conidiation-related genes, such as FgFGP1, FgVEA, GzRFX1, and FgFlbB, were also identified in the RNA-seq data. VeA is a key light-dependent developmental regulator that functions as a repressor of conidiation and activator of sexual development (Jiang et al. 2011; Kim et al. 2015; Park and Yu 2012). In our study, FgVEA was significantly down-regulated in ΔFgMed1 when compared to PH-1, suggesting that FgMed is required for the activation FgVEA gene expression.
In F. graminearum, sexual reproduction is mainly controlled by two closely linked MAT loci which are important regulators. The phenotypic changes caused by MAT deletions and gene expression patterns in F. graminearum strongly suggested that MAT genes are involved in both the early and late stages of sexual development (Kim et al. 2015). From the RNA-seq data, we found that mating-type control genes MAT1-1-1, MAT1-2-1, and MAT1-2-3 were all up-regulated in ΔFgMed1. Previous genome-wide microarray analysis showed that a putative chitin binding protein FGSG_05847 was regulated by the MAT loci and predicted to be involved in the early stage of perithecium formation during sexual development (Kim et al. 2015). In our study, FGSG_05847 was highly up-regulated in the ΔFgMed1 mutant. Since FGSG_05847 was a target gene of MAT loci, thus, we assume FgMed1 controls the expression of FGSG_05847 through MAT loci. All these results seem that FgMed1 is a negative regulator of the sexual reproduction, but the ΔFgMed1 mutant failed to generate perithecia and ascospores in our test. Therefore, the sexual development pathway regulated by FgMed1 still needed further study.
MedA orthologs are also known to govern virulence in some fungal species. For example, the MedA deletion showed an attenuated virulence in A. fumigatus, an invertebrate and a mammalian pathogen model (Gravelat et al. 2010). In M. oryzae, the deletion of ACR1 resulted in reduced ability in host surface attachment and a reduction in appressoria formation, which ultimately led to the reduction in pathogenicity (Lau and Hamer 1998). In U. maydis, the MedA mutants exhibited significantly reduced virulence as well. In our study, we also observed reduced virulence in ΔFgMed1 mutant when compared to the wild-type. There are multiple prognoses for the impairment in virulence of ΔFgMed1. First, the deletion FgMed1 led to a significant reduction in conidia production, a delay in conidial germination, and a slight inhibition in mycelium growth. Secondly, the production of DON in ΔFgMed1 was dramatically reduced.
Interesting, our RNA-seq data showed that though the expression of DON and ZEA toxin-related gene FGSG_02068 (DAL81-TF) was down-regulated in ΔFgMed1, other toxin-related genes, such as FGSG_03881, FGSG_09570 (tox1), FGSG_10566 (toxin), FGSG_02398 (ZEB2), FGSG_01790, FGSG_04488, and FGSG_12121, were up-regulated. The aurofusarin biosynthetic gene cluster was identified in our RNA-seq data; 12 aurofusarin biosynthesis genes, GIP1, GIP2, GIP3, GIP4, GIP5, PKS12, GIP6, GIP7, GIP8, GIP10, GzORF1, and GzMCT, were found. Our RNA-seq results indicate that all these genes are up-regulated in ΔFgMed1 except for GIP10, strongly suggesting that FgMed1 is a negative regulator of the aurofusarin biosynthesis gene cluster.
In this study, we mapped the DEGs in the chromosomes to have a better understanding of the overall gene expression profile. However, the results showed that the genes are distributed randomly except in chromosome 4, where we did not observe gene distribution at one terminal (Supplementary Fig S5). This is because the region of terminus of chromosome 4 represents the rDNA clusters. In conclusion, we demonstrated that the MedA ortholog FgMed1 in F. graminearum is involved in the sexual and asexual development, DON production, and virulence. Significantly, we learned that FgMed1 regulates the expression of a wide range of genes during early conidiogenesis. This functional characterization of FgMed1 expands our understanding of how conidiogenesis and toxin production are closely associated with virulence in F. graminearum.
References
Al Abdallah Q, Choe SI, Campoli P, Baptista S, Gravelat FN, Lee MJ, Sheppard DC (2012) A conserved C-terminal domain of the Aspergillus fumigatus developmental regulator MedA is required for nuclear localization, adhesion and virulence. PLoS One 7(11):e49959. https://doi.org/10.1371/journal.pone.0049959
Arnaise S, Zickler D, Poisier C, Debuchy R (2001) pah1: a homeobox gene involved in hyphal morphology and microconidiogenesis in the filamentous ascomycete Podospora anserina. Mol Microbiol 39(1):54–64
Arratia-Quijada J, Sanchez O, Scazzocchio C, Aguirre J (2012) FlbD, a Myb transcription factor of Aspergillus nidulans, is uniquely involved in both asexual and sexual differentiation. Eukaryot Cell 11(9):1132–1142. https://doi.org/10.1128/EC.00101-12
Bluhm BH, Zhao X, Flaherty JE, Xu JR, Dunkle LD (2007) RAS2 regulates growth and pathogenesis in Fusarium graminearum. Mol Plant-Microbe Interact 20(6):627–636. https://doi.org/10.1094/MPMI-20-6-0627
Cappellini RA, Peterson JL (1965) Macroconidium formation in submerged cultures by a non-sporulating strain of Gibberella zeae. Mycologia 57(6):962–966
Catlett NL, Yoder OC, Turgeon BG (2003) Whole-genome analysis of two-component signal transduction genes in fungal pathogens. Eukaryot Cell 2(6):1151–1161
Chacko N, Gold S (2012) Deletion of the Ustilago maydis ortholog of the Aspergillus sporulation regulator medA affects mating and virulence through pheromone response. Fungal Genet Biol 49(6):426–432. https://doi.org/10.1016/j.fgb.2012.04.002
Chung DW, Greenwald C, Upadhyay S, Ding S, Wilkinson HH, Ebbole DJ, Shaw BD (2011) acon-3, the Neurospora crassa ortholog of the developmental modifier, medA, complements the conidiation defect of the Aspergillus nidulans mutant. Fungal Genet Biol 48(4):370–376. https://doi.org/10.1016/j.fgb.2010.12.008
Clutterbuck AJ (1969) A mutational analysis of conidial development in Aspergillus nidulans. Genetics 63(2):317–327
Desjardins AE (2003) Gibberella from A (venaceae) to Z (eae). Annu Rev Phytopathol 41:177–198. https://doi.org/10.1146/annurev.phyto.41.011703.115501
Dutton JR, Johns S, Miller BL (1997) StuAp is a sequence-specific transcription factor that regulates developmental complexity in Aspergillus nidulans. EMBO J 16(18):5710–5721. https://doi.org/10.1093/emboj/16.18.5710
Fan G, Zhang K, Huang H, Zhang H, Zhao A, Chen L, Chen R, Li G, Wang Z, Lu GD (2017) Multiprotein-bridging factor 1 regulates vegetative growth, osmotic stress, and virulence in Magnaporthe oryzae. Curr Genet 63(2):293–309. https://doi.org/10.1007/s00294-016-0636-9
Gaffoor I, Brown DW, Plattner R, Proctor RH, Qi W, Trail F (2005) Functional analysis of the polyketide synthase genes in the filamentous fungus Gibberella zeae (anamorph Fusarium graminearum). Eukaryot Cell 4(11):1926–1933. https://doi.org/10.1128/EC.4.11.1926-1933.2005
Gravelat FN, Ejzykowicz DE, Chiang LY, Chabot JC, Urb M, Macdonald KD, al-Bader N, Filler SG, Sheppard DC (2010) Aspergillus fumigatus MedA governs adherence, host cell interactions and virulence. Cell Microbiol 12(4):473–488. https://doi.org/10.1111/j.1462-5822.2009.01408.x
Ho CY, Smith M, Houston ME, Adamson JG, Hodges RS (2002) A possible mechanism for partitioning between homo- and heterodimerization of the yeast homeodomain proteins MATa1 and MATα2. J Pept Res 59(1):34–43
Jiang J, Liu X, Yin Y, Ma Z (2011) Involvement of a velvet protein FgVeA in the regulation of asexual development, lipid and secondary metabolisms and virulence in Fusarium graminearum. PLoS One 6(11):e28291. https://doi.org/10.1371/journal.pone.0028291
Jonkers W, Dong Y, Broz K, Kistler HC (2012) The Wor1-like protein Fgp1 regulates pathogenicity, toxin synthesis and reproduction in the phytopathogenic fungus Fusarium graminearum. PLoS Pathog 8(5):e1002724. https://doi.org/10.1371/journal.ppat.1002724
Khang CH, Park SY, Lee YH, Kang S (2005) A dual selection based, targeted gene replacement tool for Magnaporthe grisea and Fusarium oxysporum. Fungal Genet Biol 42(6):483–492
Kim JE, Han KH, Jin J, Kim H, Kim JC, Yun SH, Lee YW (2005) Putative polyketide synthase and laccase genes for biosynthesis of aurofusarin in Gibberella zeae. Appl Environ Microbiol 71(4):1701–1708. https://doi.org/10.1128/AEM.71.4.1701-1708.2005
Kim JE, Jin J, Kim H, Kim JC, Yun SH, Lee YW. (2006) GIP2, a putative transcription factor that regulates the aurofusarin biosynthetic gene cluster in Gibberella zeae. Appl Environ Microbiol 72 (2):1645-1652
Kim S, Park SY, Kim KS, Rho HS, Chi MH, Choi J, Park J, Kong S, Park J, Goh J, Lee YH (2009) Homeobox transcription factors are required for conidiation and appressorium development in the rice blast fungus Magnaporthe oryzae. PLoS Genet 5(12):e1000757. https://doi.org/10.1371/journal.pgen.1000757
Kim HK, Cho EJ, Lee S, Lee YS, Yun SH (2012) Functional analyses of individual mating-type transcripts at MAT loci in Fusarium graminearum and Fusarium asiaticum. FEMS Microbiol Lett 337(2):89–96. https://doi.org/10.1111/1574-6968.12012
Kim Y, Kim H, Son H, Choi GJ, Kim JC, Lee YW (2014) MYT3, a Myb-like transcription factor, affects fungal development and pathogenicity of Fusarium graminearum. PLoS One 9(4):e94359. https://doi.org/10.1371/journal.pone.0094359
Kim HK, Jo SM, Kim GY, Kim DW, Kim YK, Yun SH (2015) A large-scale functional analysis of putative target genes of mating-type loci provides insight into the regulation of sexual development of the cereal pathogen Fusarium graminearum. PLoS Genet 11(9):e1005486. https://doi.org/10.1371/journal.pgen.1005486
Lau GW, Hamer JE (1998) Acropetal: a genetic locus required for conidiophore architecture and pathogenicity in the rice blast fungus. Fungal Genet Biol 24(1-2):228–239. https://doi.org/10.1006/fgbi.1998.1053
Lin Y, Son H, Lee J, Min K, Choi GJ, Kim JC, Lee YW (2011) A putative transcription factor MYT1 is required for female fertility in the ascomycete Gibberella zeae. PLoS One 6(10):e25586. https://doi.org/10.1371/journal.pone.0025586
Lin Y, Son H, Min K, Lee J, Choi GJ, Kim JC, Lee YW (2012) A putative transcription factor MYT2 regulates perithecium size in the ascomycete Gibberella zeae. PLoS One 7(5):e37859. https://doi.org/10.1371/journal.pone.0037859
Liu N, Fan F, Qiu D, Jiang L (2013) The transcription cofactor FgSwi6 plays a role in growth and development, carbendazim sensitivity, cellulose utilization, lithium tolerance, deoxynivalenol production and virulence in the filamentous fungus Fusarium graminearum. Fungal Genet Biol 58-59:42–52. https://doi.org/10.1016/j.fgb.2013.08.010
Lysoe E, Klemsdal SS, Bone KR, Frandsen RJ, Johansen T, Thrane U, Giese H (2006) The PKS4 gene of Fusarium graminearum is essential for zearalenone production. Appl Environ Microbiol 72(6):3924–3932. https://doi.org/10.1128/AEM.00963-05
Lysoe E, Pasquali M, Breakspear A, Kistler HC (2011) The transcription factor FgStuAp influences spore development, pathogenicity, and secondary metabolism in Fusarium graminearum. Mol Plant-Microbe Interact 24(1):54–67. https://doi.org/10.1094/MPMI-03-10-0075
Martinelli SD (1979) Phenotypes of double conidiation mutants of Aspergillus nidulans. J Gen Microbiol 114(2):277–287. https://doi.org/10.1099/00221287-114-2-277
Matar KAO, Chen X, Chen D, Anjago WM, Norvienyeku J, Lin Y, Chen M, Wang Z, Ebbole DJ, Lu GD (2017) WD40-repeat protein MoCreC is essential for carbon repression and is involved in conidiation, growth and pathogenicity of Magnaporthe oryzae. Curr Genet 63(4):685–696. https://doi.org/10.1007/s00294-016-0668-1
Mateos L, Jimenez A, Revuelta JL, Santos MA (2006) Purine biosynthesis, riboflavin production, and trophic-phase span are controlled by a Myb-related transcription factor in the fungus Ashbya gossypii. Appl Environ Microbiol 72(7):5052–5060. https://doi.org/10.1128/AEM.00424-06
McMullen M, Jones R, Gallenberg D (1997) Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis 18(12):1340–1348
Min K, Son H, Lim JY, Choi GJ, Kim JC, Harris SD, Lee YW (2014) Transcription factor RFX1 is crucial for maintenance of genome integrity in Fusarium graminearum. Eukaryot Cell 13(3):427–436. https://doi.org/10.1128/EC.00293-13
Nishimura M, Hayashi N, Jwa NS, Lau GW, Hamer JE, Hasebe A (2000) Insertion of the LINE retrotransposon MGL causes a conidiophore pattern mutation in Magnaporthe grisea. Mol Plant-Microbe Interact 13(8):892–894. https://doi.org/10.1094/MPMI.2000.13.8.892
Odenbach D, Breth B, Thines E, Weber RW, Anke H, Foster AJ (2007) The transcription factor Con7p is a central regulator of infection-related morphogenesis in the rice blast fungus Magnaporthe grisea. Mol Microbiol 64(2):293–307. https://doi.org/10.1111/j.1365-2958.2007.05643.x
Ohara T, Inoue I, Namiki F, Kunoh H, Tsuge T (2004) REN1 is required for development of microconidia and macroconidia, but not of chlamydospores, in the plant pathogenic fungus Fusarium oxysporum. Genetics 166(1):113–124
Park HS, Yu JH (2012) Genetic control of asexual sporulation in filamentous fungi. Curr Opin Microbiol 15(6):669–677. https://doi.org/10.1016/j.mib.2012.09.006
Park AR, Cho AR, Seo JA, Min K, Son H, Lee J, Choi GJ, Kim JC, Lee YW (2012) Functional analyses of regulators of G protein signaling in Gibberella zeae. Fungal Genet Biol 49(7):511–520. https://doi.org/10.1016/j.fgb.2012.05.006
Pasquali M, Kistler C (2006) Gibberella zeae ascospore production and collection for microarray experiments. J Vis Exp 30(1):115. https://doi.org/10.3791/115
Pestka JJ, Smolinski AT (2005) Deoxynivalenol: toxicology and potential effects on humans. J Toxicol Environ Health B Crit Rev 8(1):39–69. https://doi.org/10.1080/10937400590889458
Proctor RH, Hohn TM, McCormick SP (1995) Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant-Microbe Interact 8(4):593–601
Ruiz-Roldan C, Pareja-Jaime Y, Gonzalez-Reyes JA, Roncero MI (2015) The transcription factor Con7-1 is a master regulator of morphogenesis and virulence in Fusarium oxysporum. Mol Plant-Microbe Interact 28(1):55–68. https://doi.org/10.1094/MPMI-07-14-0205-R
Son H, Seo YS, Min K, Park AR, Lee J, Jin JM, Lin Y, Cao P, Hong SY, Kim EK, Lee SH, Cho A, Lee S, Kim MG, Kim Y, Kim JE, Kim JC, Choi GJ, Yun SH, Lim JY, Kim M, Lee YH, Choi YD, Lee YW (2011) A phenome-based functional analysis of transcription factors in the cereal head blight fungus, Fusarium graminearum. PLoS Pathog 7(10):e1002310. https://doi.org/10.1371/journal.ppat.1002310
Son H, Kim MG, Min K, Seo YS, Lim JY, Choi GJ, Kim JC, Chae SK, Lee YW (2013) AbaA regulates conidiogenesis in the ascomycete fungus Fusarium graminearum. PLoS One 8(9):e72915. https://doi.org/10.1371/journal.pone.0072915
Son H, Kim MG, Chae SK, Lee YW (2014a) FgFlbD regulates hyphal differentiation required for sexual and asexual reproduction in the ascomycete fungus Fusarium graminearum. J Microbiol 52(11):930–939. https://doi.org/10.1007/s12275-014-4384-6
Son H, Kim MG, Min K, Lim JY, Choi GJ, Kim JC, Chae SK, Lee YW (2014b) WetA is required for conidiogenesis and conidium maturation in the ascomycete fungus Fusarium graminearum. Eukaryot Cell 13(1):87–98. https://doi.org/10.1128/EC.00220-13
Wang G, Wang C, Hou R, Zhou X, Li G, Zhang S, Xu JR (2012) The AMT1 arginine methyltransferase gene is important for plant infection and normal hyphal growth in Fusarium graminearum. PLoS One 7(5):e38324. https://doi.org/10.1371/journal.pone.0038324
Yang C, Liu H, Li G, Liu M, Yun Y, Wang C, Ma Z, Xu JR (2015) The MADS-box transcription factor FgMcm1 regulates cell identity and fungal development in Fusarium graminearum. Environ Microbiol 17(8):2762–2776. https://doi.org/10.1111/1462-2920.12747
Ye J, Zhang Y, Cui H, Liu J, Wu Y, Cheng Y, Xu H, Huang X, Li S, Zhou A, Zhang X, Bolund L, Chen Q, Wang J, Yang H, Fang L, Shi C (2018) WEGO 2.0: a web tool for analyzing and plotting GO annotations, 2018 update. Nucleic Acids Res 46(W1):W71–W75. https://doi.org/10.1093/nar/gky400
Zhang YZ, Wei ZZ, Liu CH, Chen Q, Xu BJ, Guo ZR, Cao YL, Wang Y, Han YN, Chen C, Feng X, Qiao YY, Zong LJ, Zheng T, Deng M, Jiang QT, Li W, Zheng YL, Wei YM, Qi PF (2017) Linoleic acid isomerase gene FgLAI12 affects sensitivity to salicylic acid, mycelial growth and virulence of Fusarium graminearum. Sci Rep 7:46129. https://doi.org/10.1038/srep46129
Zheng D, Zhang S, Zhou X, Wang C, Xiang P, Zheng Q, Xu JR (2012a) The FgHOG1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum. PLoS One 7(11):e49495. https://doi.org/10.1371/journal.pone.0049495
Zheng W, Zhao X, Xie Q, Huang Q, Zhang C, Zhai H, Xu L, Lu G, Shim WB, Wang Z (2012b) A conserved homeobox transcription factor Htf1 is required for phialide development and conidiogenesis in Fusarium species. PLoS One 7(9):e45432. https://doi.org/10.1371/journal.pone.0045432
Zheng H, Zheng W, Wu C, Yang J, Xi Y, Xie Q, Zhao X, Deng X, Lu G, Li G, Ebbole D, Zhou J, Wang Z (2015) Rab GTPases are essential for membrane trafficking-dependent growth and pathogenicity in Fusarium graminearum. Environ Microbiol 17(11):4580–4599. https://doi.org/10.1111/1462-2920.12982
Funding
This research is supported by the International scientific and technological cooperation and exchange project of Fujian Agriculture and Forestry University (KXGH17007) to Guo-dong Lu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 770 kb)
Rights and permissions
About this article
Cite this article
Fan, G., Zhang, K., Zhang, J. et al. The transcription factor FgMed1 is involved in early conidiogenesis and DON biosynthesis in the plant pathogenic fungus Fusarium graminearum. Appl Microbiol Biotechnol 103, 5851–5865 (2019). https://doi.org/10.1007/s00253-019-09872-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09872-2