Abstract
Metallic nanoparticles (MNPs) with their diverse physical and chemical properties have been applied in various biomedical domains. The increasing demand for MNPs has attracted researchers to develop straightforward, inexpensive, simple, and eco-friendly processes for the enhanced production of MNPs. To discover new biomedical applications first requires knowledge of the interactions of MNPs with target cells. This review focuses on plant and microbial synthesis of biological MNPs, their cellular uptake, biocompatibility, any biological consequences such as cytotoxicity, and biomedical applications. We highlighted the involvement of biomolecules in capping and stabilization of MNPs and the effect of physicochemical parameters particularly the pH on the synthesis of MNPs. Recently achieved milestones to understand the role of synthetic biology (SynBiol) in the synthesis of tailored MNPs are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different physiochemical methods including aerosol technologies, laser ablation, ultraviolet irradiation, lithography, photochemical reduction, and ultrasonic have been developed for the synthesis of metallic nanoparticles (MNPs), which require the use of reactive and toxic chemicals causing disruptive effects on the ecosystem. Therefore, scientific community is continuously trying to develop environment friendly, cost-efficient, and straightforward biological processes for the synthesis of MNPs. Like other scientific endeavors, inspiration has been taken from nature where different forms of nanostructures such as encapsulin, dodecameric (Dps), and ferritins are performing vital role in biomineralization of metals. Richard Feynman described the basic idea of nanotechnology in a lecture entitled “there is plenty of room at the bottom” at the American Institute of Technology in 1959. MNPs vary in their physicochemical properties with the variation in size from 0.1–1000 nm. Generally, MNPs are synthesized using two approaches including bottom-up and top-down (Fawcett et al. 2017; Schöbel et al. 2017; Zhang and Liu 2017). In the bottom-up approach, atoms and molecules assemble to form nanostructures, whereas in the top-down approach, bulk materials are broken down to nano-sized particles (Fig. 1). Preferably, the bottom-up approach has been practiced for the biological synthesis of MNPs.
Biological synthesis of MNPs by using microbes and plants is simple, straightforward, and eco-friendly approach (Varma 2012; Zeth et al. 2016). The use of unicellular microorganisms and multicellular plant tissue extracts for the synthesis of MNPs has been reported as a facile and inexpensive approach (Maliszewska 2011; Ovais et al. 2018; Rajesh et al. 2012). Microorganisms can produce MNPs both intracellularly as well as extracellularly. However, extracellular synthesis of MNPs is easy and cost-efficient. Unicellular magnetotactic bacteria are known for their natural ability to produce magnetite (Fe3O4) (Descamps et al. 2016; Marcano et al. 2017). Also, the extract of different plant tissues has been extensively used for the synthesis of MNPs (Ankamwar et al. 2017; MubarakAli et al. 2011). Plant and microbial-mediated synthesis of MNPs is a bottom-up approach where oxidation and reduction are the two principle reactions involved in the formation of nanoparticles. Biomolecules such as proteins, phenolic compounds, sugars, polysaccharides, and others are considered to be responsible for the conversion of charged metallic ions to their zero-valent nano-forms (Ovais et al. 2018).
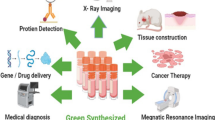
Even though many advancements have been made in the synthesis and use of MNPs, much is yet unknown about the toxicity induced by MNPs. Based on the available knowledge, the use of biologically synthesized MNPs in biomedical sciences is safer than those produced by traditional physicochemical approaches (Ahmed et al. 2016; Duan et al. 2015). The better biocompatibility of BMNPs is due to the encapsulation of metallic core of the nanoparticles with non-toxic biomolecules. Due to this reason, synthesis of MNPs using biological and biotechnological approaches opened a new paradigm for the production of biocompatible, non-toxic, reproducible, easy scaling-up, and well-defined nanostructures. In the recent era, there is a growing interest among the scientific community to develop synthetic biology (SynBiol) techniques to produce tailored BMNPs (Giessen and Silver 2016; Maddinedi et al. 2017; Roopan et al. 2013). Furthermore, metabolic alleyways can be designed, assembled, and integrated within a cell using biotechnological tools. Specifically, microorganisms, plants, and nonhazardous biocompatible agents (Mirzaei and Darroudi 2017; Narayanan and Sakthivel 2010; Wang et al. 2017) having biogenic origin have been explored as a potential resource for the biosynthesis of MNPs (Fig. 2).
This review provides ease for the readers to understand the process of plant and microbial synthesis of biological MNPs and the effect of experimental factors affecting the process. Recently, the role of synthetic biology in the synthesis of tailored MNPs has been described. We highlighted existing knowledge regarding interaction of MNPs with cells, in particular, how the unique properties of MNPs influence their cellular uptake and cytotoxicity. Furthermore, biocompatibility of plant and microbe-assisted synthesized biological MNPs with their biomedical applications has been elaborated.
Microbial synthesis of MNPs
Microbes have immense potential for the synthesis of environmentally benign MNPs, without using conventional physical as well as chemical approaches. Microbes have the capability biomineralized inorganic minerals either intracellularly or extracellularly to produce materials of different size and morphologies at nano-scale (Fig. 3). Microbes are ubiquitously present that can quickly acclimatize themselves in the environment and show resistance to the toxic metals. Microbial resistance against toxic metals is based on two basic approaches: (1) chemical detoxification and (2) ionic efflux from the cell that might be concentration gradient or energy dependent. These functions are actively carried out by the membrane proteins functioning as chemiosmotic cations, ATPase, and anti-transporters. Microbes can produce MNPs both intracellularly as well as extracellularly. Briefly for the extracellular synthesis of MNPs, under optimum conditions, targeted microbe is grown in a rotary shaker for 1–2 days, the biomass is removed through centrifugation, and the supernatant is collected. Subsequently mixing the filter-sterilized metallic salt solution and cell-free culture supernatant in a particular ration followed by incubation at optimal temperature will produce MNPs. Contrastingly, for the synthesis of intracellular MNPs, the microbial biomass is collected after centrifugation following thorough washing with sterilized water, then the biomass is dissolved in a filter-sterilized metallic salt solution. The reaction mixture is incubated and monitored for a visual change of the color. After repeated cycles of sonication, the biomass is removed by centrifugation and the synthesized MNPs are measured using a UV-spectrophotometer. Ultrasonication helps to break the microbial cell wall/membrane and allow the MNPs to move out of the cell. Extracellular synthesis of MNPs is considered as an inexpensive, rapid, and scalable approach because it eliminates the processing steps, including sonication, centrifugation, and washing required in the case of intracellular synthesis and recovery of MNPs.
Microbes, including fungi, yeast, virus, and bacteria have been studied for intra- and extracellular synthesis of MNPs (Table 1) (Chen et al. 2015; Priyadarshini et al. 2013). Recently, different bacterial types such as Vibrio parahaemolyticus (Elayaraja et al. 2017), Bacillus methylotrophicus (Wang et al. 2016), Pseudomonas deceptionensis DC5 (Jo et al. 2016), Rhodococcus spp. (Otari et al. 2015), and Shewanella oneidensis MR-1 (Ishiki et al. 2017) have been studied for the synthesis of gold and silver NPs. Similarly, some reports have been published showing the potential of Bacillus flexus, Staphylococcus lentus, Escherichia coli, Magnetospirillum gryphiswaldense, and Rhodospirillum rubrum to produce MNPs (Apte et al. 2016; Das et al. 2014; Kushwaha et al. 2015; Priyadarshini et al. 2013). Furthermore, SynBiol represents a step forward for biotechnologically sustainable production of tailored MNPs (Lin et al. 2012) such as magnetite (Fe3O4) nanostructures (Kolinko et al. 2014). A range of bacterial genera including Lactobacillus, Brevibacterium, Pseudomonas, Rhodobacter, Weissella, Rhodococcus, Bacillus, Corynebacterium, Pseudomonas, Aeromonas, Klebsiella, Enterobacter, Escherichia, Shewanella, Streptomyces, Sargassum, Trichoderma, Rhodopseudomonas, Desulfovibrio, Plectonema boryanum, and Pyrobaculum have been reported for the synthesis of MNPs (Li et al. 2011).
Mycosynthesis of MNPs has been explored by utilizing diverse metabolites of fungi showing enhanced oxidation/reduction potential and better bioaccumulation potential with straightforward eco-friendly method (Castro-Longoria 2016; Kashyap et al. 2017; Kitching et al. 2016). Three different phenomena including electron shuttle quinones, the activity of nitrate reductase, and the combination of both have been reported for the myco-mediated synthesis of MNPs (Alghuthaymi et al. 2015). Different enzymes such as nitrate reductase in case of Penicillium sp. and NADPH-dependent reductases of Fusarium oxysporum have been reported for the formation of different MNPs (Kumar et al. 2007). Although, few reports are available showing the involvement of actinomycetes in the formation of MNPs (Sanjenbam et al. 2014). However, there is room to explore the potential of actinomycetes for the synthesis of stable and monodispersed MNPs. Synthesis of copper, zinc, and silver NPs using reductase enzyme obtained from Streptomyces sp. have been reported for the synthesis of MNPs (Undabarrena et al. 2017). The yeast has also been studied for the synthesis of MNPs on a large scale with simple downstream methods (AbdelRahim et al. 2017; Apte et al. 2013; Barabadi et al. 2017; Mourato et al. 2011; Waghmare et al. 2015; Yang et al. 2016).
Tobacco mosaic virus (TMV)-mediated synthesis of iron oxide were achieved due to the involvement of amino acids such as aspartate, and glutamate present on the external surface of the virus (Shenton et al. 1999). The capsids of genetically engineered viruses were also investigated as biotemplates for the synthesis of nanostructures of titanium and quantum dot nanowires (Chen et al. 2015). Furthermore, some biological molecules such as amino acids, polyphosphates, and fatty acids were applied as a template for the synthesis of semiconductor nanoparticles. Some other biological approaches are also available for green synthesis of nanostructures, including protein cages (Palchoudhury et al. 2017; Uchida et al. 2015), DNA (Lodha et al. 2017; Vial et al. 2017), biolipid cylinders (Charcosset 2016), multicellular superstructures (Mann et al. 1997), and viroid capsules (Chen et al. 2015), which have been applied for the template-mediated formation of MNPs.
Phyto-mediated synthesis of MNPs
Phytonanotechnology is also an environment friendly, rapid, cost-effective, stable, and straightforward approach for the synthesis of MNPs (Fig. 3). Phytonanotechnology has many advantages such as the use of a universal solvent (water) as a reducing medium, scalability, biocompatibility, lack of pathogenicity, and medical applicability of phyto-synthesized MNPs (Noruzi 2015). Thus, the non-toxic nature of plant-derived materials seems to be suitable to accomplish the high demand for the synthesis of MNPs with applications in biomedical areas. Gold and silver NPs were successfully synthesized by using fruit, leaf, root, and bark extracts of aquatic and herbal plants (Ankamwar et al. 2017; Mittal et al. 2013; Salari et al. 2016; Singh et al. 2016). Different plant tissues, such as roots, stems, bark, leaves, flowers, fruits, and seed extracts have been exploited for the synthesis of various MNPs (Table 2) (Bankar et al. 2010; Dhand et al. 2016; Edison et al. 2016; Gogoi et al. 2015; Jafarirad et al. 2016; Malaikozhundan et al. 2017; Mo et al. 2015; Murugan et al. 2015; Rajakumar et al. 2017; Rajan et al. 2017; Rao et al. 2016; Tahir et al. 2017; Wang et al. 2014).
The process of phyto-mediated synthesis of MNPs is simple and proceeds from washing the plant tissues, cutting into small pieces, crushing, and heating in boiling water to extract plant biomolecules out of the cell. Subsequently, purification of the extract can be performed through centrifugation and filtration process. Synthesis of MNPs can be carried out by mixing the metallic salt solution and plant extract in particular ratios followed by incubation of the reaction mixture at ambient temperatures. The change in color of the reaction mixture is the visual indication of MNP synthesis. Usually, red-colored gold and transparent silver salt solutions change to purple and brown respectively when mixed with plant extracts. Synthesized MNPs can be extracted out of the solution through centrifugation of the nanoparticles suspension at high-speed such as 22,000-24,000g for 30 to 40 min. Un-reacted metallic ions and small biomolecules can be removed by washing the separated MNPs with water and repeated cycles of ultra-high centrifugation.
Although it is possible to utilize living plants as biofermenters for the synthesis of MNPs, however, this domain remains as less explored. To date, synthesis of silver and gold NPs was performed in vitro using legume mung bean (Vigna radiata L.), and alfalfa (Medicago sativa) plants respectively (Gardea-Torresdey et al. 2002; Kumari et al. 2017). Furthermore, Parker et al. successfully used Arabidopsis thaliana plants growing in medium containing potassium tetrachloropalladate (K2PdCl4) for the synthesis of palladium NPs (Parker et al. 2014). Briefly, reports about the plant-mediated synthesis of MNPs with their applications are shown in (Table 2) and applications are explained in the “Biomedical applications” section.
Phyto-synthesis of MNPs may occur by oxidation and reduction of metallic ions into their non-toxic, stable, and zero-valent form. However, the exact components and the mechanisms responsible for the phyto-mediated synthesis of MNPs yet remain unclear. It has been studied that amino acids, vitamins, proteins, organic acids, and also secondary metabolic products, such as polyphenols, flavonoids, terpenoids, alkaloids, polysaccharides, heterocyclic compounds, and lipids, have a vital role in the reduction of metallic ions to stabilize MNPs (Devi et al. 2017; Duan et al. 2015). Recently, Ovais et al. discussed the role of phytochemical and microbial enzymes in the synthesis of metallic nanoparticles. Authors emphasized on explaining the biosynthesis pathways of phenolics, flavonoids, terpenoids, mevalonate, and methylerythritol phosphate; however, less emphasis was given on how these biomolecules interact with different metals for the synthesis of their corresponding zero-valent nanoparticles (Ovais et al. 2018). Hence, there is room to investigate how these biomolecules play their role in the synthesis of MNPs. Therefore, the role of phytochemicals that takes part in the synthesis of MNPs is elaborated in the following sections.
Phytochemicals and synthesis of MNPs
Flavonoids
They are secondary water-soluble metabolites containing 15 carbons and comprising of six major subgroups including anthoxanthins, flavans, flavanones, anthocyanidins, flavanonols, and isoflavonoid. Flavonoids are considered to be the major reducing agent of plant extracts involved in the reduction of metallic ions to their corresponding zero-valent nanoparticles. The oxidation and reduction potential of flavonoids is attributed to the molecular oxygen of flavonoids (Zhou et al. 2010). Pietta (2000) has reported that the removal of hydrogen during keto-enol conversion in flavonoids results in the formation of rosmarinic acid and luteolin which are responsible for the metal ion reduction to their MNPs (Ahmad et al. 2010). Ghoreishi et al. documented the reduction of hydroxyl groups of myricetin and quercetin into carbonyl groups during metallic (gold) ion reduction and the formation of AuNPs (Ghoreishi et al. 2011). In another study, microwave-assisted guava (Psidium guajava) leaf extract-mediated synthesis of AuNPs has been documented. Authors concluded that the flavonoids present in guava leaves are responsible for the synthesis of biogenic AuNPs (Raghunandan et al. 2009).
Phenolic acids
Phenolic acids are polyphenols containing a phenolic ring and a carboxylic functional group, and their metal reduction potential is attributed to their nucleophilic aromatic rings (Wang et al. 2007). The synthesis of Terminalia chebula fruit extract-mediated AgNPs is attributed to the phenolic hydroxyl group of gallic acid that undergoes oxidation during interaction with silver ions leading to the formation of AgNPs (Edison and Sethuraman 2012). In another study, AuNPs were synthesized using aqueous extract of Macrotyloma uniflorum and showed that the release of hydrogen during conversion of caffeic acid to ferulic acid is responsible for the reduction of gold ions to AuNPs (Aromal et al. 2012). Due to the presence of propanoic acid side chain, caffeic acid is acting as a strong reducing agent by delocalization of electron between the propanoic chain and aromatic ring. The metal reduction potential of lignin, tannin, and flavonoid glycosides has also been reported in the literature (Kasthuri et al. 2009a; Kasthuri et al. 2009b; Lopes et al. 2018).
Terpenoids
Also called isoprenoids are the abundant organic compounds responsible for color, taste, and aroma in various plant species. Recently, phytochemicals are reported for the synthesis of AuNPs using bark extract of Pterocarpus santalinus L. (Srinath and Rai 2018). Authors reported the involvement of terpenoids in the reduction of gold ions to synthesize biocompatible AuNPs. Similarly, Cinnamon zeylanicum bark extract harboring terpenoids such as eugenol, linalool, and methyl chavicol are also reported for the reduction of metallic ions for the synthesis of AgNPs (Sathishkumar et al. 2009). Sing et al. reported the synthesis of amine group functionalized gold and silver nanoparticles using eugenol of clove (Syzygium aromaticum) extract (Singh et al. 2010). The release of the electron from hydroxyl group of eugenol transforms eugenol to its anionic form, making it a reducing agent. Also, the reduction potential of eugenol further increased due to the inductive effect of electron withdrawing allyl and methoxy functional groups present at ortho and para positions of the OH group. The simultaneous release of electrons is considered to be responsible for the reduction of metallic ions to for NPs.
Proteins
To date, proteins are considered to be the ultimate working force of the living system. Proteins play a vital role in the synthesis of metallic nanoparticles through their carboxylate and/or amino groups (Cuellar-Cruz 2017). Tan et al. studied the peptide-mediated synthesis of AuNPs and investigated the metal binding and reducing potential of 20 amino acids (Tan et al. 2010). AgNPs were synthesized using the latex of Jatropha curcas, and cyclic peptides present in the latex were proved to be responsible for the synthesis of AgNPs (Bar et al. 2009a). The silver ion-reducing potential of tyrosine has been described, through ionization of phenolic group of tyrosine. This ionization resulted in the conversion of tyrosine in the semi-quinones (Roy et al. 2014). Reports are also available showing the involvement of tryptophan in the reduction of metallic ions by transient transformation of tryptophan into tryptophol radical by releasing an electron (Tamuly et al. 2013; Veerakumar et al. 2013).
Organic acids
Plant secondary metabolites such as alkaloids and organic acids are reported for their metal reduction and synthesis of MNPs. Tamuly et al. reported the biosynthesis of AgNPs by using pedicellamide (A) isolated from Piper pedicellatum C.DC leaf (Tamuly et al. 2014). Mechanism involved in the pedicellamide-mediated synthesis of AgNPs is attributed to the release of reactive hydrogen from pedicellamide. The synthesis of AgNPs by ascorbic acid of orange peel extract has been reported (Konwarh et al. 2011). In another study, the tautomerization of benzoquinone derivatives obtained from mesophyte Cyperus sp. has been reported for the reduction of metals.
Similarly, phytochemicals of Hydrilla sp., including catechol, protocatecheuic acid, and ascorbic acid have been reported for the synthesis of AgNPs through the release of reactive hydrogen. Also, malic and pyruvic acid synthesized during redox reaction of glycolytic pathway in Bryophyllum sp., (a xerophyte) are reported for the reduction of silver ions to AgNPs (Jha et al. 2009). Gonzalez-Ballesteros et al. found that the carbonyl and hydroxyl groups of protein from plant extracts could help in the production of stable NPs (González-Ballesteros et al. 2017; M Joseph et al. 2016).
Parameters affecting biological synthesis of MNPs
Although biological synthesis of MNPs has numerous advantages, a polydispersity of the nanoparticles remains a disadvantage. Therefore, the scientific community has focused on process optimization for enhanced production of monodispersed uniformly sized MNPs (Tables 1 and 2). Optimization of the growth medium composition and parameters such as temperature, pH, salt concentration, incubation period, mixing ratio, redox conditions, irradiation, and aeration are the necessary steps in almost every type of biological process (Rao and Paria 2015; Ahmad et al. 2018; Verma and Mehata 2016; El-Naggar and Abdelwahed 2014; Kammoun et al. 2008). Among others, the pH has reported as a critical parameter responsible for the variation in the size of synthesized MNPs. Kumari et al. found that the smallest gold NPs of 4 nm size were synthesized by Trichoderma viride when the pH of the reaction mixture was set at 9.5 (Kumari et al. 2017). However, optimum synthesis of AgNPs by Microbacterium sp. MV4 was observed at pH 7. The change in the pH of the reaction mixture alters the charged species of natural biomolecules both in microbial and phyto-extracts and affects their metal ion reduction capability. Avena sativa extract was used to develop small sized gold NPs by adjusting pH at 3.0, and 4.0, however, further decrease in the pH up to 2.0 resulted in the aggregation of synthesized NPs. Authors proposed that this effect may be because of nucleation and binding of metal ions to the numerous functional groups that become possible at 4.0 and 3.0 instead of pH 2.0 (Sankar et al. 2017). In another study, the extract of pears was exploited for the synthesis of gold NPs. Authors reported that the triangular and hexagonal gold NPs were observed at alkaline pH instead of acidic pH (Ghodake et al. 2010). Likewise, salt concentrations, incubation time and chances for NPs synthesis depend on the source and nature of extracts (Pereira et al. 2015).
Current understanding of the biological synthesis of MNPs
Potentially microorganisms can synthesize MNPs both intra- and extracellularly. Microbial extracellular formation of MNPs occurs when metallic ions come in contact with reduced sugars of cell wall/membrane. Previously, the use of Fusarium oxysporum and Verticillium sp. ruled out the possible involvement of reduced sugars present in the cell wall for the synthesis of gold NPs by reducing Au+3 ions. However, negatively charged AuCl4− ions interact with positively charged amino acids of the enzymes localized on the cell wall (Kitching et al. 2015). Contrastingly, formation of intracellular Au3+ NPs, the ions diffused inside the cell through the cell membrane, is reduced by intracellular redox mediators. Though, it is not clear either the cross-membrane diffusion is active bioaccumulation or passive biosorption. The biosorption of gold ions could be due to their toxicity to the viable microbial cell that induces pores in the cell wall/membrane. Intracellularly, nicotinamide adenine dinucleotide phosphate (NADPH) or nicotinamide adenine dinucleotide (NADH) is usually involved in the reduction of metallic ions. X-ray diffraction analysis of various microorganisms revealed that the intracellular concentration of zero-valent metals increases and the concentration of charged ions decreases with increased incubation time. This observation suggested that the increased incubation allows more ions to move into the cell where they get reduced to their zero-valent state. Also, the formation of ethylated metallic ions suggested the possible defense mechanism of viable cells against metal toxicity (Gholami-Shabani et al. 2016; Otari et al. 2015).
The available reports showed that metal-induced toxicity trigger intracellular signaling pathway for the activation of stress response genes resulting in the production of reductases and oxygenases. The upregulation of such genes takes place at sub-toxic concentrations of metal ions; however, at higher concentrations metal toxicity hinders the expression of proteins and inhibits cellular growth (Demidchik 2015; Gupta et al. 2017). For example, results of SDS-PAGE showed upregulated proteins from Rhizopus oryzae when exposed to Au3+ ions. Further, investigation on the biosynthesis of gold NPs from extracts of fungi, bacteria, and plants demonstrated the involvement of NADH/NADPH-dependent reductase in metal bioreduction process (Prasad et al. 2016). Also, the possible involvement of aromatic amino acids, for example, tryptophan and tyrosine in the reduction of metal ions to produce MNPs was confirmed by Fourier transform infrared spectroscopy (FTIR).
Synthetic biology and MNPs
Recently, there is a growing interest among the scientific community to explore the potential use of synthetic biology (SynBiol) for the synthesis of tailored MNPs (Chessher et al. 2015; Kolinko et al. 2014) (Fig. 4). It is challenging to produce monodisperse single magnetic domain NPs at ambient temperatures. Magnetotactic bacteria have shown a huge potential for the mineralization of magnetosomes (membrane-bounded magnetic nano-crystalline particles depicting unprecedented magnetic features). Because of the difficult handling of magnetotactic bacteria, Kolinko et al. tried to express the underlying biosynthetic pathway from these fastidious bacteria into non-magnetic host using synthetic biology approaches. They successfully transferred a minimal set of genes from magnetotactic donor bacterium Magnetospirillum gryphiswaldense into a non-magnetic photosynthetic model bacterium Rhodospirillum rubrum. Authors concluded that this approach will enable the sustainable production of tailored biogenic nanoparticles in non-native biotechnologically important hosts. Also, they suggested that this is a step towards the endogenous magnetization of different organisms using synthetic biology (Kolinko et al. 2014).
Chessher and co-workers discovered a gene controlling the synthesis of magnetosome and clustered in 115 kb large genomic island interspersed with many genes of unknown functions (Ullrich et al. 2005). Later functions of different genes of this island were determined (Lohbe et al. 2011). Afterward, authors stitched together many expression cassettes containing 29 genes (25 kb) of four operons in different combinations and deleted one gene (ftsZm) from mamXY operon because of its known interference function with cell division during cloning. Transformation of recombinant expression cassettes into non-magnetic strains of M. gryphiswaldense resulted in the formation of stable native type magnetosome biomineralization showing the success of cloning and functionality of transferred operons. Also, authors transferred expression cassettes into non-magnetic photosynthetic alphaproteobacterium R. rubrum foreign host bacterium. After transformation of the optimized expression cassette (Fig. 5a), the strain ABG6X of R. rubrum encompassed all 29 relevant genes of magnetosome island except ftsZm. The cell of recombinant ABG6X strain showed magnetic response within several hours, and a pellet of bacterial cells become visible near the magnet placed at the edge of culture flask showing that the strain ABG6X has attained magnetotactic features (Fig. 5b) (Kolinko et al. 2014).
a Schematic presentation of molecular arrangement of gene cassettes introduced into the chromosome of R. rubrum in a stepwise manner. Broad arrows indicate the extensions and transcriptional directions of individual genes. Different colors illustrate the cassettes inserted into the chromosome (oval shape, not to scale) as indicated by their gene names in the figure. Shown in yellow are antibiotic resistance genes (kmR, kanamycin resistance; tcR, tetracycline resistance; apR, ampicillin resistance; gmR, gentamicin resistance). Thin red arrows indicate different promoters (P) driving transcription of inserted genes (Pkm, Pgm, Ptc, promoters of antibiotic resistance cassettes; PlacI, promoter lac repressor; Pmms, PmamDC, PmamH, PmamXY, native promoters of the respective gene clusters from M. gryphiswaldense; Plac, lac promoter). Crossed lines indicate sites of gene deletions of mamI and mamJ in strains R. rubrum_ABG6X_dI and R. rubrum_ABG6X_dJ, respectively. IR, inverted repeat defining the boundaries of the sequence inserted by the transposase (Kolinko et al. 2014). b Cells of R. rubrum_ABG6X accumulated as a visible red spot near the pole of a permanent magnet at the edge of a culture flask (Kolinko et al. 2014)
Possible mechanisms for cellular uptake and cytotoxicity of MNPs
Possible mechanisms for cellular uptake of MNPs
Cell membranes are semipermeable allowing only small molecules/ions to pass through it while hindering others. Communication among or between the cells and their microenvironment and uptake of nutrients take place through the cell membrane by different mechanisms. Small nonpolar or hydrophobic molecules, water, carbon dioxide, and oxygen can diffuse through the cell membrane based on their concentration gradients. Molecules like amino acids and various ions move across the cell membrane through active transport mediated by membrane protein pumps (Jentsch 2016); however, hydrophilic biomacromolecules at the nano-scale conventionally entered the cell through endocytosis, a plasma membrane-driven process that encapsulates the cargo into transport vesicles (Conner and Schmid 2003).
How do MNPs apt in the cellular uptake system? Findings of various experiments showed how engineered MNPs could pass through the plasma membrane of diverse cell types, such as human alveolar epithelial (Park et al. 2007), human mesenchymal stem cells (Greulich et al. 2011), and human epidermal keratinocyte (HEK) cells (Monteiro-Riviere et al. 2013). Based on the experimental findings, the mechanisms involved in the cellular uptake of nanoparticles have been summarized in (Fig. 6). Nevertheless, the mechanisms of cellular uptake of nanoparticles proposed by different research groups are sometimes inconsistent or even contradicting with each other. A systematic understanding of the process of uptake and intracellular trafficking of NPs hence became a topic of great interest among the scientific community.
Different physicochemical properties such as size and shape, surface chemistry, and surface charges play a vital role in cellular uptake of metallic nanoparticles (Carnovale et al. 2019). Figure 6 summarizes the cellular uptake of MNPs through endocytosis that is a form of active transport where cells encapsulate objects with cytoplasmic membrane resulting in the formation of vesicles. Such endocytotic processes known for their involvement in the uptake and intracellular dissemination of MNPs are (1) phagocytosis, (2) pinocytosis, and (3) clathrin-mediated or caveolae-dependent endocytosis (Nel et al. 2009; Verma and Stellacci 2010; Ishimoto et al. 2008).
Phagocytosis is a process usually operated by particular mammalian cells such as macrophages, neutrophils, and monocytes. It plays a vital role to engulf solid particles with a diameter greater than 750 nm to form internal phagosomes (Ishimoto et al. 2008). Macropinocytosis or pinocytosis that occurs in almost every cell type is responsible for the uptake of particles varying in size from few to several hundred nanometers. Possibly, the primarily characterized pathway for the internalization of nanoparticles is active uptake through clathrin-mediated endocytosis, and the cargo is encapsulated into small vesicles with a diameter smaller than 100 nm (Harush-Frenkel et al. 2007; Li et al. 2008) that fuses with endosomes (Zhang et al. 2009). Also, clathrin-independent mechanisms rely on the cholesterol-dependent grouping of lipid-attached proteins into various domains at microscale (called lipid raft carriers) (Kirkham and Parton 2005). These lipid rafts/caveolae comprise of 50–80 nm invaginations of plasma membrane harboring sphingolipids, caveolins, and cholesterol (McIntosh et al. 2002). Caveolae-mediated endocytosis is a significant mechanism for the cellular uptake of MNPs in endothelial cells (Partlow et al. 2008; Wang et al. 2009).
Almost every type of nanoparticle adapted a specific mechanism for their entrance into the cell. For example, incubation of gold nanoparticles with HeLa cells in their growth medium results in the adsorption of serum proteins on the surface of gold nanoparticles leading the nanoparticles towards cells through receptor-mediated endocytosis (RME) (Khan et al. 2007). Clathrin-dependent endocytosis and RME are names of the same process where cells encapsulate molecules by inward growth of plasma membrane to make vesicles at the particular receptor site specific to the proteins attached with the nanoparticles being internalized. Internalization of Herceptin encapsulated gold nanoparticles is carried out through RME with the help of ErbB2 receptor of the plasma membrane. During this process binding of gold particles, activation of the ErbB2 receptor and expression of the protein is strictly dependent on the size of the nanoparticles (Jiang et al. 2008).
The encapsulation of gold nanoparticles with various organic/biological molecules usually alters their mode of cellular internalization. Such internalization of particles with polymeric surfaces involves dynamin as well as F-action that is usually energy-dependent. For the uptake of nanoparticles with positive charges on their surfaces, micropinocytosis seems to be a significant pathway. However, clathrin or caveolae-independent endocytosis seems to be an important pathway for the uptake of MNPs with negative charge of their surfaces (Dausend et al. 2008; Van Haute et al. 2018). For example, positively charged gold nanoparticles with plain surface were internalized by clathrin and caveolin-dependent endocytosis as well as micropinocytosis, while negatively charged gold nanoparticles coated with polyethylene glycol (PEG) were internalized through caveolin or clathrin-dependent endocytosis without the involvement of micropinocytosis (Brandenberger et al. 2010).
Autophagy is a conserved process involved in the degradation of cytoplasmic organelles and cellular proteins by encapsulating them with phagophores to form autophagosomes. Recently, the idea of nanoparticle-induced autophagy has attracted the intention of many researchers (Klionsky 2007; Mishra et al. 2016). Various MNPs such as neodymium oxide, silver (Ag), and gold (Au) are reported for inducing autophagy in different cells (Chen et al. 2005; Li et al. 2010; Mishra et al. 2016). Reports are published showing the successful use of different metallic nanoparticles such as zinc oxide, iron oxide, and gold for the treatment of cancerous T cells, lung epithelial cells, and oral cancer cells respectively (Hanley et al. 2008; Khan et al. 2012; Wu et al. 2011). However, the actual process of autophagy and mechanisms involved in the nanoparticles mediated treatment of cancer remains unknown which need to be addressed.
Possible mechanism of MNP-induced cytotoxicity
Different types of MNPs with diverse physical and chemical properties have been shown to induce reactive oxygen species (ROS)-mediated cytotoxicity. ROS constitute a pool of oxidative species containing hydroxyl radical (OH•), singlet oxygen (1O2), superoxide anion (O2•−), hypochlorous acid (HOCL), and hydrogen peroxide (H2O2). These oxygen species are known to induce cytotoxicity through oxidative stress both in vivo and in vitro (Donaldson et al. 2001; Yang et al. 2009). Generation of primary ROS species (O2•−) is intrinsically catalyzed by nicotinamide adenine dinucleotide phosphate (NADPH) via one-electron reduction from molecular oxygen. Subsequently, the reduction of oxygen may lead to the formation of OH• or H2O2 via metal-induced Fenton reaction and dismutation respectively (Forrester et al. 2018; Thannickal and Fanburg 2000).
Several reports showed that ROS generation and oxidative stress are the events leading towards MNPs-induced cytotoxicity. Oxidative stress is correlated with the physicochemical properties of MNPs. The key factors involved in the NP-induced cytotoxicity are (1) activation of NADPH-dependent oxidation system, (2) mitochondrial respiration and apoptosis, (3) depletion of antioxidant enzymes, and (4) alteration in cell homeostasis. MNP-mediated ROS generation triggers cell signaling pathways, expressions of chemokine and cytokines, and activation of transcription factors. Triggering of these pathways is linked with the transcription of genes responsible for inflammation, fibrosis, genotoxicity, and cancer. Thereafter, observed cytotoxicity during MNPs exposure could be endorsed to the generation of ROS. To access the toxic effects of MNPs, the inclusion of these cellular responses is an essential screening tool. For example, biological system undergoes mild oxidative stress when antioxidant enzymes show their overexpression, whereas severe oxidative stress results in mitochondrial apoptosis. ROS provides a scale to access the cytotoxic effects upon exposure to the MNPs. To access the cytotoxic effects of MNPs, a rigorous characterization of MNPs and in detail investigation of oxidative stress markers both in vivo and in vitro are vital.
Biocompatibility of biologically synthesized MNPs
The biocompatibility of biologically synthesized MNPs has been widely ascribed to molecules encapsulating the metallic core of the nanoparticles (Aditya et al. 2017). Knowledge about the biocompatibility of synthesized MNPs is vital before their applications. Generally, a range of biomolecules such as phenolics, carbohydrates, vitamins, flavonoids, tannins, terpenoids, proteins, and polycerids are responsible for different properties of MNPs. These biomolecules control the net charge on the surface of MNPs that is the key factor responsible for determining the mechanism of action of MNPs (Sendra et al. 2017). Various biomolecules are functionally divergent and may act as nonspecific reducing agents, legends, and free radical scavengers to bind with the metallic core of the MNPs (Fig. 2). Different cell types depict varying levels of tolerance against biomolecules derived from both plants and microbes.
However, the question is how a crust of biomolecules can eradicate the toxic effects of the metallic core of MNPs. To answer this question, the epigenetic effect of capped biomolecules on mammalian gene expression for disease prevention and protection has been investigated in cellular models. The involvement of plant-derived phenolic antioxidants in the prevention of human diseases such as Alzheimer’s disease, cancer, and cardiovascular diseases through epigenetic modulation has been reported (Malireddy et al. 2012; Tang and Tsao 2017; Thakur et al. 2014). Cell viability assay of the cervical cancer cell line (SiHa) with biologically synthesized platinum NPs encapsulated with tea polyphenols (TPP@Pt) and bimetallic next-generation Pt@Cu synthesized using polyphenon 60 showed that the cell proliferation of SiHa cells was inhibited. Treatment with TPP@Pt and Pt@Cu nanoparticles significantly increased the percentage of cells in G2/M phase, indicating cell cycle arrest in the G2/M phase and an increased cell number in sub-G0 cell death phase (Alshatwi et al. 2015; Athinarayanan et al. 2016). In two separate studies, authors suggested that both TPP@Pt and Pt@Cu can be used for cervical cancer treatment. Separately, a study conducted on Parkinsonism in zebrafish model reported that green synthesized Pt-NPs had successfully inverted the neurotoxic impact of MPTP by optimizing levels of mitochondrial complex I, superoxide dismutase, dopamine, glutathione peroxidase, and glutathione (Ganaie et al. 2017; Nellore et al. 2013). Dedicated research is still required to be carried out to understand whether or not encapsulating biomolecules or metallic core of biologically synthesized MNPs has been involved in inducing epigenetic effects.
Panda et al. reported the use of aromatic spath of male inflorescence of screw pine (Pandanus odorifer) mediated biologically synthesized silver nanoparticles (24-55 nm), commercial silver nanoparticles (70 nm) procured from Sigma-Aldrich, USA, and other silver species including silver ions, colloidal silver chloride, to investigate their genotoxicity effects in their own established Allium cepa assay. Authors found no effect on genotoxicity when both types of AgNPs were applied at 10 μg/ml. Also, the evaluation of MNP-mediated cytotoxicity-associated biomarkers including the generation of ROS species, mitotic index, DNA damage (using comet assay at 20 to 80 μg/ml), mitotic aberrations, micronucleus, and cell death revealed that both biologically synthesized and commercial AgNPs showed lesser cytotoxicity and greater genotoxicity as compared with Ag+ ions alone. Cell death induction potential of different species of silver in roots of Allium cepa were arranged as; Ag+ > colloidal AgCl > commercial AgNPs > biologically synthesized AgNPs. DNA damage and cell death caused by biogenic AgNPs was prevented through dimethyl thiourea and Tiron by scavenging H2O2 and O2−. ROS was suggested as the potential source for inducing AgNPs mediated DNA damage and cell death (Panda et al. 2011).
Separately Sarkar et al. investigated toxicity induced by Alternaria alternata-mediated synthesized protein-capped biogenic AgNPs (25 to 45 nm) and evaluated the DNA damage in human lymphocytes. Non-significant changes in cell viability of cells treated with 400 μg/ml of AgNPs as compared with control (untreated cells) cells were observed through trypan blue dye exclusion method. DNA damage analysis of in vitro treatment of lymphocytes via comet assay showed no DNA damage up to 40 μg/ml of AgNPs, while an increase in DNA damage was observed in dose-dependent manner up to 300 μg/ml. This DNA damage was represented in terms of olive tail moment test and percentage of DNA in the tail of the comet assay tail. Comet assay revealed that the DNA damage was approximately five times higher in the positive control (cell treated with 100 μmol/l methyl methanesulphonate) as compared with the lowest treatment dose of biogenic AgNPs (Sarkar et al. 2011).
Available methods for biocompatibility analysis of MNPs
At present, different qualitative and quantitative assays have been utilized to study the biocompatibility of MNPs. For qualitative analysis, these assays include live or dead cell staining assays such as acridine orange and ethidium bromide cellular staining assays. However, for quantitative analysis of biocompatibility, 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and lactate dehydrogenase (LDH) leakage tests are most widely used dye-conversion assays (Patil and Kim 2017; Sriranjani et al. 2016). The LDH leakage assay indicates the damage to the cell membrane, while the MTT assay is the sign of mitochondrial viability. Further, the protein content of a viable cell and lysosome activity can be measured by protein assay and neutral red assay respectively (Fotakis and Timbrell 2006; Lobner 2000). Type of metallic nanoparticles (gold, iron, silver, platinum, copper oxide), dose (concentration × time), selection of cell line, size, shape, and surface properties of MNPs have a significant role in overall biocompatibility of the MNPs (Kononenko et al. 2017; Muthiah et al. 2013; Shang et al. 2014).
Biomedical applications
Biologically synthesized metallic nanoparticles (MNPs) have many applications in the biomedical field with a vast potential for continued growth in this area. Various MNPs have been widely implemented for their antibacterial, optical, and anticancer properties in diagnostic, therapeutic, and hyperthermia applications. Further, applications of different MNPs are described with examples in the following sections.
Antimicrobial activity
Antibacterial activity
Exposure to AgNPs causes cytotoxicity in bacteria. Silver ions and AgNPs interact with plasma membrane resulting in disturbance of cell permeability, cellular respiration, and prevention of DNA replication by denaturing the ribosomes or by binding the DNA (Chaloupka et al. 2010). The mechanism of AuNPs induced antibacterial potential against E. coli is illustrated by Cui et al. (2012). Authors reported that AuNPs distorted membrane potential and inhibited activity of ATPase resulting in the significant decrease of cellular ATP. Also, Sadhasivam et al. reported that the exposure to AuNPs showed inhibition of rRNA binding with ribosomes. Antimicrobial potential of bacteria-mediated synthesized AgNPs and AuNPs was documented by Sadhasivam et al. (2012) and Sadhasivam et al. (2010). In these studies, Streptomyces hygroscopicus-mediated biologically synthesized NPs were used as an antimicrobial agent against Enterococcus faecalis, Bacillus subtilis, Staphylococcus epidermidis, E. coli, S. aureus, and Salmonella typhimurium. Recently, Saraca asoca plant leaf extract was used for the synthesis of AgNPs and antibacterial potential of synthesized NPs was investigated against Staphylococcus aureus, Salmonella typhi, and Streptococcus pyogenes (Fatema et al. 2019).
Antifungal activity
Biologically synthesized MNPs have been reported for the antifungal properties. Alternaria alternata-mediated extracellularly synthesized biological AgNPs showed increment in antifungal activity of fluconazole against Phoma herbarum, Phoma glomerata, Candida albicans, Trichoderma sp., and Fusarium semitectum (Gajbhiye et al. 2009). Ocimum vasilicum leaf extract-mediated synthesized AgNPs were applied as antibacterial as well as antifungal agent. Synthesized AgNPs showed higher antifungal activity against Aspergillus flavus, Aspergillus niger, Aspergillus terreus, and Aspergillus fumigatus (Elumalai et al. 2019).
Antiviral activity
Viruses are the causative agents of various diseases of plants and animals and a potential source of diverse problems in medicine. Relatively less number of reports on antiviral potential of biogenic metallic nanoparticles has been reported. Aspergillus ochraceus-mediated synthesized intracellular AgNPs have been reported for potential antiviral agent (Vijayakumar and Prasad 2009). In this report, authors isolated the biogenic AgNPs by heat treatment and applied against M13 phage and antiviral potential was determined through plaque count method. Gaikwad et al. reported the feasibility for mycosynthesis of AgNPs, and their antiviral activity varies with the production system used. Authors demonstrated the interaction of AgNPs with human parainfluenza virus type 3 and herpes simplex virus types 1 and 2 are dependent on the size of AgNPs. In this study, the reduction of viral infectivity has attributed the potential of AgNPs to hinder the interaction between virus and cell, which might be dependent on zeta potential and size of the AgNPs (Gaikwad et al. 2013).
MNPs as anticancer agents
Cytotoxicity of biologically synthesized MNPs in various cancer cell lines has been well documented. Amarnath et al. reported the facile synthesis of AuNPs using phytochemicals present in grapes (Vitis vinifera) acting as reducing and stabilizing agents. AuNPs showed a strong affinity towards human breast cancer cells (HBL-100) and induced apoptosis in exposed cells (Amarnath et al. 2011). Joseph et al. synthesized medicinal plant Indigofera tinctoria leaf extract induced biogenic AgNPs and AuNPs (Joseph and Mathew 2018). Both silver and gold NPs exhibited dose-dependent cytotoxicity to lung cancer cell line A549, where NPs showed more toxic effect on cancer cell lines as compared to the control (pure plant leaf extract). In another study, cytotoxic properties of fungus (Penicillium brevicompactum)-mediated synthesized biogenic AuNPs were analyzed in mouse myoblast cancer (C2C12) cells (Mishra et al. 2011). Recently, Pugazhendhi et al. reported the biogenic synthesis of magnesium oxide nanoparticles (MgONPs) by using brown algae Sargassum wightii as reducing and capping agent. Cytotoxicity of biogenic MgONPs in exposed lung cancer cell line A549 was dose-dependent with IC50 value of 77.5 ± 0.34 μg/ml. Safety evaluation using peripheral blood mononuclear cells (PBMCs) were used for safety evaluation of synthesized MgONPs, and results illustrated that MgONPs to be non-toxic (Pugazhendhi et al. 2019).
MNPs as drug carriers
Naturally crystalline magnetic greigite Fe3S4 and/or magnetite Fe3O4 are biomineralized by magnetotactic bacteria (MTB) in a phospholipid bilayer membrane encapsulated structure called magnetosomes. It is documented that researchers isolated and purified the magnetosomes from a magnetotactic bacterium (Magnetospirillum gryphiswaldense) and encapsulated/loaded with a chemotherapy drug doxorubicin (DOX) (Sun et al. 2008). Anticancer potential of DOX-loaded bacterial magnetosomes was checked by performing cytotoxicity assay in EMT-6 and HL60 cancer cells. In this study, authors concluded that DOX-loaded magnetosomes have successfully inhibited the proliferation of carcinoma cells and suppressed the level of mRNA of the c-myc gene, suggesting that DOX-loaded magnetosomes are important for cancer treatment. In another study, Li et al. revealed in vitro biocompatibility of phospholipid bilayer membrane encapsulated purified and sterilized bacterial magnetosomes in mouse fibroblast cells (Xiang et al. 2007). Recently, magnetosomes derived from Magnetospirillum gryphiswaldense are investigated in anti-tumor immunotherapy (Tang et al. 2019). In this study, authors selected syngeneic TC-1 mouse models of cancer to investigate whether anti-4-1BB agonistic antibody-loaded bacterial magnetosomes could enhance the therapeutic effects of anti-4-1BB in localized disease settings. Intravenous injection of antibody-coupled bacterial magnetosomes along with magnetic treatment showed significantly (p < 0.05) higher tumor protection than other treatment approaches. Anti-4-1BB antibody-loaded bacterial magnetosomes may have potential for their clinical application to anti-tumor antibody therapy.
MNPs in medical diagnostics and biosensors
The combined use of biogenic MNPs and chemical sensors has been documented for the detection of medically relevant compounds such as glucose and peroxides. For example, Zheng et al. reported the synthesis of AuNPs using eggshell membrane (ESM) (AuNPs/ESM) at ambient temperature and immobilized glucose oxidase (GOx) on AuNPs/ESM to investigate glucose biosensing. Range of glucose biosensor was 20 μM to 0.80 mM with detection limit 17 μM, indicating that biosynthesized AuNPs on ESM can be used in the biosensing field (Zheng et al. 2010). Subsequently, the same material was used as glucose biosensor to determine the level of glucose in blood serum, and results obtained showed an agreement with standard spectrophotometric tests (Zheng et al. 2011). An environmental isolate Bacillus pumilus sp. BAB-3706-mediated extracellularly synthesized biogenic selenium nanoparticle (SeNPs) was reported as low-cost H2O2 biosensor (Prasad et al. 2015). Wang et al. utilized Bacillus subtilis-mediated synthesized SeNPs for the preparation of H2O2 sensor. Based on bacterial extracellular proteins, spherical SeNPs could be converted into trigonal wires. An electrode (glassy carbon) was dipped in a drop of SeNPs suspension and horseradish peroxidase. Subsequently, voltammetry analysis showed that SeNPs increased the detection of H2O2 with 80 nM detection limit (Wang et al. 2010).
MNPs and medical imaging
Due to the diverse optical properties, MNPs have been attracting the attention of the scientific community for centuries. Various optical features linked with MNPs are low- and high-refractive index, photoluminescence properties, surface plasmon resonance, and photonic crystals. In nanophotonics where light interacts with nanoparticles, localized surface plasmon resonances and size-dependent semiconductor band gap are the significantly important properties.
Microalgae Klebsormidium flaccidum encapsulated with silica-mediated synthesis of AuNPs resulted in the construction of biohybrid material. In this work, in situ images of silica-encapsulated cells were obtained through Raman spectroscopy to study the effect of AuNPs on the photosynthetic potential of algae. Photosynthesis-based biosensors might be developed by coupling the sol-gel encapsulation and Raman imaging (Sicard et al. 2010). Iron oxide and superparamagnetic iron oxide NPs have dual capacity to act as photothermal and magnetic agents that can be used for hyperthermia, cancer therapy, tissue repair, targeted drug delivery, and immunoassays such as cell labeling, detoxification of biological fluids, and magnetic resonance imaging (Espinosa et al. 2016; Hajba and Guttman 2016; Laurent et al. 2017; Mou et al. 2015; Wu et al. 2015). Biogenic AgNPs synthesized by Trichoderma viride extracellular filtrate are reported for blue and orange light emission. These NPs showed photoluminescence in the range of 320 to 520 nm after laser excitation making them a promising candidate for imaging and labeling purposes (Fayaz et al. 2010).
Conclusion and future prospects
Synthesis of MNPs by microbes and plants has emerged with many advantages such as lack of toxic chemicals, monodispersity, and efficient eco-friendly and rapid approach. The factors such as temperature, incubation time, and pH play an essential role in the synthesis of MNPs. Biologically synthesized MNPs showed higher biocompatibility as compared with the MNPs synthesized through various physicochemical methods. The available knowledge to understand the mechanism of interaction between biomolecules (phytochemicals) and metal ions during synthesis of MNPs has been summarized to bridge the gap in this domain. Recently, the use of synthetic biology (SynBiol), which is a step forward in the synthesis of tailored biological MNPs, has been explained. Biologically synthesized MNPs have been extensively used to solve the problems or to enhance the process efficiency in industries and biomedical sciences. During in vivo application of MNPs, the problems like excretion, clearance, and non-targeted distribution of MNPs are required to be solved. Also, the availability of insufficient knowledge of signaling pathways and biochemical agents responsible for the synthesis of MNPs remains an open challenge to explore the potential of microbe and plants for the efficient synthesis of MNPs. The knowledge of biocompatibility of MNPs is still at the juvenile stage and dedicated research is required in this domain.
References
AbdelRahim K, Mahmoud SY, Ali AM, Almaary KS, Mustafa AE-ZMA, Husseiny SM (2017) Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi J Biol Sci 24(1):208–216. https://doi.org/10.1016/j.sjbs.2016.02.025
Aditya N, Espinosa YG, Norton IT (2017) Encapsulation systems for the delivery of hydrophilic nutraceuticals: food application. Biotechnol Adv 35(4):450–457. https://doi.org/10.1016/j.biotechadv.2017.03.012
Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, Sastry M (2003a) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B 28(4):313–318. https://doi.org/10.1016/S0927-7765(02)00174-1
Ahmad A, Senapati S, Khan MI, Kumar R, Ramani R, Srinivas V, Sastry M (2003b) Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 14(7):824
Ahmad F, Anwar S, Firdous S, Da-Chuan Y, Iqbal S (2018) Biodegradation of bispyribac sodium by a novel bacterial consortium BDAM: optimization of degradation conditions using response surface methodology. J Hazard Mater 349:272–281. https://doi.org/10.1016/j.jhazmat.2017.12.065
Ahmad N, Sharma S, Alam MK, Singh VN, Shamsi SF, Mehta BR, Fatma A (2010) Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids Surf B 81(1):81–86. https://doi.org/10.1016/j.colsurfb.2010.06.029
Ahmed S, Ahmad M, Swami BL, Ikram S (2016) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res 7(1):17–28. https://doi.org/10.1016/j.jare.2015.02.007
Alghuthaymi MA, Almoammar H, Rai M, Said-Galiev E, Abd-Elsalam KA (2015) Myconanoparticles: synthesis and their role in phytopathogens management. Biotechnol Biotechnol Equip 29(2):221–236
Aljabali AA, Barclay JE, Lomonossoff GP, Evans DJ (2010) Virus templated metallic nanoparticles. Nanoscale 2(12):2596–2600
Alshatwi AA, Athinarayanan J, Subbarayan PV (2015) Green synthesis of platinum nanoparticles that induce cell death and G2/M-phase cell cycle arrest in human cervical cancer cells. J Mater Sci Mater Med 26(1):1–9
Amarnath K, Mathew NL, Nellore J, Siddarth CRV, Kumar J (2011) Facile synthesis of biocompatible gold nanoparticles from Vites vinefera and its cellular internalization against HBL-100 cells. Cancer Nanotechnol 2(1):121–132. https://doi.org/10.1007/s12645-011-0022-8
Ankamwar B, Salgaonkar M, Sur UK (2017) Room temperature green synthesis of anisotropic gold nanoparticles using novel biological fruit extract. Inorg Nano-Metal Chem 47(9):1359–1363. https://doi.org/10.1080/24701556.2017.1284121
Apte M, Chaudhari P, Vaidya A, Kumar AR, Zinjarde S (2016) Application of nanoparticles derived from marine Staphylococcus lentus in sensing dichlorvos and mercury ions. Colloids Surf A Physicochem Eng Asp 501:1–8. https://doi.org/10.1016/j.colsurfa.2016.04.055
Apte M, Sambre D, Gaikawad S, Joshi S, Bankar A, Kumar AR, Zinjarde S (2013) Psychrotrophic yeast Yarrowia lipolytica NCYC 789 mediates the synthesis of antimicrobial silver nanoparticles via cell-associated melanin. AMB Express 3(1):32
Aromal SA, Vidhu VK, Philip D (2012) Green synthesis of well-dispersed gold nanoparticles using Macrotyloma uniflorum. Spectrochim Acta, Part A 85(1):99–104. https://doi.org/10.1016/j.saa.2011.09.035
Athinarayanan J, Periasamy VS, Alshatwi AA (2016) Eco-friendly synthesis and characterization of platinum-copper alloy nanoparticles induce cell death in human cervical cancer cells. Process Biochem 51(7):925–932
Bankar A, Joshi B, Kumar AR, Zinjarde S (2010) Banana peel extract mediated synthesis of gold nanoparticles. Colloids Surf B 80(1):45–50
Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Misra A (2009a) Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf A Physicochem Eng Asp 339(1):134–139. https://doi.org/10.1016/j.colsurfa.2009.02.008
Bar H, Bhui DK, Sahoo GP, Sarkar P, Pyne S, Misra A (2009b) Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf A Physicochem Eng Asp 348(1):212–216. https://doi.org/10.1016/j.colsurfa.2009.07.021
Barabadi H, Honary S, Mohammadi MA, Ahmadpour E, Rahimi MT, Alizadeh A, Naghibi F, Saravanan M (2017) Green chemical synthesis of gold nanoparticles by using Penicillium aculeatum and their scolicidal activity against hydatid cyst protoscolices of Echinococcus granulosus. Environ Sci Pollut Res 24(6):5800–5810. https://doi.org/10.1007/s11356-016-8291-8
Behravan M, Hossein Panahi A, Naghizadeh A, Ziaee M, Mahdavi R, Mirzapour A (2019) Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int J Biol Macromol 124:148–154. https://doi.org/10.1016/j.ijbiomac.2018.11.101
Bhainsa KC, D'Souza SF (2006) Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B 47(2):160–164. https://doi.org/10.1016/j.colsurfb.2005.11.026
Brandenberger C, Mühlfeld C, Ali Z, Lenz AG, Schmid O, Parak WJ, Gehr P, Rothen-Rutishauser B (2010) Quantitative evaluation of cellular uptake and trafficking of plain and polyethylene glycol-coated gold nanoparticles. Small 6(15):1669–1678. https://doi.org/10.1002/smll.201000528
Carnovale C, Bryant G, Shukla R, Bansal V (2019) Identifying trends in gold nanoparticle toxicity and uptake: size, shape, capping ligand, and biological corona. ACS Omega 4(1):242–256. https://doi.org/10.1021/acsomega.8b03227
Castro-Longoria E (2016) Fungal biosynthesis of nanoparticles, a cleaner alternative. In: Purchase D (ed) Fungal applications in sustainable environmental biotechnology. Springer International Publishing, Cham, pp 323–351
Chaloupka K, Malam Y, Seifalian AM (2010) Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol 28(11):580–588. https://doi.org/10.1016/j.tibtech.2010.07.006
Charcosset C (2016) Preparation of nanomaterials for food applications using membrane emulsification and membrane mixing. Emulsions 3:37
Chen P-Y, Dang X, Klug MT, Courchesne N-MD, Qi J, Hyder MN, Belcher AM, Hammond PT (2015) M13 virus-enabled synthesis of titanium dioxide nanowires for tunable mesoporous semiconducting networks. Chem Mater 27(5):1531–1540. https://doi.org/10.1021/cm503803u
Chen Y, Yang L, Feng C, Wen L-P (2005) Nano neodymium oxide induces massive vacuolization and autophagic cell death in non-small cell lung cancer NCI-H460 cells. Biochem Biophys Res Commun 337(1):52–60
Chessher A, Breitling R, Takano E (2015) Bacterial microcompartments: biomaterials for synthetic biology-based compartmentalization strategies. ACS Biomater Sci Eng 1(6):345–351
Conner SD, Schmid SL (2003) Regulated portals of entry into the cell. Nature 422:37. https://doi.org/10.1038/nature01451
Cuéllar-Cruz M (2017) Synthesis of inorganic and organic crystals mediated by proteins in different biological organisms. A mechanism of biomineralization conserved throughout evolution in all living species. Prog Cryst Growth Charact Mater 63(3):94–103. https://doi.org/10.1016/j.pcrysgrow.2017.07.001
Cui Y, Zhao Y, Tian Y, Zhang W, Lü X, Jiang X (2012) The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 33(7):2327–2333. https://doi.org/10.1016/j.biomaterials.2011.11.057
Das RK, Bhuyan D (2019) Microwave-mediated green synthesis of gold and silver nanoparticles from fruit peel aqueous extract of Solanum melongena L. and study of antimicrobial property of silver nanoparticles. Nanotechnol Environ Eng 4(1):5. https://doi.org/10.1007/s41204-018-0052-0
Das VL, Thomas R, Varghese RT, Soniya EV, Mathew J, Radhakrishnan EK (2014) Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech 4(2):121–126 https://doi.org/10.1007/s13205-013-0130-8
Dausend J, Musyanovych A, Dass M, Walther P, Schrezenmeier H, Landfester K, Mailänder V (2008) Uptake mechanism of oppositely charged fluorescent nanoparticles in HeLa cells. Macromol Biosci 8(12):1135–1143
Demidchik V (2015) Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Exp Bot 109:212–228
Descamps EC, Abbé JB, Pignol D, Lefèvre CT (2016) Controlled biomineralization of magnetite in bacteria. iron oxides: From nature to applications:99–116
Devi GK, Kumar KS, Parthiban R, Kalishwaralal K (2017) An insight study on HPTLC fingerprinting of Mukia maderaspatna: mechanism of bioactive constituents in metal nanoparticle synthesis and its activity against human pathogens. Microb Pathog 102:120–132. https://doi.org/10.1016/j.micpath.2016.11.026
Dhand V, Soumya L, Bharadwaj S, Chakra S, Bhatt D, Sreedhar B (2016) Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater Sci Eng C 58:36–43
Donaldson K, Stone V, Seaton A, MacNee W (2001) Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect 109(Suppl 4):523
Duan H, Wang D, Li Y (2015) Green chemistry for nanoparticle synthesis. Chem Soc Rev 44(16):5778–5792
Dubey SP, Lahtinen M, Sillanpaa M (2010) Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem 45(7):1065–1071. https://doi.org/10.1016/j.procbio.2010.03.024
Edison TJI, Sethuraman MG (2012) Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochem 47(9):1351–1357. https://doi.org/10.1016/j.procbio.2012.04.025
Edison TNJI, Lee YR, Sethuraman MG (2016) Green synthesis of silver nanoparticles using Terminalia cuneata and its catalytic action in reduction of direct yellow-12 dye. Spectrochim Acta, Part A 161:122–129. https://doi.org/10.1016/j.saa.2016.02.044
El-Naggar NE-A, Abdelwahed NA (2014) Application of statistical experimental design for optimization of silver nanoparticles biosynthesis by a nanofactory Streptomyces viridochromogenes. J Microbiol 52(1):53–63
Elayaraja S, Zagorsek K, Li F, Xiang J (2017) In situ synthesis of silver nanoparticles into TEMPO-mediated oxidized bacterial cellulose and their antivibriocidal activity against shrimp pathogens. Carbohydr Polym 166:329-337 https://doi.org/10.1016/j.carbpol.2017.02.093
Elumalai D, Sathiyaraj M, Vimalkumar E, Kaleena PK, Hemavathi M, Venkatesh P (2019) Bio fabricated of silver nanoparticles using Ocimum basilicum and its efficacy of antimicrobial and antioxidant activity. Asian J Green Chem 3(1. pp. 1-124):103–124
Espinosa A, Di Corato R, Kolosnjaj-Tabi J, Flaud P, Pellegrino T, Wilhelm C (2016) Duality of iron oxide nanoparticles in cancer therapy: amplification of heating efficiency by magnetic hyperthermia and photothermal bimodal treatment. ACS Nano 10(2):2436–2446
Fatema S, Shirsat M, Farooqui M, Pathan MA (2019) Biosynthesis of Silver nanoparticle using aqueous extract of Saraca asoca leaves, its characterization and antimicrobial activity. Int J Nano Dimension 10(2):163–168
Fawcett D, Verduin JJ, Shah M, Sharma SB, Poinern GEJ (2017) A review of current research into the biogenic synthesis of metal and metal oxide nanoparticles via marine algae and seagrasses. J Nanosci 2017:15. https://doi.org/10.1155/2017/8013850
Fayaz M, Tiwary CS, Kalaichelvan PT, Venkatesan R (2010) Blue orange light emission from biogenic synthesized silver nanoparticles using Trichoderma viride. Colloids Surf B 75(1):175–178. https://doi.org/10.1016/j.colsurfb.2009.08.028
Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK (2018) Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 122(6):877–902
Fotakis G, Timbrell JA (2006) In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett 160(2):171–177
Gaikwad S, Ingle A, Gade A, Rai M, Falanga A, Incoronato N, Russo L, Galdiero S, Galdiero M (2013) Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomedicine 8:4303–4314. https://doi.org/10.2147/IJN.S50070
Gajbhiye M, Kesharwani J, Ingle A, Gade A, Rai M (2009) Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine 5(4):382–386. https://doi.org/10.1016/j.nano.2009.06.005
Ganaie S, Abbasi T, Abbasi S (2017) Biomimetic synthesis of platinum nanoparticles utilizing a terrestrial weed Antigonon leptopus. Part Sci Technol 36(6):681–688. https://doi.org/10.1080/02726351.2017.1292336
Gardea-Torresdey JL, Parsons JG, Gomez E, Peralta-Videa J, Troiani HE, Santiago P, Yacaman MJ (2002) Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett 2(4):397–401. https://doi.org/10.1021/nl015673+
Ghodake G, Deshpande N, Lee Y, Jin E (2010) Pear fruit extract-assisted room-temperature biosynthesis of gold nanoplates. Colloids Surf B 75(2):584–589
Gholami-Shabani M, Shams-Ghahfarokhi M, Gholami-Shabani Z, Razzaghi-Abyaneh M (2016) Microbial enzymes: current features and potential applications in nanobiotechnology advances and applications through fungal nanobiotechnology. Springer, pp:91–127
Ghoreishi SM, Behpour M, Khayatkashani M (2011) Green synthesis of silver and gold nanoparticles using Rosa damascena and its primary application in electrochemistry. Phys E 44(1):97–104. https://doi.org/10.1016/j.physe.2011.07.008
Giessen TW, Silver PA (2016) Encapsulation as a strategy for the design of biological compartmentalization. J Mol Biol 428(5):916–927
Gogoi N, Babu PJ, Mahanta C, Bora U (2015) Green synthesis and characterization of silver nanoparticles using alcoholic flower extract of Nyctanthes arbortristis and in vitro investigation of their antibacterial and cytotoxic activities. Mater Sci Eng C 46:463–469. https://doi.org/10.1016/j.msec.2014.10.069
Gonzalez-Ballesteros N, Prado-Lopez S, Rodriguez-Gonzalez J, Lastra M, Rodriguez-Arguelles M (2017) Green synthesis of gold nanoparticles using brown algae Cystoseira baccata: its activity in colon cancer cells. Colloids Surf B 153:190–198
Greulich C, Diendorf J, Simon T, Eggeler G, Epple M, Köller M (2011) Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomater 7(1):347–354. https://doi.org/10.1016/j.actbio.2010.08.003
Gupta S, Rautela P, Maharana C, Singh K (2017) Priming host defense against biotic stress by arbuscular mycorrhizal fungi agro-environmental sustainability. Springer, pp:255–270
Hajba L, Guttman A (2016) The use of magnetic nanoparticles in cancer theranostics: toward handheld diagnostic devices. Biotechnol Adv 34(4):354–361
Hanley C, Layne J, Punnoose A, Reddy K, Coombs I, Coombs A, Feris K, Wingett D (2008) Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 19(29):295103
Harush-Frenkel O, Debotton N, Benita S, Altschuler Y (2007) Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem Biophys Res Commun 353(1):26–32. https://doi.org/10.1016/j.bbrc.2006.11.135
Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, Wang H, Wang Y, Shao W, He N (2007) Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 18(10):105104
Ishiki K, Okada K, Shiigi H, Nagaoka T (2017) Investigation concerning the formation process of gold nanoparticles by Shewanella oneidensis MR-1. Anal Sci 33(2):129–131
Ishimoto H, Yanagihara K, Araki N, Mukae H, Sakamoto N, Izumikawa K, Seki M, Miyazaki Y, Hirakata Y, Mizuta Y (2008) Single-cell observation of phagocytosis by human blood dendritic cells. Jap J Infect Dis 61(4):294–297
Jafarirad S, Mehrabi M, Divband B, Kosari-Nasab M (2016) Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: a mechanistic approach. Mater Sci Eng C 59:296–302. https://doi.org/10.1016/j.msec.2015.09.089
Jayaseelan C, Rahuman AA, Kirthi AV, Marimuthu S, Santhoshkumar T, Bagavan A, Gaurav K, Karthik L, Rao KVB (2012) Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim Acta, Part A 90:78–84. https://doi.org/10.1016/j.saa.2012.01.006
Jayaseelan C, Ramkumar R, Rahuman AA, Perumal P (2013) Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Ind Crop Prod 45:423–429. https://doi.org/10.1016/j.indcrop.2012.12.019
Jentsch TJ (2016) VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat Rev Mol Cell Biol 17:293. https://doi.org/10.1038/nrm.2016.29 https://www.nature.com/articles/nrm.2016.29#supplementary-information
Jha AK, Prasad K, Prasad K, Kulkarni AR (2009) Plant system: nature’s nanofactory. Colloids Surf B 73(2):219–223. https://doi.org/10.1016/j.colsurfb.2009.05.018
Jiang W, Kim BYS, Rutka JT, Chan WCW (2008) Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol 3:145. https://doi.org/10.1038/nnano.2008.30 https://www.nature.com/articles/nnano.2008.30#supplementary-information
Jo JH, Singh P, Kim YJ, Wang C, Mathiyalagan R, Jin C-G, Yang DC (2016) Pseudomonas deceptionensis DC5-mediated synthesis of extracellular silver nanoparticles. Artif Cells Nanomed Biotechnol 44(6):1576–1581. https://doi.org/10.3109/21691401.2015.1068792
Joseph S, Mathew B (2018) Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties AU - Vijayan, Remya. Artif Cells Nanomed Biotechnol 46(4):861–871. https://doi.org/10.1080/21691401.2017.1345930
Kammoun R, Naili B, Bejar S (2008) Application of a statistical design to the optimization of parameters and culture medium for α-amylase production by Aspergillus oryzae CBS 819.72 grown on gruel (wheat grinding by-product). Bioresour Technol 99(13):5602–5609
Kashyap PL, Kumar S, Srivastava AK (2017) Nanodiagnostics for plant pathogens. Environ Chem Lett 15(1):7–13. https://doi.org/10.1007/s10311-016-0580-4
Kasthuri J, Kathiravan K, Rajendiran N (2009a) Phyllanthin-assisted biosynthesis of silver and gold nanoparticles: a novel biological approach. J Nanopart Res 11(5):1075–1085. https://doi.org/10.1007/s11051-008-9494-9
Kasthuri J, Veerapandian S, Rajendiran N (2009b) Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf B 68(1):55–60. https://doi.org/10.1016/j.colsurfb.2008.09.021
Khan JA, Pillai B, Das TK, Singh Y, Maiti S (2007) Molecular effects of uptake of gold nanoparticles in HeLa cells. Chembiochem 8(11):1237–1240
Khan MI, Mohammad A, Patil G, Naqvi S, Chauhan L, Ahmad I (2012) Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 33(5):1477–1488
Kirkham M, Parton RG (2005) Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta, Mol Cell Res 1745(3):273–286. https://doi.org/10.1016/j.bbamcr.2005.06.002
Kitching M, Choudhary P, Inguva S, Guo Y, Ramani M, Das SK, Marsili E (2016) Fungal surface protein mediated one-pot synthesis of stable and hemocompatible gold nanoparticles. Enzym Microb Technol 95:76–84. https://doi.org/10.1016/j.enzmictec.2016.08.007
Kitching M, Ramani M, Marsili E (2015) Fungal biosynthesis of gold nanoparticles: mechanism and scale up. Microb Biotechnol 8(6):904–917
Klionsky DJ (2007) Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 8:931. https://doi.org/10.1038/nrm2245 https://www.nature.com/articles/nrm2245#supplementary-information
Knez M, Bittner AM, Boes F, Wege C, Jeske H, Maib E, Kern K (2003) Biotemplate synthesis of 3-nm nickel and cobalt nanowires. Nano Lett 3(8):1079–1082. https://doi.org/10.1021/nl0342545
Kolinko I, Lohbe A, Borg S, Raschdorf O, Jogler C, Tu Q, Posfai M, Tompa E, Plitzko JM, Brachmann A (2014) Biosynthesis of magnetic nanostructures in a foreign organism by transfer of bacterial magnetosome gene clusters. Nat Nanotechnol 9(3):193–197
Kononenko V, Repar N, Marusic N, Drasler B, Romih T, Hocevar S, Drobne D (2017) Comparative in vitro genotoxicity study of ZnO nanoparticles, ZnO macroparticles and ZnCl 2 to MDCK kidney cells: size matters. Toxicol in Vitro 40:256–263
Konwarh R, Gogoi B, Philip R, Laskar MA, Karak N (2011) Biomimetic preparation of polymer-supported free radical scavenging, cytocompatible and antimicrobial “green” silver nanoparticles using aqueous extract of Citrus sinensis peel. Colloids Surf B 84(2):338–345. https://doi.org/10.1016/j.colsurfb.2011.01.024
Kowshik M, Ashtaputre S, Kharrazi S, Vogel W, Urban J, Kulkarni SK, Paknikar K (2002) Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 14(1):95
Kumar SA, Abyaneh MK, Gosavi S, Kulkarni SK, Pasricha R, Ahmad A, Khan M (2007) Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol Lett 29(3):439–445
Kumar V, Yadav SC, Yadav SK (2010) Syzygium cumini leaf and seed extract mediated biosynthesis of silver nanoparticles and their characterization. J Chem Technol Biotechnol 85(10):1301–1309
Kumari R, Singh JS, Singh DP (2017) Biogenic synthesis and spatial distribution of silver nanoparticles in the legume mungbean plant (Vigna radiata L.), Plant Physiol Biochem. 110:158–166. https://doi.org/10.1016/j.plaphy.2016.06.001
Kushwaha A, Singh VK, Bhartariya J, Singh P, Yasmeen K (2015) Isolation and identification of E. coli bacteria for the synthesis of silver nanoparticles: characterization of the particles and study of antibacterial activity. Eur J Exp Biol 5(1):65–70
Laurent S, Henoumont C, Stanicki D, Boutry S, Lipani E, Belaid S, Muller RN, Vander Elst L (2017) Superparamagnetic iron oxide nanoparticles MRI contrast agents. Springer, pp:55–109
Lee KD, Kuppusamy P, Kim DH, Govindan N, Maniam GP, Choi KC (2018) Forage crop Lolium multiflorum assisted synthesis of AgNPs and their bioactivities against poultry pathogenic bacteria in in vitro. Indian J Microbiol 58(4):507–514. https://doi.org/10.1007/s12088-018-0755-8
Li JJ, Hartono D, Ong C-N, Bay B-H, Yung L-YL (2010) Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 31(23):5996–6003
Li W, Chen C, Ye C, Wei T, Zhao Y, Lao F, Chen Z, Meng H, Gao Y, Yuan H (2008) The translocation of fullerenic nanoparticles into lysosome via the pathway of clathrin-mediated endocytosis. Nanotechnology 19(14):145102
Li X, Xu H, Chen Z-S, Chen G (2011) Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater 2011. https://doi.org/10.1155/2011/270974
Lin M, Zhao Y, Wang S, Liu M, Duan Z, Chen Y, Li F, Xu F, Lu T (2012) Recent advances in synthesis and surface modification of lanthanide-doped upconversion nanoparticles for biomedical applications. Biotechnol Adv 30(6):1551–1561
Lobner D (2000) Comparison of the LDH and MTT assays for quantifying cell death: validity for neuronal apoptosis? J Neurosci Methods 96(2):147–152
Lodha A, Ansari N, Shah S, Rao M, Menon SK (2017) Isolation of PCR ready-human DNA using copper nanoparticles from skeletal remains. Forensic Sci Int 270:146–152
Lohbe A, Ullrich S, Katzmann E, Borg S, Wanner G, Richter M, Voigt B, Schweder T, Schuler D (2011) Functional analysis of the magnetosome island in Magnetospirillum gryphiswaldense: the mamAB operon is sufficient for magnetite biomineralization. PLoS One 6(10):e25561
Lopes L, Brito LM, Bezerra TT, Gomes KN, Carvalho FA, Chaves MH, Cantanhede W (2018) Silver and gold nanoparticles from tannic acid: synthesis, characterization and evaluation of antileishmanial and cytotoxic activities. An Acad Bras Cienc 90(3):2679–2689
M Joseph M, K George S, T Sreelekha T (2016) Bridging ‘green’ with nanoparticles: biosynthesis approaches for cancer management and targeting of cancer stem cells. Curr Nanosci 12(1):47–62
Maddinedi SB, Mandal BK, Anna KK (2017) Environment friendly approach for size controllable synthesis of biocompatible silver nanoparticles using diastase. Environ Toxicol Pharmacol 49:131–136
Malaikozhundan B, Vaseeharan B, Vijayakumar S, Pandiselvi K, Kalanjiam MAR, Murugan K, Benelli G (2017) Biological therapeutics of Pongamia pinnata coated zinc oxide nanoparticles against clinically important pathogenic bacteria, fungi and MCF-7 breast cancer cells. Microb Pathogen 104:268–277. https://doi.org/10.1016/j.micpath.2017.01.029
Malireddy S, Kotha SR, Secor JD, Gurney TO, Abbott JL, Maulik G, Maddipati KR, Parinandi NL (2012) Phytochemical antioxidants modulate mammalian cellular epigenome: implications in health and disease. Antioxid Redox Signal 17(2):327–339
Maliszewska I (2011) Microbial synthesis of metal nanoparticles metal nanoparticles in microbiology. Springer, pp 153–175
Mann S, Burkett SL, Davis SA, Fowler CE, Mendelson NH, Sims SD, Walsh D, Whilton NT (1997) Sol−gel synthesis of organized matter. Chem Mater 9(11):2300–2310
Marcano L, García-Prieto A, Muñoz D, Fernández Barquín L, Orue I, Alonso J, Muela A, Fdez-Gubieda ML (2017) Influence of the bacterial growth phase on the magnetic properties of magnetosomes synthesized by Magnetospirillum gryphiswaldense. Biochim Biophys Acta Gen Subj 1861(6):1507–1514. https://doi.org/10.1016/j.bbagen.2017.01.012
McIntosh DP, Tan X-Y, Oh P, Schnitzer JE (2002) Targeting endothelium and its dynamic caveolae for tissue-specific transcytosis in vivo: a pathway to overcome cell barriers to drug and gene delivery. Proc Natl Acad Sci 99(4):1996. https://doi.org/10.1073/pnas.251662398
Mirzaei H, Darroudi M (2017) Zinc oxide nanoparticles: biological synthesis and biomedical applications. Ceram Int 43(1):907–914
Mishra A, Tripathy SK, Wahab R, Jeong S-H, Hwang I, Yang Y-B, Kim Y-S, Shin H-S, Yun S-I (2011) Microbial synthesis of gold nanoparticles using the fungus Penicillium brevicompactum and their cytotoxic effects against mouse mayo blast cancer C2C12 cells. Appl Microbiol Biotechnol 92(3):617–630
Mishra AR, Zheng J, Tang X, Goering PL (2016) Silver nanoparticle-induced autophagic-lysosomal disruption and NLRP3-inflammasome activation in HepG2 cells is size-dependent. Toxicol Sci 150(2):473–487
Mittal AK, Chisti Y, Banerjee UC (2013) Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv 31(2):346–356
Mo Y-y, Tang Y-k, Wang S-y, Lin J-m, Zhang H-b, Luo D-y (2015) Green synthesis of silver nanoparticles using eucalyptus leaf extract. Mater Lett 144:165–167. https://doi.org/10.1016/j.matlet.2015.01.004
Momin B, Rahman S, Jha N, Annapure US (2019) Valorization of mutant Bacillus licheniformis M09 supernatant for green synthesis of silver nanoparticles: photocatalytic dye degradation, antibacterial activity, and cytotoxicity. Bioprocess Biosyst Eng. https://doi.org/10.1007/s00449-018-2057-2
Monteiro-Riviere NA, Samberg ME, Oldenburg SJ, Riviere JE (2013) Protein binding modulates the cellular uptake of silver nanoparticles into human cells: implications for in vitro to in vivo extrapolations? Toxicol Lett 220(3):286–293. https://doi.org/10.1016/j.toxlet.2013.04.022
Mou X, Ali Z, Li S, He N (2015) Applications of magnetic nanoparticles in targeted drug delivery system. J Nanosci Nanotechnol 15(1):54–62
Mourato A, Gadanho M, Lino AR, Tenreiro R (2011) Biosynthesis of crystalline silver and gold nanoparticles by extremophilic yeasts. Bioinorg Chem Appl 2011. https://doi.org/10.1155/2011/546074
MubarakAli D, Thajuddin N, Jeganathan K, Gunasekaran M (2011) Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf B 85(2):360–365
Murugan K, Benelli G, Panneerselvam C, Subramaniam J, Jeyalalitha T, Dinesh D, Nicoletti M, Hwang J-S, Suresh U, Madhiyazhagan P (2015) Cymbopogon citratus-synthesized gold nanoparticles boost the predation efficiency of copepod Mesocyclops aspericornis against malaria and dengue mosquitoes. Exp Parasitol 153:129–138
Muthiah M, Park I-K, Cho C-S (2013) Surface modification of iron oxide nanoparticles by biocompatible polymers for tissue imaging and targeting. Biotechnol Adv 31(8):1224–1236
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interf Sci 156(1):1–13
Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M (2009) Understanding biophysicochemical interactions at the nano–bio interface. Nat Mater 8:543. https://doi.org/10.1038/nmat2442
Nellore J, Pauline C, Amarnath K (2013) Bacopa monnieri phytochemicals mediated synthesis of platinum nanoparticles and its neurorescue effect on 1-methyl 4-phenyl 1, 2, 3, 6 tetrahydropyridine-induced experimental parkinsonism in zebrafish. J Neurodegenerative Dis 2013. ID 972391
Noruzi M (2015) Biosynthesis of gold nanoparticles using plant extracts. Bioprocess Biosyst Eng 38(1):1–14
Otari SV, Patil RM, Ghosh SJ, Thorat ND, Pawar SH (2015) Intracellular synthesis of silver nanoparticle by actinobacteria and its antimicrobial activity, Spectrochim Acta, Part A. 136(Part B):1175–1180. https://doi.org/10.1016/j.saa.2014.10.003
Ovais M, Khalil AT, Islam NU, Ahmad I, Ayaz M, Saravanan M, Shinwari ZK, Mukherjee S (2018) Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl Microbiol Biotechnol 102(16):6799–6814. https://doi.org/10.1007/s00253-018-9146-7
Palchoudhury S, Zhou Z, Ramasamy K, Okirie F, Prevelige PE, Gupta A (2017) Self-assembly of P22 protein cages with polyamidoamine dendrimer and inorganic nanoparticles. J Mater Res 32(2):465–472
Pallela PNVK, Ummey S, Ruddaraju LK, Pammi SVN, Yoon S-G (2018) Ultra small, mono dispersed green synthesized silver nanoparticles using aqueous extract of Sida cordifolia plant and investigation of antibacterial activity. Microb Pathog 124:63–69. https://doi.org/10.1016/j.micpath.2018.08.026
Panda KK, Achary VMM, Krishnaveni R, Padhi BK, Sarangi SN, Sahu SN, Panda BB (2011) In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol in Vitro 25(5):1097–1105. https://doi.org/10.1016/j.tiv.2011.03.008
Park S, Lee YK, Jung M, Kim KH, Chung N, Ahn E-K, Lim Y, Lee K-H (2007) Cellular toxicity of various inhalable metal nanoparticles on human alveolar epithelial cells. Inhalation Toxicol 19(sup1):59-65. https://doi.org/10.1080/08958370701493282
Parker HL, Rylott EL, Hunt AJ, Dodson JR, Taylor AF, Bruce NC, Clark JH (2014) Supported palladium nanoparticles synthesized by living plants as a catalyst for Suzuki-Miyaura reactions. PLoS One 9(1):e87192
Partlow KC, Lanza GM, Wickline SA (2008) Exploiting lipid raft transport with membrane targeted nanoparticles: a strategy for cytosolic drug delivery. Biomaterials 29(23):3367–3375. https://doi.org/10.1016/j.biomaterials.2008.04.030
Patil MP, Kim G-D (2017) Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl Microbiol Biotechnol 101(1):79–92
Pereira L, Mehboob F, Stams AJ, Mota MM, Rijnaarts HH, Alves MM (2015) Metallic nanoparticles: microbial synthesis and unique properties for biotechnological applications, bioavailability and biotransformation. Crit Rev Biotechnol 35(1):114–128
Pietta P-G (2000) Flavonoids as antioxidants. J Nat Prod 63(7):1035–1042. https://doi.org/10.1021/np9904509
Prasad KS, Vaghasiya JV, Soni SS, Patel J, Patel R, Kumari M, Jasmani F, Selvaraj K (2015) Microbial selenium nanoparticles (SeNPs) and their application as a sensitive hydrogen peroxide biosensor. Appl Biochem Biotechnol 177(6):1386–1393. https://doi.org/10.1007/s12010-015-1814-9
Prasad R, Pandey R, Barman I (2016) Engineering tailored nanoparticles with microbes: quo vadis? Wiley Interdiscip Rev Nanomed Nanobiotechnol 8(2):316–330
Priyadarshini S, Gopinath V, Meera Priyadharsshini N, MubarakAli D, Velusamy P (2013) Synthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus and its biomedical application. Colloids Surf B 102:232–237. https://doi.org/10.1016/j.colsurfb.2012.08.018
Pugazhendhi A, Prabhu R, Muruganantham K, Shanmuganathan R, Natarajan S (2019) Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J Photochem Photobiol B 190:86–97. https://doi.org/10.1016/j.jphotobiol.2018.11.014
Pugazhenthiran N, Anandan S, Kathiravan G, Prakash NKU, Crawford S, Ashokkumar M (2009) Microbial synthesis of silver nanoparticles by Bacillus sp. J Nanopart Res 11(7):1811
Raghunandan D, Basavaraja S, Mahesh B, Balaji S, Manjunath SY, Venkataraman A (2009) Biosynthesis of stable polyshaped gold nanoparticles from microwave-exposed aqueous extracellular anti-malignant guava (Psidium guajava) leaf extract. NanoBiotechnology 5(1):34–41. https://doi.org/10.1007/s12030-009-9030-8
Rajakumar G, Gomathi T, Thiruvengadam M, Devi Rajeswari V, Kalpana VN, Chung I-M (2017) Evaluation of anti-cholinesterase, antibacterial and cytotoxic activities of green synthesized silver nanoparticles using from Millettia pinnata flower extract. Microb Pathog 103:123–128. https://doi.org/10.1016/j.micpath.2016.12.019
Rajan A, Rajan AR, Philip D (2017) Elettaria cardamomum seed mediated rapid synthesis of gold nanoparticles and its biological activities. OpenNano 2:1–8. https://doi.org/10.1016/j.onano.2016.11.002
Rajesh S, Raja DP, Rathi J, Sahayaraj K (2012) Biosynthesis of silver nanoparticles using Ulva fasciata (Delile) ethyl acetate extract and its activity against Xanthomonas campestris pv. malvacearum. J Biopesticides 5:119–128
Ramar K, Gnanamoorthy G, Mukundan D, Vasanthakumari R, Narayanan V, Jafar Ahamed A (2018) Environmental and antimicrobial properties of silver nanoparticles synthesized using Azadirachta indica Juss leaves extract. SN Appl Sci 1(1):128. https://doi.org/10.1007/s42452-018-0143-3
Rao NH, Lakshmidevi N, Pammi S, Kollu P, Ganapaty S, Lakshmi P (2016) Green synthesis of silver nanoparticles using methanolic root extracts of Diospyros paniculata and their antimicrobial activities. Mater Sci Eng C 62:553–557
Rao KJ, Paria S (2015) Aegle marmelos leaf extract and plant surfactants mediated green synthesis of au and ag nanoparticles by optimizing process parameters using Taguchi method. ACS Sustain Chem Eng 3(3):483–491. https://doi.org/10.1021/acssuschemeng.5b00022
Roopan SM, Bharathi A, Prabhakarn A, Abdul Rahuman A, Velayutham K, Rajakumar G, Padmaja RD, Lekshmi M, Madhumitha G (2012) Efficient phyto-synthesis and structural characterization of rutile TiO2 nanoparticles using Annona squamosa peel extract. Spectrochim Acta, Part A 98:86–90. https://doi.org/10.1016/j.saa.2012.08.055
Roopan SM, Madhumitha G, Rahuman AA, Kamaraj C, Bharathi A, Surendra T (2013) Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind Crop Prod 43:631–635
Roy B, Mukherjee S, Mukherjee N, Chowdhury P, Sinha Babu SP (2014) Design and green synthesis of polymer inspired nanoparticles for the evaluation of their antimicrobial and antifilarial efficiency. RSC Adv 4(65):34487–34499. https://doi.org/10.1039/C4RA03732D
Sadhasivam S, Shanmugam P, Veerapandian M, Subbiah R, Yun K (2012) Biogenic synthesis of multidimensional gold nanoparticles assisted by Streptomyces hygroscopicus and its electrochemical and antibacterial properties. BioMetals 25(2):351–360. https://doi.org/10.1007/s10534-011-9506-6
Sadhasivam S, Shanmugam P, Yun K (2010) Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids Surf B 81(1):358–362. https://doi.org/10.1016/j.colsurfb.2010.07.036
Salari Z, Danafar F, Dabaghi S, Ataei SA (2016) Sustainable synthesis of silver nanoparticles using macroalgae Spirogyra varians and analysis of their antibacterial activity. J Saudi Chem Soc 20(4):459–464
Sanjenbam P, Gopal JV, Kannabiran K (2014) Anticandidal activity of silver nanoparticles synthesized using Streptomyces sp. VITPK1. J Med Mycol 24(3):211–219. https://doi.org/10.1016/j.mycmed.2014.03.004
Sankar R, Rahman PKSM, Varunkumar K, Anusha C, Kalaiarasi A, Shivashangari KS, Ravikumar V (2017) Facile synthesis of Curcuma longa tuber powder engineered metal nanoparticles for bioimaging applications. J Mol Struct 1129:8–16. https://doi.org/10.1016/j.molstruc.2016.09.054
Sarkar J, Chattopadhyay D, Patra S, Singh Deo S, Sinha S, Ghosh M, Mukherjee A, Acharya K (2011) Alternaria alternata mediated synthesis of protein capped silver nanoparticles and their genotoxic activity. Digest J Nanomater Biostructures (DJNB) 6(2):563–573
Sathishkumar M, Sneha K, Won SW, Cho CW, Kim S, Yun YS (2009) Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf B 73(2):332–338. https://doi.org/10.1016/j.colsurfb.2009.06.005
Schöbel J, Burgard M, Hils C, Dersch R, Dulle M, Volk K, Karg M, Greiner A, Schmalz H (2017) Bottom-up meets top-down: patchy hybrid nonwovens as an efficient catalysis platform. Angew Chem Int Ed 56(1):405–408
Sendra M, Yeste PM, Moreno-Garrido I, Gatica JM, Blasco J (2017) CeO2 NPs, toxic or protective to phytoplankton? Charge of nanoparticles and cell wall as factors which cause changes in cell complexity. Sci Total Environ 590-591:304–315. https://doi.org/10.1016/j.scitotenv.2017.03.007
Shang L, Nienhaus K, Nienhaus GU (2014) Engineered nanoparticles interacting with cells: size matters. J Nanobiotechnol 12(1):5. https://doi.org/10.1186/1477-3155-12-5
Shenton W, Douglas T, Young M, Stubbs G, Mann S (1999) Inorganic–organic nanotube composites from template mineralization of tobacco mosaic virus. Adv Mater 11(3):253–256
Sicard C, Brayner R, Margueritat J, Hemadi M, Coute A, Yepremian C, Djediat C, Aubard J, Fievet F, Livage J, Coradin T (2010) Nano-gold biosynthesis by silica-encapsulated micro-algae: a “living” bio-hybrid material. J Mater Chem 20(42):9342–9347. https://doi.org/10.1039/C0JM01735C
Singh AK, Talat M, Singh DP, Srivastava ON (2010) Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. J Nanopart Res 12(5):1667–1675. https://doi.org/10.1007/s11051-009-9835-3
Singh P, Kim YJ, Wang C, Mathiyalagan R, Yang DC (2016) The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif Cells Nanomed Biotechnol 44(4):1150–1157
Soliman H, Elsayed A, Dyaa A (2018) Antimicrobial activity of silver nanoparticles biosynthesised by Rhodotorula sp. strain ATL72. Egypt J Basic Appl Sci 5(3):228–233. https://doi.org/10.1016/j.ejbas.2018.05.005
Spagnoletti FN, Spedalieri C, Kronberg F, Giacometti R (2019) Extracellular biosynthesis of bactericidal Ag/AgCl nanoparticles for crop protection using the fungus Macrophomina phaseolina. J Environ Manag 231:457–466. https://doi.org/10.1016/j.jenvman.2018.10.081
Srinath BS, Rai VR (2018) Phytochemicals-mediated green synthesis of gold nanoparticles using Pterocarpus santalinus L. (Red Sanders) bark extract and their antimicrobial properties. Part Sci Technol 36(7):785–790. https://doi.org/10.1080/02726351.2017.1302533
Sriranjani R, Srinithya B, Vellingiri V, Brindha P, Anthony SP, Sivasubramanian A, Muthuraman MS (2016) Silver nanoparticle synthesis using Clerodendrum phlomidis leaf extract and preliminary investigation of its antioxidant and anticancer activities. J Mol Liq 220:926–930
Sun J-B, Duan J-H, Dai S-L, Ren J, Guo L, Jiang W, Li Y (2008) Preparation and anti-tumor efficiency evaluation of doxorubicin-loaded bacterial magnetosomes: magnetic nanoparticles as drug carriers isolated from Magnetospirillum gryphiswaldense. Biotechnol Bioeng 101(6):1313–1320. https://doi.org/10.1002/bit.22011
Tahir K, Nazir S, Ahmad A, Li B, Khan AU, Khan ZUH, Khan FU, Khan QU, Khan A, Rahman AU (2017) Facile and green synthesis of phytochemicals capped platinum nanoparticles and in vitro their superior antibacterial activity. J Photochem Photobiol B 166:246–251. https://doi.org/10.1016/j.jphotobiol.2016.12.016
Tamuly C, Hazarika M, Bordoloi M, Bhattacharyya PK, Kar R (2014) Biosynthesis of Ag nanoparticles using pedicellamide and its photocatalytic activity: an eco-friendly approach. Spectrochim Acta, Part A 132:687–691. https://doi.org/10.1016/j.saa.2014.05.024
Tamuly C, Hazarika M, Bordoloi M, Das MR (2013) Photocatalytic activity of Ag nanoparticles synthesized by using Piper pedicellatum C.DC fruits. Mater Lett 102-103:1–4. https://doi.org/10.1016/j.matlet.2013.03.090
Tan YN, Lee JY, Wang DIC (2010) Uncovering the design rules for peptide synthesis of metal nanoparticles. J Am Chem Soc 132(16):5677–5686. https://doi.org/10.1021/ja907454f
Tang Y-S, Wang D, Zhou C, Zhang S (2019) Preparation and anti-tumor efficiency evaluation of bacterial magnetosome–anti-4-1BB antibody complex: bacterial magnetosome as antibody carriers isolated from Magnetospirillum gryphiswaldense. Biotechnol Appl Biochem 0(0) https://doi.org/10.1002/bab.1724
Tang Y, Tsao R (2017) Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: a review. Mol Nutr Food Res 61(7):1600767. https://doi.org/10.1002/mnfr.201600767
Thakur S, Sarkar B, Cholia RP, Gautam N, Dhiman M, Mantha AK (2014) APE1/Ref-1 as an emerging therapeutic target for various human diseases: phytochemical modulation of its functions. Exp Mol Med 46(7):e106
Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signaling. Am J Physiol Cell Mol Physiol 279(6):L1005–L1028
Uchida M, Qazi S, Edwards E, Douglas T (2015) Use of protein cages as a template for confined synthesis of inorganic and organic nanoparticles. Protein cages: methods protocols:17–25
Ullrich S, Kube M, Schübbe S, Reinhardt R, Schüler D (2005) A hypervariable 130-kilobase genomic region of Magnetospirillum gryphiswaldense comprises a magnetosome island which undergoes frequent rearrangements during stationary growth. J Bacteriol 187(21):7176–7184. https://doi.org/10.1128/jb.187.21.7176-7184.2005
Umar H, Kavaz D, Rizaner N (2018) Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int J Nanomedicine 14:87–100. https://doi.org/10.2147/IJN.S186888
Undabarrena A, Ugalde JA, Seeger M, Camara B (2017) Genomic data mining of the marine actinobacteria Streptomyces sp. H-KF8 unveils insights into multi-stress related genes and metabolic pathways involved in antimicrobial synthesis. PeerJ 5:e2912
Van Haute D, Liu AT, Berlin JM (2018) Coating metal nanoparticle surfaces with small organic molecules can reduce nonspecific cell uptake. ACS Nano 12(1):117–127
Varma RS (2012) Greener approach to nanomaterials and their sustainable applications. Curr Opin Chem Eng 1(2):123–128. https://doi.org/10.1016/j.coche.2011.12.002
Veerakumar K, Govindarajan M, Rajeswary M (2013) Green synthesis of silver nanoparticles using Sida acuta (Malvaceae) leaf extract against Culex quinquefasciatus, Anopheles stephensi, and Aedes aegypti (Diptera: Culicidae). Parasitol Res 112(12):4073–4085. https://doi.org/10.1007/s00436-013-3598-6
Verma A, Stellacci F (2010) Effect of surface properties on nanoparticle–cell interactions. Small 6(1):12–21
Verma A, Mehata MS (2016) Controllable synthesis of silver nanoparticles using neem leaves and their antimicrobial activity. J Radiat Res Appl Sci 9(1):109–115. https://doi.org/10.1016/j.jrras.2015.11.001
Vial S, Berrahal Y, Prado M, Wenger J (2017) Single-step DNA detection assay monitoring dual-color light scattering from individual metal nanoparticle aggregates. ACS Sens 2(2):251–256. https://doi.org/10.1021/acssensors.6b00737
Vijayakumar PS, Prasad BLV (2009) Intracellular biogenic silver nanoparticles for the generation of carbon supported antiviral and sustained bactericidal agents. Langmuir 25(19):11741–11747. https://doi.org/10.1021/la901024p
Waghmare SR, Mulla MN, Marathe SR, Sonawane KD (2015) Ecofriendly production of silver nanoparticles using Candida utilis and its mechanistic action against pathogenic microorganisms. 3 Biotech 5(1):33–38
Wang C, Kim YJ, Singh P, Mathiyalagan R, Jin Y, Yang DC (2016) Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artif Cells Nanomed Biotechnol 44(4):1127–1132
Wang J, Hao S, Luo T, Cheng Z, Li W, Gao F, Guo T, Gong Y, Wang B (2017) Feather keratin hydrogel for wound repair: preparation, healing effect and biocompatibility evaluation. Colloids Surf B 149:341–350
Wang T, Jin X, Chen Z, Megharaj M, Naidu R (2014) Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci Total Environ 466–467:210–213. https://doi.org/10.1016/j.scitotenv.2013.07.022
Wang T, Yang L, Zhang B, Liu J (2010) Extracellular biosynthesis and transformation of selenium nanoparticles and application in H2O2 biosensor. Colloids Surf B 80(1):94–102. https://doi.org/10.1016/j.colsurfb.2010.05.041
Wang W, Chen Q, Jiang C, Yang D, Liu X, Xu S (2007) One-step synthesis of biocompatible gold nanoparticles using gallic acid in the presence of poly-(N-vinyl-2-pyrrolidone). Colloids Surf A Physicochem Eng Asp 301(1):73–79. https://doi.org/10.1016/j.colsurfa.2006.12.037
Wang Z, Tiruppathi C, Minshall RD, Malik AB (2009) Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano 3(12):4110–4116. https://doi.org/10.1021/nn9012274
Wu W, Wu Z, Yu T, Jiang C, Kim W-S (2015) Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater 16(2):023501
Wu Y-N, Yang L-X, Shi X-Y, Li I-C, Biazik JM, Ratinac KR, Chen D-H, Thordarson P, Shieh D-B, Braet F (2011) The selective growth inhibition of oral cancer by iron core-gold shell nanoparticles through mitochondria-mediated autophagy. Biomaterials 32(20):4565–4573
Xiang L, Wei J, Jianbo S, Guili W, Feng G, Ying L (2007) Purified and sterilized magnetosomes from Magnetospirillum gryphiswaldense MSR-1 were not toxic to mouse fibroblasts in vitro. Lett Appl Microbiol 45(1):75–81. https://doi.org/10.1111/j.1472-765X.2007.02143.x
Yang H, Liu C, Yang D, Zhang H, Xi Z (2009) Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol 29(1):69–78
Yang N, Li W-H (2013) Mango peel extract mediated novel route for synthesis of silver nanoparticles and antibacterial application of silver nanoparticles loaded onto non-woven fabrics. Ind Crop Prod 48:81–88. https://doi.org/10.1016/j.indcrop.2013.04.001
Yang Z, Li Z, Lu X, He F, Zhu X, Ma Y, He R, Gao F, Ni W, Yi Y (2016) Controllable biosynthesis and properties of gold nanoplates using yeast extract. Nano-Micro Letters 9(1):5. https://doi.org/10.1007/s40820-016-0102-8
Zeth K, Hoiczyk E, Okuda M (2016) Ferroxidase-mediated iron oxide biomineralization: novel pathways to multifunctional nanoparticles. Trends BiochemSci 41(2):190–203
Zhang H, Liu S (2017) A combined self-assembly and calcination method for preparation of nanoparticles-assembled cobalt oxide nanosheets using graphene oxide as template and their application for non-enzymatic glucose biosensing. J Colloid Interface Sci 485:159–166
Zhang LW, Yang J, Barron AR, Monteiro-Riviere NA (2009) Endocytic mechanisms and toxicity of a functionalized fullerene in human cells. Toxicol Lett 191(2):149–157. https://doi.org/10.1016/j.toxlet.2009.08.017
Zheng B, Qian L, Yuan H, Xiao D, Yang X, Paau MC, Choi MMF (2010) Preparation of gold nanoparticles on eggshell membrane and their biosensing application. Talanta 82(1):177–183. https://doi.org/10.1016/j.talanta.2010.04.014
Zheng B, Xie S, Qian L, Yuan H, Xiao D, Choi MMF (2011) Gold nanoparticles-coated eggshell membrane with immobilized glucose oxidase for fabrication of glucose biosensor. Sensors Actuators B Chem 152(1):49–55. https://doi.org/10.1016/j.snb.2010.09.051
Zhou Y, Lin W, Huang J, Wang W, Gao Y, Lin L, Li Q, Lin L, Du M (2010) Biosynthesis of gold nanoparticles by foliar broths: roles of biocompounds and other attributes of the extracts. Nanoscale Res Lett 5(8):1351. https://doi.org/10.1007/s11671-010-9652-8
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. U1632126).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, F., Ashraf, N., Ashraf, T. et al. Biological synthesis of metallic nanoparticles (MNPs) by plants and microbes: their cellular uptake, biocompatibility, and biomedical applications. Appl Microbiol Biotechnol 103, 2913–2935 (2019). https://doi.org/10.1007/s00253-019-09675-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09675-5